
EPR/81/28A
September 1981


 | FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS | ESN: FAO/WHO/UNU EPR/81/28A September 1981 |
 | WORLD HEALTH ORGANIZATION | |
 | THE UNITED NATIONS UNIVERSITY |
Item 3.2.2 of the Provisional Agenda
Joint FAO/WHO/UNU Expert Consultation on
Energy and Protein Requirements
Rome, 5 to 17 October 1981
PHYSICAL ACTIVITY: IMPACT ON PROTEIN AND AMINO ACID
METABOLISM AND IMPLICATIONS FOR NUTRITIONAL REQUIREMENTS
by
Vernon R. Young and Benjamin Torún
Department of Nutrition and Food Science, MIT, Cambridge, USA
and
INCAP, Guatemala
INTRODUCTION
Although the molecular basis for the events leading to the contraction of muscles remains to be defined in detail, it is generally accepted that they include an interaction between actin and myosin, during which ATP is hydrolyzed to ADP (e.g. Bessman and Geiger, 1981). Thus, an essential metabolic aspect of exercise is the generation of utilization of chemical energy in the course of muscular work or its transformation with a generation of heat. But because the concentration of ATP in muscle is small, as indicated in Table 1, and the store of high energy phosphate bonds in the form of creatine phosphate is sufficient for only a limited number of contractions, a continuous generation of these bonds, through anaerobic and oxidative metabolism of fuels, is necessary to support a continuous output of muscular work for any significant period of time. In this brief review, we will consider the extent to which amino acid catabolism participates in the formation of the ATP required for muscular activity and if so whether this is of significance in relation to the protein and amino acid requirement of physically active human subjects.
| Fuel | Amount | |
|---|---|---|
| kcal | kJ | |
| ATP | 1.5 | 6 |
| Creatine phosphate | 3.5 | 15 |
| Glycogen | 1,200 | |
| Fat | 140,000 | |
In addition to the acute effects of physical exercise on the sources and utilization of major energy substrates, it is necessary also to consider the more prolonged effects of muscular activity on the status of tissue and body protein and amino acid metabolism. This is important because chronic periods of altered physical activity as, for example, in the case of an extended bed rest (e.g. MacDougal et al., 1977) bring about changes in body protein mass and may also influence the nutritional requirement for nitrogen and indispensable amino acids.
In the following sections we review selectively various observations reported in the literature and consider some of our unpublished work in an attempt to assess the relationships between physical activity, protein and amino acid metabolism and nutrition in the human. Our purpose is to highlight areas that appear to deserve more complete study, particularly because national (e.g. US/FNB, 1980) and international (FAO/WHO, 1973) allowances for dietary protein and amino acids do not make specific recommendations for persons whose physical activity differs widely.
Major Sources of Energy
Recognition of the importance of carbohydrate and fat as the major fuels in overall energy expenditure during exercise is based on numerous studies in the intact organism, including man, and in isolated muscles (reviewed by di Prompero, 1981). In summary, various factors influence the relative contribution of these two fuel sources, including intensity and duration of the exercise, body carbohydrate stores, physical conditioning, whether carbohydrate is consumed during the period of exercise and the prior nutritional history of the subject (e.g. Lemon and Nagle, 1981). The prevailing view, however, is expressed by Astrand (1979) who states, “the choice of fuels for working muscles, therefore, is limited to carbohydrate and fat” and by di Prompero (1981) who concludes that “the energy for muscular work is entirely derived from carbohydrates and fat”.
The importance of carbohydrate in relation to meeting the energy need of the exercised muscle is revealed by studies, involving application of a needle biopsy technique, that show a reduction in muscle glycogen with the continuation of exercise (e.g. Bergstrom and Hultman, 1972) and that the concentration of muscle glycogen may limit work performance (Costill and Miller, 1980). It should be noted, however, that reduced glycogen stores do not inevitably prevent prolonged exercise, provided the supply of FFA is adequate (Phinney et al., 1980).
Based on the arteriovenous difference studies, Ahlborg et al. (1974) estimated, as depicted in Figure 1, that the contribution of blood glucose to total oxidative metabolism by the exercising leg reached a peak of 90 minutes and then declined as exercise continued. On the other hand, the fraction of total metabolism attributed to the uptake of free fatty acids (FFA) exceeded the relative contribution made by glucose as the exercise period continued. The combined uptake of glucose and FFA by the exercising muscle accounted for approximately 65% of the total metabolism at 40 min and by 90 min the contribution approached more than 90%. (See also Felig and Wahren, 1975).
