INTRODUCTION
Industrial and domestic wastes, insecticides, herbicides and other pesticides, radioactive wastes, silt from gravel washing or soil erosion, cooling water used in steam or atomic energy plants may adversely affect aquatic life if these pollutants are not properly treated or excluded from streams, reservoirs or lakes.
Effective methods of waste treatment are known for practically all industrial and domestic wastes. There are guidelines available for the treatment of many kinds of wastes. Recovery of chemicals in some waste treatment processes actually pay for the cost of treatment.
Extensive research on the biological and physiological effects of industrial wastes, pesticides, radioactive materials, detergents, temperature, etc., has been carried out in many parts of the world. The general result of this research has been the establishment of more rigid water quality requirements for fish and other aquatic life than has formerly been deemed necessary.
Industries are encouraged to determine by means of bioassays the toxicity of their effluents to fish and other aquatic life. The toxic components of many industrial wastes are already well known. These components may be directly toxic, they may taint fish flesh rendering them inedible, or they may be oxygen-demanding materials that use up the available oxygen supply of streams or other waters.
In the following sections are listed water quality criteria that may be affected by many kinds of wastes, both industrial and domestic. They are the water quality objectives which if maintained in Iranian waters will protect nearly all forms of fish and other aquatic life.
The preparation of this manual for the Government Iran was suggested by H.E. Dr. A.A. Ahmadi, Under Secretary, Parliamentary and Technical Affairs, Ministry of Natural Resources on 22 September 1968. In its preparation the writer, has followed the recent recommendations of the United States' Report of the National Technical Advisory Committee to the Secretary of the Interior entitled “Water Quality Criteria” published 1 April 1968 by the Federal Water Pollution Control Administration, Washington, D.C., U.S.A. (234 p.)
For these industries, institutions or persons wishing to follow standard methods developed in the United States of America for determining the toxicity of pollutants to fish and other aquatic life, the methods are described in "Standard Methods for the Examination of Water and Waste Water including Bottom Sediment and Sludges. 1965, 12th Ed., American Public Health Association, Inc., New York. The section on “Bioassays to Evaluate Toxicity to Fish” has been revised this year to include continuous-flow bioassays as well as static bioassays.
A “Definition of Terms” section has been prepared to assist the reader in understanding the use of words or technical terms. It appears as Appendix A.
Eugene W. Surber
Inland Fishery Biologist
FAO/UNDP/TA Advisor to the Ministry of Natural Resources
Water Quality Criteria
The criteria that follow are those that are necessary for the protection of fish and other aquatic life. They are the limits, within our present knowledge, that permit not only survival, but growth and reproduction as well.
pH, Alkalinity, Acidity
(1) No highly dissociated materials should be added in quantities sufficient to lower the pH below 6.0 or to raise the pH above 9.0.
(2) To protect the carbonate system and thus the productivity of the water, acid should not be added in sufficient quantity to lower the total alkalinity to less than 20 mg/l.
(3) The addition of weakly dissociated acids and alkalies should be regulated in terms of their own toxicities as established by bioassay procedures.
Temperature
Warm-water Biota
To maintain a well rounded population of warm-water fishes, the following restrictions on temperature extremes and temperature increases are recommended:
(1) During any month of the year heat should not be added to a stream in excess of the amount that will raise the temperature of the water (at the expected minimum flow-for that month) more than 3.0°C. In lakes, the temperature of the epilimnion in those areas where important organisms are most likely to be adversely affected should not be raised more that 2.0°C above that which existed before the addition of heat of artificial origin. The increases should be based on the monthly average of the maximum daily temperatures. Unless a special study shows that a discharge of a heated effluent into the hypolimnion will be desirable, such practice is not recommended and water for cooling should not be pumped from the hypolimnion to be discharged to the same body of water.
(2) The normal daily and seasonal temperature variations that were present before the addition of heat due to other than natural causes should be maintained.
Cold-water Biota
Inland trout streams, headwaters of salmon streams, trout and salmon lakes, and the hypolimnion of lakes and reservoirs containing salmonids and other cold water forms should not be warmed or used for cooling water. No heated effluent should be discharged in the vicinity of spawning areas.