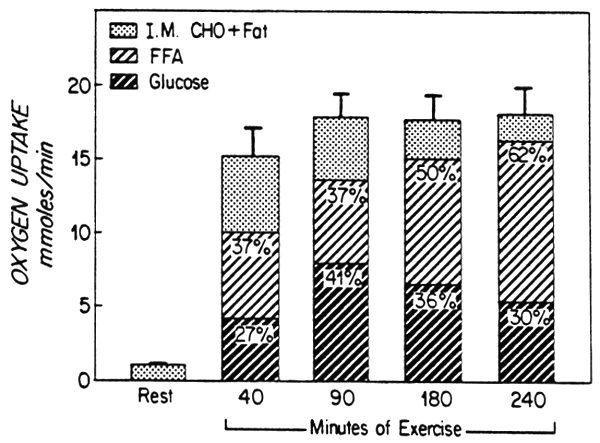
Fig. 1. Leg uptake of oxygen (O2) and contribution to oxidative metabolism made by substrates at rest and during exercise. The percent values represent proportion of total O2 uptake accounted for by these substrates. Drawn from Ahlborg et al. (1974).
ALAN R. LISS, INC.
150 Fifth Avenue
New York, NY 10011
At the time when the utilization of blood-borne glucose is markedly increased, hypoglycemia does not usually occur, due to a balanced increase in the rate of glucose output by the liver (Wahren et al., 1971), which originates via glycogenolysis in the short term, as evidenced by a depletion of liver glycogen stores (Bergstrom and Hultman, 1942), and from increased gluconeogenesis in the longer term (Ahlborg et al., 1974). However, exercise-induced hypoglycemia may occur occasionally in the insulin-dependent diabetic patient undergoing unusually strenuous exercise within 12h of administration of long-acting or intermediate-acting insulin preparations (Felig and Wahren, 1975).
From these various observations, it can be concluded that the quantitatively important components of the pattern of fuel utilization during prolonged periods of mild to moderate exercise involve utilization of muscle glycogen, blood glucose and free fatty acids.
The exercise induced increase in hepatic glucose output is associated with multiple changes in plasma hormone levels (Table 2), including a reduction in insulin and elevations in glucagon, growth hormone, glucocorticoids and catecholamines. The extent of these changes in hormonal levels are complex and depend upon the intensity of exercise, degree of training and the nutritional, metabolic and health state of the subject. Nevertheless, these hormonal changes serve to modulate the alterations in hepatic glucose output in response to exercise (e.g. Felig and Wahren, 1979) as well as the enhanced lipolysis and fatty acid oxidation.
Muscle Protein Metabolism
Because the protein and amino acid metabolism of body cells and organs is responsive to changes in the availability and source of energy substrates (Munro, 1964; Young et al., 1981) and to alterations in hormonal balance (Munro, 1964), it is important to consider the impact of exercise on protein and amino acid metabolism.First, this should be made in reference to muscle protein metabolism because this is known to be affected by numerous hormones (Young, 1970) and this tissue shows responses in vitro to changes in physical activity (reviewed by Young, 1981). Furthermore, a brief discussion of protein and amino acid metabolism in skeletal muscles in relation to exercise is important because it plays an important role in total body protein and amino acid metabolism. For example, in a series of studies in which we have estimated simultaneoulsy rates of muscle protein breakdown and whole body protein breakdown in adult subjects, muscle was found to account for 25–30% of total body protein turnover in young adults, declining to about 20% in elderly subjects (Young and Munro, 1978).
| Exercise Response (Plasma concentration) | |
|---|---|
| Norepinephrine | ↑ |
| Growth Hormone | ↑ |
| Epinephrine | ↑ |
| Cortisol | ↑ |
| Glucagon | ↑ |
| Insulin | ↓ |
| Training: Modifies degree of change | |
| Dietary Condition: Modifies degree of change | |
| Health Status: Modifies the responses. | |
A further indication of the importance of skeletal muscles in whole body protein and amino acid metabolism is revealed by studies of the distribution of enzymes involved in branched chain amino acid oxidation. Specifically, the dehydrogenases of α-keto-isocaproic, and α-keto-isovaleric acid, the keto acid analogues of leucine and valine, respectively, catalyze the irreversible decarboxylation of the two branched chain amino acids. These enzymes are considered to be the rate limiting enzymes in the pathway of branched chain amino acid oxidation in muscle (Hutson et al., 1978), and Khatra et al., (1977) estimated that of the total body, activity of these enzymes is distributed with about 2/3 associated with the skeletal musculature and 1/3 in the liver in adult humans.
The responsiveness of muscle protein metabolism to physical exercise is indicated from the findings that exercise patterns influence the size and distribution of the different fiber types (Gollnick et al., 1972; Saltin, 1973; Holloszy and Booth, 1976; Costill et al., 1976) and the types of proteins (Holloszy and Booth, 1976), as revealed by measurement of enzyme activities (Table 3) in muscle samples obtained from individuals with differing physical activity patterns. Although such measures of enzyme activity indicate changes in muscle protein metabolism in response to exercise, they give little insight into the biochemical changes that are responsible for these adaptations.
| Group | % ST2 | Fiber Area3 | Enzyme Activity3 | ||
|---|---|---|---|---|---|
| ST | FT | LDH | SDH | ||
| Elite Distance | 79 ± 3.5 | 8.3 | 6.5 | 746 | 21.6 |
| Middle Distance | 62 ± 2.9 | 6.4 | 6.3 | 788 | 17.7 |
| Untrained | 58 ± 2.5 | 5.4 | 4.9 | 843 | 6.4 |
1 Summarized from Costill et al. (1976).
2 % of Slow Twitch fibers.ST = Slow Twitch; FT = FastTwitch.
3 Values are μ2 × 10-3
4 μmoles/g/min (LDH = lactate dehydrogenase; SDH = succinicdehydrogenase).
= p < 0.05 from elite distance runners.
The effects of exercise on muscle protein metabolism may be explored by various approaches and different models have been used to induce changes in muscle protein mass for this purpose (see Millward, 1980). A summary of some studies, carried out with these various models, concerned with various aspects of muscle protein and amino acid metabolism, is given in Table 4. As shown here the findings reveal that amino acid transport is enhanced with increased work, and that there is an increased rate of incorporation of labeled amino acids into muscle proteins, including both the sarcoplasmic and fibrillar protein fractions. Furthermore, there are also changes in the metabolism and content of nucleic acids (RNA and DNA) and these favor increased rates of protein synthesis and growth of muscle with higher work loads. Although the available data are more extensive than indicated here, it is clear that there are a complex series of changes in nucleic acid metabolism and in the protein synthetic machinery of muscle cells. These changes are regulated co-ordinately in response to altered work loads in this organ. However, the signals and primary events that lead to this complex series of changes in muscle protein metabolism remain to be determined (for example, see Young, 1981).
| Process | Author |
|---|---|
| Amino Acid Transport | |
| ↑ AIB Accumulation (Tenotomy) | Goldberg et al. (1974) |
| ↑ AIB Accumulation (Stimulation in vitro | Goldberg et al. (1974) |
| ↑ AIB Accumulation (Denervation, diaphragm) | Buse et al. (1975) |
| Protein Synthesis (in vitro, in vivo) | |
| ↑ 14C-amino acid incorporation | Laurent & Sparrow (1977) |
| ↑ 14C-amino acid incorporation | Goldberg et al. (1975) |
| ↑ 14C-amino acid incorporation | Laurent et al. (1978) |
| ↑ Ribosome activity | Turner & Manchester (1973) |
| ↑ Cell-sap activity | Turner & Manchester (1973) |
| ↑ Microsomal activity | Hamosh et al. (1967) |
| Nucleic Acid | |
| ↑ RNA polymerase | Sobel & Kaufman (1970) |
| ↑ 3H-thymidine into DNA | Goldberg et al. (1975) |
| ↑ DNA, RNA content | Various authors |
Changes in protein synthesis may also be accompanied by alterations in the rate of protein breakdown and in Table 5 a summary is given of the conclusions by various authors concerning the effects of exercise on protein breakdown. It is evident that there is little consistency among the various studies; some have concluded that breakdown does not change, others that it is decreased and still others that it is increased. The reasons for these variable conclusions may relate to the use of different models and/or the different methods used to estimate actual breakdown rates.