For other types and reaches of cold-water streams, reservoirs and lakes, the following restrictions are recommended:
(1) During any month of the year heat should not be added to a stream in excess of the amount that will raise the temperature of the water more than 3.0°C, based on the minimum expected flow for that month. In lakes, the temperature of the epilimnion should not be raised more than 2.0°C by the addition of heat of artificial origin.
(2) The normal daily and seasonal temperature fluctuations that existed before the addition of heat due to other than natural causes should be maintained.
(3) The recommended maximum temperatures that are not to be exceeded for some species of fish are listed below:
| °C | |
| 36.0 | Growth of tilapia |
| 32.0 | Growth of carp |
| 29.0 | Growth of pike-perch, perch |
| 27.0 | Spawning and egg development of sturgeon |
| 20.0 | Growth and migration routes of salmonids and for egg development of perch |
| 13.0 | Spawning and egg development of salmon and trout (other than lake trout) |
| 9.0 | Spawning and egg development of lake trout, pike-perch, pike |
Dissolved Oxygen
(1) For a diversified warm-water biota, including food fishes, the dissolved oxygen (DO) concentration should be above 5 mg/l, assuming normal seasonal and daily variations are above this concentration. Under extreme conditions, however, they may range between 5 and 4 mg/l for short periods during any 24 hour period, provided that the water quality is favourable in all other respects. In stratified lakes, the DO requirements may not apply to the hypolimnion. In shallow unstratified lakes, they should apply to the entire circulation water mass.
These requirements should apply to all waters except administratively established mixing zones. In lakes, such zones must be restricted so as to limit the effect on the biota. In streams, there must be adequate and safe passageways for migrating forms. These must be extensive enough so that the majority of plankton and other drifting organisms are protected.
(2) For the cold-water biota, it is desirable that DO concentration be at or near saturation. This is especially important in spawning areas where DO levels must not be below 7 mg/l at any time.
For good growth and the general well-being of trout, salmon, and their associated biota, DO concentrations should not be below 6 mg/l. Under extreme conditions they may range between 6 and 5 mg/l for short periods provided the water quality is favourable in all other respects. In large streams that have some stratification or that serve principally as migratory routes, DO levels may range between 4 and 5 mg/l for periods up to 6 hours, but should be below 4 mg/l at any time or place.
(3) DO levels in the hypolimnion of oligotrophic small inland lakes and in large lakes should not be lowered below 6 mg/l at any time due to the addition of oxygen demanding water or other materials.
Carbon Dioxide
“Free” carbon dioxide concentration should not exceed 25 mg/l.
Oil
Oil or petrochemicals should not be added in such quantities to the receiving waters that they will:-
produce a visible color film on the surface;
impart an oily odor to the water or an oily or noxious taste to fish and edible invertebrates;
coat the banks and bottoms of the water course or taint any of the associated biota;
become effective toxicants.
Turbidity
(1) Turbidity in the receiving waters due to the discharge of wastes should not exceed 50 Jackson units in warm-water streams or 10 Jackson units in cold-water streams.
(2) There should be no discharge to warm-water lakes which would cause turbidity exceeding 25 Jackson units. The turbidity of cold-water or oligotrophic lakes should not exceed 10 units.
Dissolved Materials
(1) Dissolved materials that are relatively innocuous; i.e., their harmful effect is due to osmotic effects at high concentrations, should not be increased by more than one-third of the concentration that is characteristic of the natural condition of the subject water. In no instance should the concentration of total dissolved materials exceed 50 milliosmoles (the equivalent of 1,500 mg/l NaCl).
(2) When dissolved materials are being increased, bioassays and field studies should be used to determine how much of the materials may be tolerated without reducing the number of the desired organisms.
Settleable Materials
Since it is known that even minor deposits of settleable materials inhibit the growth of normal stream and lake flora, no such materials should be added to these waters in quantities that adversely affect the natural biota.