| Muscle & Condition | Response of Breakdown | Author |
|---|---|---|
| Soleus-tenotomy | Reduced+ | Goldberg (1969) |
| Soleus-stimulated,stretched | Reduced* | Goldberg (1979) |
| EDL-Immobilized,lengthened | Increased* | Goldspink (1977) |
| EDL-Denervated,stretched | Increased* | Goldspink (1978) |
| Diaphragm-Denerv. | Increased+ | Turner & Garlick (1974) |
| ALD (Weighting) | No change+ | Laurent & Sparrow (1977) |
| ALD (Weighting) | Increased+ | Laurent et al. (1978) |
+ In vivo measurement.
* In vitro measurement.
ALAN R. LISS, INC.
150 Fifth Avenue
New York, NY 10011
From these studies it is to be concluded that the rate of protein synthesis is increased, possibly accompanied by changes in protein breakdown, in response to a continuous period of increased muscular work, whether or not an actual hypertrophy of the muscle occurs. It is also evident that the changes in protein metabolism are associated with many of the various phases of amino acid utilization and protein anabolism. Overall these changes favor protein anabolism and, possibly, a more efficient use of dietary derived amino acids for incorporation into muscle proteins. Thus, an increased dietary protein requirement would not be predicted from these biochemical observations, except perhaps in relation to maintenance of a larger muscle mass. An argument against this, and as shown in Table 6, is that Dohm et al. (1977) have concluded that endurance training in rats results in an increased oxidation of leucine in muscle which is paralleled by an enhanced urea N output in trained rats as compared to untrained, pair-fed controls.
| Group | Body Wt. (g) | Urea N Excretion (g/kg0.73/d) | 14CO2 produced (nmol/min/g) from U-14C-Leucine |
|---|---|---|---|
| Untrained | 358 | 0.55 | 0.21 |
| Pair-fed Untrained | 353 | 0.57 | |
| Trained | 328 | 0.80 | 0.31 |
In contrast to these extensive, although not entirely consistent, observations on the longer-term effects of repeated exercise on muscle protein and amino acid metabolism, the acute effects of exercise on muscle protein and amino acid metabolism have received less investigation. However, acute changes in physical activity do appear to influence muscle protein and amino acid metabolism. For example, as summarized in Table 7, Dohm et al. (1980) found a reduced rate of muscle protein breakdown when rats were forced to swim for 1h. Furthermore, Booth and Seider (1979) observed an early decrease in protein synthesis when muscular activity was reduced by immobilization of the hind limbs in rats. Thus, muscle protein metabolism is responsive to brief as well as more sustained periods of altered physical activity.
| Group | Synthesis | Breakdown Rate3 | |
|---|---|---|---|
| 3H-Tyrosine incorporation2 (nmole/h/mg protein) | 14C-leucine incorporation by polyribosomes (pmole/min/mg RNA) | ||
| Rested | 0.32 | 3.4 | 8.9 |
| Exercised | 0.26 | 2.5 | 13.8 |
1 Summarized from Dohm et al. (Biochem. J. 188:255 (1980)).
2Soluble protein fraction of perfused muscle.
3μmol tyrosine release/h per hemicorpus.
Studies of Protein and Amino Acid Metabolism in Man:
Alterations in protein and amino acid metabolism in the muscles and whole body of rats would imply that exercise also affects the status of tissue and organ protein metabolism in human subjects. In addition to differences in the morphological and enzymatic characteristics of muscle fibers in subjects of varying fitness that were referred to above, studies have been made in intact human subjects of various aspects of protein, amino acid and N metabolism.
By measuring arteriovenous amino acid differences across the skeletal muscles of the leg and the splanchnic bed, Felig, Wahren and colleagues (Felig and Wahren, 1971, 1975; Ahlborg et al., 1974) have found that arterial levels of alanine increase during exercise (Table 8) and that this rise is associated with an increased muscle alanine output and enhanced uptake of this amino acid by the liver (Ahlborg et al., 1974). In contrast, as also shown in Table 8, there is a selective uptake of branched chain amino acids by the exercising limb and an equivalent output from the splanchnic bed. This implies a possible increase in the rate of oxidation of these branched chain amino acids in the active muscles. Furthermore, because alanine is considered to be a vehicle for nitrogen transport from muscles to liver (e.g. Chochinov et al., 1978), where it may donate its N to urea (Lund, 1981), acute exercise would be expected to lead an increased rate of urea formation. This possibility will be discussed further below because of the implications for changes in the N balance and protein requirement.
| Amino Acid | Arterial Concentration | Exchange | ||||
|---|---|---|---|---|---|---|
| Rest | 240min Exercise | Splanchnic | Leg | |||
| Rest | @240min | Rest | 240min Exercise | |||
| μmol/l | μmol/min | |||||
| Glycine | 188 | 160 | 8.3 | 31.8* | -8 | 17.8* |
| Alanine | 192 | 233 | 57.6 | 119.0* | -30.4 | -95.4* |
| Leucine | 126 | 151* | -2.2 | -30.2* | -0.8 | 28.6* |
| Valine | 242 | 243 | -3.2 | -31.6* | -0.6 | 43.4* |
| Isoleucine | 60 | 81* | -1.0 | -17.2* | -0.4 | 21.8* |
1 Partial summary of data of Ahlborg et al. (1974).
*Significantly different p < 0.05 from value at rest.
There have been relatively few studies designed to quantify dynamic aspects of whole body amino acid metabolism in relation to exercise. Therefore, we have begun to explore this problem with the aid of stable isotope probes (e.g. Young and Bier, 1981a). Aspects of whole body amino acid metabolism are being examined in adult subjects while at rest and in response to a 2 h bicycle ergometer ride at an energy level equivalent to 55% of the VO2 max and our preliminary findings reveal a number of important points as follows; free amino acid levels in venous plasma showed little change in response to the exercise, although fatty acid and glycerol levels, reflective of increased adipose tissue lipolysis, rose during this time (Table 9). However, because plasma amino acid levels represent the balance between their rates of inflow and outflow in the circulation, measurements of these levels fail to indicate the possible changes in amino acid metabolism that occur during exercise. Therefore, using a 1-13C-leucine as a stable isotope probe and applying the model described by Waterlow and colleagues (Waterlow, 1967; Waterlow et al., 1978), that we have used in previous studies on the adaptations of whole body amino acid metabolism to dietary factors (Young and Bier, 1981a, 1981b), we examined whole body leucine kinetics before and during a 2h period of moderate exercise.Our initial findings are summarized in Table 10 and they indicate that the rate of leucine oxidation is markedly increased during exercise in these adult subjects. This change in leucine oxidation, as determined in both trained and untrained subjects who had received only a small breakfast before exercise, was associated with a reduced rate of leucine incorporation into body proteins and a reduction in leucine flux, the latter indicative of a decline in the rate of total body protein breakdown. Using a similar approach, Rennie and co-workers (1981) have also observed an increased rate of whole body leucine oxidation although they did not find a reduction in total body protein breakdown. These investigators have concluded, however, that the rate of muscle protein breakdown is reduced during exercise (Millward et al., 1981).
| Metabolite | Condition | Post-Exercise (15 min) | |
|---|---|---|---|
| Rest | Exercise (at 120 min) | ||
| Amino Acids (mM) | |||
| Alanine | 410 | 384 | 325 |
| Glutamine | 594 | 638 | 569b |
| Leucine | 126 | 135 | 134 |
| Isoleucine | 65 | 61 | 62 |
| Threonine | 161 | 144a | 129b |
| Urea (mg/dl) | 16 | 15 | 16 |
| Free fatty acid (mM) | 0.42 | 1.47a | 2.49b |
| Glycerol (mM) | 0.11 | 0.44a | 0.36b |
1 Unpublished data of Wright et al. (1981).Exercise was a bicycle ergometer ride for 2h at 55% VO2 max.
a Significantly different from value at rest.
b Significantly different from value at 120' exercise.