Color and Transparency
For effective photosynthetic production of oxygen, it is required that 10 percent of the incident light reach the bottom of any desired photosynthetic zone in which adequate dissolved oxygen concentrations are to be maintained.
Floating Materials
All floating materials of foreign origin should be excluded from streams and lakes.
Tainting Substances
All materials that will impart odor or taste to fish or edible invertebrates should be excluded from receiving waters at levels that produce tainting.
Plant Nutrients and Nuisance Growths
(1) In order to limit nuisance growths, the addition of all organic wastes such as sewage, food processing, cannery, and industrial waste containing nutrients, vitamins, trace elements, and growth stimulants should be carefully controlled. The addition of sulphates or manganese oxide to a lake should be limited if iron is present in the hypolimnion as they may increase the quantity of available phosphorus.
(2) Nothing should be added that causes an increased zone of anaerobic decomposition of a lake or reservoir.
(3) The naturally occuring ratios and amounts of nitrogen (particularly NO3 and NH3) to total phosphorus should not be radically changed by the addition of materials, the concentration of total phosphorus should not be increased to levels exceeding 100 micrograms per litre in flowing streams or 50 micrograms per litre where streams enter lakes or reservoirs.
With periodic monitoring, undesirable trends can be detected and more stringent regulation of added organic materials imposed.
Toxic Substances
(1) Substances of Unknown Toxicity
All effluents containing foreign materials should be considered harmful and not permissible until bioassay tests have shown otherwise.
(2) Pesticides
Any addition of chlorinated hydrocarbon insecticides is likely to cause damage to some desired organisms and should be avoided.
Table I lists the 48-hour TLm (level at which half of the fish die) for a number of pesticides. To provide reasonably safe concentrations of these materials in receiving waters, application factors ranging from one tenth to one hundred should be used with these values depending on the characteristic of the pesticide in question.
Detergent and Surfactants
With continuous exposure, the concentration of ABS (Alkyl Benzene Sulphonate) should not exceed one-seventh of the 48-hour TLm concentration. Concentration as high as 1 mg/l may be tolerated infrequently for periods not exceeding 24 hours. ABS may increase the toxicity of other materials.
The concentration of LAS (Linear Alkyl Sulphonate) should not exceed 0.2 mg/l or one-seventh of the 48-hour TLm concentration, whichever is the lower.
TABLE I
48-hour TLm Values for Fish from Static Bioassays of Some Pesticides in common use*
micrograms per litre (1 microgram = 0.001 mg/l)
| Chlorinated Hydrocarbons | |||||
| TLm | Species | TLm | Species | ||
| Aldrin | 3.0 | Rainbow trout Salmo gairdnerii) | Toxaphene | 2.8 | Rainbow trout |
| Benzine Hezachloride | 18.0 | Rainbow trout | D.D.T. | 2.1 | Bass (Micropterus salmoides) |
| Chlordane | 10.0 | Rainbow trout | Dieldrin | 3.4 | Bluegill |
| Endrin | 0.2 | Bluegill (Lepomis macrochirus) | Endosulfan | 1.2 | Rainbow trout |
| Heptachlor | 9.0 | Rainbow trout | Malathion | 19.5 | Brook trout |
| Lindane | 0.2 | (?) | Methoxychlor | 7.2 | Rainbow trout |
| Perthane | 7.0 | Rainbow trout | TDE (DDD) | 9.0 | Rainbow trout |
| Organic Phosphorus Pesticides | Other Pesticides | ||||
| TLm | Species | TLm | Species | ||
| Coumaphos | 2.0 | (?) | Abate | 1,500.0 | Brook trout (Salvelinus fontinalis) |
| Dursban | 20.0 | Rainbow trout | Baytex | 80.0 | Brown trout (Salmo trutta) |
| Fenthion | 0.03 | (?) | Rotenone | 22.0 | Bluegill |
| Parathion | 47.0 | Bluegill | Trichlorofon (Dipterex) | 160 | Rainbow trout |
* Adapted from Table III - 5A, p. 62 Water Quality Criteria (1968)
Cyanide
Recent work on fish has demonstrated that HCN rather than CN is the toxic component, and that the toxicity of metallo-cyanide complexes are greatly affected by pH. For example, a thousand-fold increase in the toxicity of a nickelo-cyanide complex was associated with a drop in pH from 8.0 to 6.5. For lethal levels of Cyanide see Table II.