Relation of Changes in Metabolism to Nutritional Requirements
In view of the enhanced rate of leucine oxidation in exercised subjects, it is instructive to consider how this response relates to the substantial increase in total energy expenditure under these conditions. Thus, although the oxidation of leucine and probably other amino acids (e.g. White and Brooks, 1981) is increased with exercise, we have calculated that the contribution made by total amino acid catabolism to the total energy expenditure may actually be lower, in relative terms, during exercise than at rest (Table 11). These data are entirely consistent with the notion that carbohydrate and fats account for the major contribution to the rise in oxidative catabolism associated with exercise, as discussed above.
| Parameters | Condition | P | |
|---|---|---|---|
| Rest | Exercise | ||
| Leucine Flux | 120.4±6.2 (15)2 | 97.0±7.13 (15) | < 0.001 |
| Leucine Oxidation | 14.8±1.3 (8) | 46.1±9.7 (8) | <0.01 |
| Leucine Incorporation into protein | 113.2±5.4 (8) | 58.2±9.5 (8) | <0.01 |
1 Unpublished data of Wright, Evans, Phinney and Young (1981).
2 Values are μmole.kg-1h-1.Mean ± SEM.Number of subjects in parentheses.
On the other hand, the rise in leucine oxidation may have considerable significance for the economy of N metabolism and the requirements for protein and indispensable amino acids. This possibility is further underscored when it is considered that a major source of the N of the alanine liberated at an increased rate from exercised muscle (Ahlborg et al., 1974) might arise from leucine (e.g. Goldberg and Chang, 1978) and that the alanine nitrogen is subsequently transferred to urea via urea enzyme cycle activity in the liver. An evaluation of this scheme requires direct estimates of urea synthesis in the exercised subject and these have not yet been performed. However, recent studies, expecially those of Lemon and Mullin (1980) showing plasma urea changes during exercise (Fig. 2) are highly suggestive of enhanced rates of urea formation during exercise and, furthermore, that a prior period of low dietary carbohydrate intake accentuates the change in plasma urea. The findings of Refsun and Stromme (1974) and those of other investigators, reviewed by Lemon and Nagle (1981), further suggest an enhanced output of urinary urea during brief or prolonged periods of physical activity. Thus, it would be instructive to quantify directly the rate of urea production, using 15N or 13C urea, during exercise in adult subjects in an attempt to improve our understanding of the relationships between the changes in interorgan amino acid flow, discussed earlier, and urea metabolism during and immediately following periods of exercise of varying intensity and duration.
| Subjects | No. | Pre-Exercise | During Exercise |
|---|---|---|---|
| Untrained | 3 | 14.1 | 4.8 |
| Trained | 5 | 12.2 | 2.9 |
1 Unpublished data of Wright et al. (1981).
a Based upon leucine content in whole body protein of 590 μmol
g and mean values for leucine oxidation of 14.8 μmol.kg-1
h-1 (pre-exercise) and 46.1 μmol.kg-1 h-1 (during exercise).
b Total energy expenditure derived from indirect calorimetry
using measured values of VO2 and “R” during a 2 h period.
Because urea accounts for the major proportion of total urinary N excretion in well-nourished subjects, changes in urea production with exercise should be reflected by changes in body N balance. Indeed, there is evidence that the latter becomes more negative when moderate exercise is required in healthy men receiving a generous intake of protein and adequate energy (Gontzea et al., 1974; 1975) (e.g. Fig. 3). Hence, it can be suggested that moderate to heavy exercise might increase the requirement for total N, at least during the initial weeks of a program of moderate physical activity. This increased requirement appears even more likely if the increased losses of N via sweat are also taken into account (e.g. Lemon and Mullin, 1980).
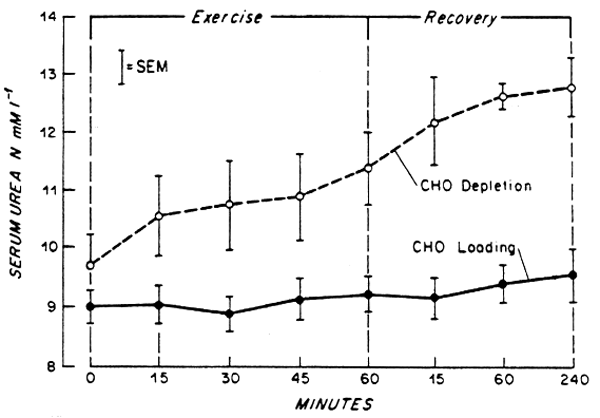
Fig. 2. Change in serum urea concentration during and following recovery from exercise (for 60 min at 61% VO2 max on a bicycle ergometer) in adult male subjects who had received previously a high carbohydrate diet (CHO-loaded) for 3 days or who were carbohydrate depleted. Drawn from Lemon and Mullin (1980).
There have been an insufficient number of studies to examine this issue in much greater depth but we have shown, as depicted in Figure 4, that a period of training of isometric exercise in healthy adult men results in a reduction in body potassium, and probably body cell mass, when the test subjects consume a diet approximating the FAO/WHO (1973) dietary protein allowance. In contrast these changes did not occur when subjects were first adapted to a diet providing a more generous intake of 1 g egg protein/kg/day (Torun et al., 1977). Furthermore, a change in protein requirement as a consequence of exercise is also suggested by the data of Yoshimura et al. (1980). These investigators have shown that during training to strenuous physical exercise, the content of hemoglobin in circulating blood decreased when subjects received a diet containing about 1.3 to 1.5 g protein/kg/day, but that this did not occur when the level of protein intake was 2.5 g/kg/day. These observations were interpreted by Yoshimura et al. (1980) to indicate that a high intake protein is required during the course of physical training.
EFFECT OF PHYSICAL EXERCISE ON
NITROGEN BALANCE
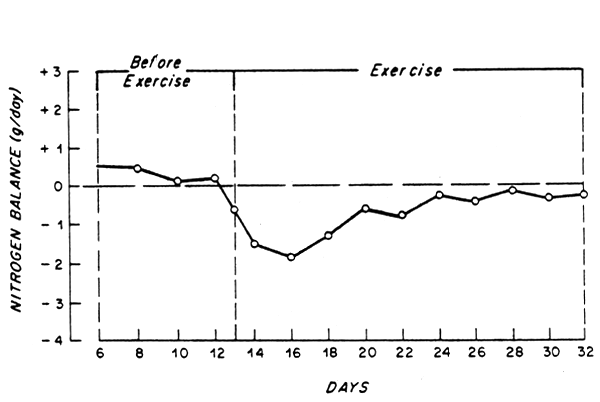
Fig. 3. Mean nitrogen balance for 12 healthy young men before and during a 3-week period of increased physical activity (9.9 Kcal/min for 6 of each 20 min periods per day). Subjects received 1 g protein/kg/day (35% from animal origen). Drawn from Gontzea et al. (1975).
In spite of the early work suggesting the lack of an effect of physical exercise per se on the protein requirement of the host (see FAO/WHO, 1973), the more recent studies referred to here indicate that intermittent bouts of exercise disturb the nitrogen metabolism of the host and that the biochemical observations suggest it is likely that the requirement for total nitrogen and/or for specific dispensable amino acids is increased above that of the sedentary individual. This possibility can be examined further in reference to our findings on the rate of whole body leucine oxidation during exercise. Thus, as shown in Table 12, the oxidation of leucine during two hours of moderate exercise on a bicycle ergometer represents an amount equivalent to the upper range of the estimated dietary requirement for this amino acid (FAO/WHO, 1973). Recent studies from our laboratory have shown that the requirement for leucine may be estimated from the rate of whole body leucine (Young and Bier, 1981a,b). Hence, from the results of our recent studies, and assuming that there is not an adaptive reduction in the rate of leucine oxidation during the post-exercise period, in comparison with that for non-exercised subjects, the minimum physiological requirement for this amino acid would be higher for physically active than for sedentary subjects. Of course, another possibility that must be considered in the interpretation of these data is that the rate of leucine oxidation during exercise may be determined, in part, by the previous dietary intake level of leucine. It is possible that subjects who had been adapted to a lower protein or essential amino acid intake may not show the marked increase in leucine oxidation that was observed in our subjects who had received a generous protein intake for a number of days prior to the exercise test. Furthermore, because leucine oxidation rates, during both the post-absorptive and absorptive phases of amino acid metabolism, fall when the intake of dietary leucine or protein is lowered (Motil et al., 1981), it would also be of interest to determine whether a period of exercise induces a significant increase in leucine oxidation in subjects adapted to a diet that provides leucine at levels approximating current estimates of the requirement for this amino acid in adults. If an increase in leucine oxidation was observed in trained subjects under these dietary conditions, this would provide convincing evidence that the physiological requirement for this amino acid is determined, in part, by physical activity per se.