Metallo-cyanide complexes decompose in sunlight to become highly toxic due to the release of cyanide.
Permissible concentrations of cyanides should be determined by flow-through bioassays.
Ammonia
At pH levels of 8.0 and above, total ammonia expressed as N should not exceed 1.5 mg/l. It has been found that 2.5 mg/l total ammonia expressed as N is acutely toxic.
Heavy Metals
Zinc
For a given calcium and magnesium concentration, the acute toxicity of zinc increases (TLm concentration decreases) as pH is raised from 5 to 9.
The toxicity of zinc is also affected by the oxygen content of the water, increasing as the oxygen content decreases.
The toxicity of zinc to aquatic organisms should be determined by bioassay. It has been demonstrated that one-hundreth of the 96-hour TLm value is a safe concentration for continuous exposure.
Copper
The maximum copper (expressed as Cu) concentration (not including copper attached to silt particles or in stable organic combination) at any time or place should not be greater than one-tenth the 96-hour TLm value, nor should any 24-hour average concentration exceed one-thirtieth of the 96-hour TLm value.
Cadmium
The concentration of cadmium should not exceed one-thirtieth of the 96-hour TLm concentration at any time or place and the maximum 24-hour average concentration should not exceed one-five hundreth of the 96-hour TLm concentration.
Hexavalent Chromium
The toxicity of this compound to fish and other aquatic life is in need of further study. A concentration of 0.02 mg/l in soft water has been found safe for salmonoid fishes.
The growth of phytoplankton organisms has been inhibited by concentrations as low as 0.032–0.208 mg/l as Cr.
In Table III, are given lethal concentrations of a number of heavy metal compounds to fish.
TABLE II
Lethal Levels* of Cyanide (determined as CN, without Reference to pH) to Several Species of Fish
| Species of Fish | Lethal concentration mg/l | Exposure time in hours | |
| Rainbow trout (Salmo gairdnerii) | 0.07 | 74 | |
| Brook trout (Salvelinus fontinalis) | 0.05 | 120 | |
| Perch (Perca fluviatilis) | 0.13 | 17 | |
| Tench (Tinca tinca) | 0.20 | 48 | |
| Bluegill (Lepomis macrochirus) | 0.09 | 24 | |
| Largemouth bass (Micropterus salmoides) | 0.11 | 24 | |
| Fathead minnow (Pimephales promelas) | 0.23 | 96 |
* Adapted from Jones (1964, p. 94).
TABLE III
Toxicity of Several Heavy Metal Coumpounds to Fish*
| Compound | Species of fish | Lethal concentration mg/l | Exposure time in hours |
| Beryllium sulphate | Fathead minnow | 0.2 Be | 96 |
| " " | Bluegill sunfish (Lepomis macrochirus) | 1.3 Be | 96 |
| Cadmium chloride | Goldfish (Carassius carassius) | 0.017 | 9–18 |
| " " | Fathead minnow | 0.9 | 96 |
| Copper sulphate | Fathead minnow | 0.05 Cu | 96 |
| " " | Bluegill | 0.2 Cu | 96 |
| Lead chloride | Fathead minnow | 2.4 Pb | 96 |
| Lead nitrate | Rainbow trout | 1.0 Pb | 100 |
| Nickel chloride | Fathead minnow | 4.0 Ni | 96 |
| " " | Goldfish | 10.0 | 200 |
| Sodium chloride | Green sunfish (Lepomis cyanellus) | 10,713.0 | 96 |
| " " | Goldfish | 10,000.0 | 240 |
| " " | Fathead minnow | 8,718.0 | 96 |
| Sodium sulphate | Goldfish | 100.0 | 96 |
| Zinc sulphate | Stickleback (Gasterosteus aculeatus) | 0.3 Zn | 204 |
| " " | Goldfish | 100.0 | 120 |
| " " | Rainbow trout | 0.5 | 64 |
* Data from Jones (1964), pp. 74, 75.
Radionuclides
(1) No radioactive materials should be present in natural waters as a consequence of the failure of an installation to exercise appropriate controls to minimize releases.
(2) No radionuclide or mixture of radionuclides should be present at concentrations greater than those permitted in drinking water:
| Radioactivity | Permissible | Desirable |
|---|---|---|
| Gross beta | 1,000 | pc/l < 100 |
| Radium - 226 | 3 | pc/l < 1 |
| Strontium - 90 | 10 | pc/l < 2 |
REFERENCES
American Public Health Association, 1965 Standard methods for the examination of water and waste water including bottom sediment and sludges. New York, American Public Health Association Inc., 12th ed., 769 p.
Hynes, H.B.N., 1960 The biology of polluted waters. Liverpool University Press, 202 p.
Jones, J.R., 1964 Fish and river pollution. London, Butterworth and Co. (Publishers) Ltd., 203 p.
McKee, J.E. and H.W. Wolf, 1963 Water quality criteria. Sacramento, California, State Water Quality Control Board (3-A):548 p.
Mount, D.I. and W.A. Brungs, 1967 A simplified dosing apparatus for fish toxicology studies. Wat.Res., 1:21–2
Nemerow, N.L., 1963 Theories and practices of industrial waste treatment. Reading, Mass., Addison-Wesley Publishing Co., 557 p.
APPENDIX A
Definition of Terms
Application Factor: In the determination of the TLm of an industrial effluent, for example, it was found that the 48-hour TLm was 2.4 percent of the industrial effluent when mixed with unpolluted water from the receiving stream. Then the ratio of toxic effluent to diluent water is 100.0 minus 2.4 percent or 2.4 : 97.6 or 1:40. Total volume of the plant effluent amounted to 20 l/sec. Then 20 l/sec. × 40 or 800 l/sec. of diluted water will be required if half of the fish in the receiving stream are to live. But it is desired to have all of the fish live so a factor (application factor) of at least 2 must be applied. In this case, the volume of water required for the safe disposal of this waste becomes at least 800 × 2 or 1,600 l/sec. Usually an application factor of 10 or 12 is used to assure the protection of all forms of aquatic life.
Another method is to determine the concentration of the waste or material that does not adversely affect the productivity of the aquatic biota on continuous exposure, in water of known quality, and under environmental conditions (DO, temperature, pH, etc.) at which it is most toxic. This concentration is then divided by the 96-hour TLm value obtained under the same conditions to give the application factor:

For example, if the 96-hour TLm is 0.5 mg/l and the concentration of the waste found to be safe is 0.01 mg/l the application factor would be
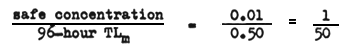
Bioassay: refers to the exposure of fish under certain prescribed conditions to toxic materials or effluents to determine the level at which they are killed or damaged physiologically.
There are two types of bioassays; (1) static in which a certain number of fish (usually 10) are placed under uniform conditions in jars or containers of water and exposed to a logarithmic series of concentrations of the toxic materials added only once to each container and (2) flow through (continuous flow) bioassays where the toxic materials are added continuously for periods of 48, 96 hours or for 30, 90 days or more to determine the long-term effects on growth and natural reproduction of the test organisms.
Biota: aquatic animals and plants.
Epilimnion: surface waters of lakes and reservoirs that when warmed may be circulated by winds independently of the deeper colder waters.
Hypolimnion: refers to the deeper, colder waters of lakes and reservoirs beneath the epilimnion.
Oligotrophic: refers to a class of lakes poor in nutrients and low in dissolved salts and biological productivity.
Plankton: Free-floating or free-swimming plants or animals usually microscopic in size.
TLm or TL50: refers to the toxic level at which half of the fish or other aquatic organisms die in bioassay tests. Most static bioassays are carried out for a period of 96 hours, but 24-hour and 48-hour TLm may be determined in the same series of tests.
Toxicity: the level at which water is poisonous to fish or other aquatic life.