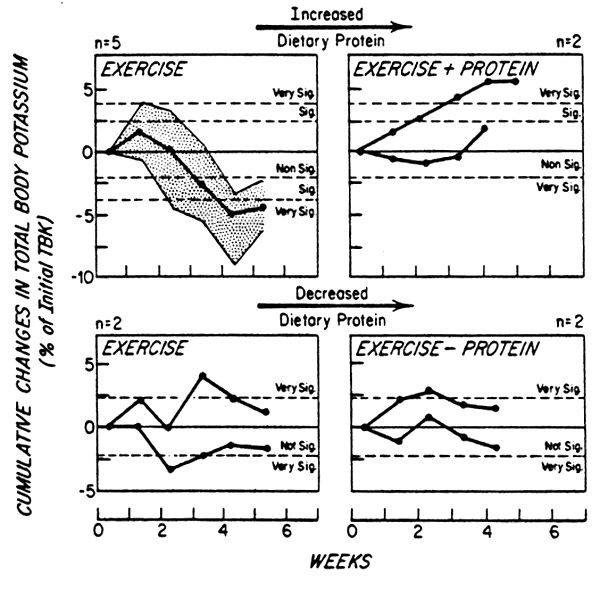
Fig. 4. Weekly mean cumulative changes in total body potassium (TBK) in young men, receiving 0.5 or 1.0 g egg protein/ kg/day, while performing a 75 min isometric exercise program daily for 4 to 6 weeks. Drawn from Torun et al., (1977).
| Status | Rate | Total Quantity |
|---|---|---|
| Rest: 2 hrs (recumbent) | ||
| Leucine Oxidation | 14.8μmol.kg-1h-1 | 2087μmol = 274 mg |
| Leucine Requirement | 14.0 mg.kg-1d-1 | 987 mg |
| Oxidation: Percent of Requirement | 28 | |
| Exercise: 2 hrs (55% VO2 max) | ||
| Leucine Oxidation | 46.1 μmol.kg-1h-1 | 6500μmol = 853 mg |
| Leucine Requirement | 987 mg | |
| Oxidation: Percent of Requirement | 86 | |
1 Untrained subjects (mean wt. 70.5 kg).
2 FAO/WHO (1973); WHO Tech. Rept. Ser. No. 522, World HealthOrganization, Geneva, Switzerland.
Finally, it is important to emphasize that the metabolism of leucine is closely associated with the skeletal muscles (e.g. Miller, 1962). Although it is tempting to interpret a change in leucine oxidation during exercise as indicative of a general increase in the oxidation of indispensable (essential) amino acids, it will be important to determine directly whether exercise alters significantly the rate of oxidation of amino acids that are predominantly catabolized in the liver, such as lysine (Hutzler and Dancis, 1975) and tyrosine (Miller, 1962).
Comment on Chronic Patterns of Physical Activity.
In the foregoing section we have considered changes in human body protein and amino acid metabolism that occur in response to brief periods of exercise. We have argued that the metabolic findings suggest increased amino acid requirements in subjects who are physically active. Here, the effects of more continuous patterns of physical activity should also be mentioned.
Studies with animal models, reviewed above, show that continuous periods of work induce muscular hypertrophy and that there is an overall anabolic effect on body protein metabolism and this is accompanied by an improvement in somatic growth (e.g. Boreer, 1980). In contrast, muscle atrophy is a characteristic of hypokinesia, arising from restricted movement (e.g. Booth, 1977) and the findings of Musacchia et al. (1980) (Figure 5), based on measurement of the output of NT-methylhistidine (3-methylhistidine) in the urine suggest, that with the loss of muscle mass and increased excretion of urea, there is an increased rate of muscle protein catabolism in hypokinetic rats. Thus between these extremes in muscular activity there is a pattern of physical activity that is consistent with and necessary for maintenance of an adequate state of body protein and amino acid metabolism and function. Although this pattern cannot be defined in precise quantitative terms, studies carried out at INCAP by B. Torun and F. Viteri (manuscript in preparation) in young children recovering from protein-energy malnutrition reveal the beneficial effects of continued mild exercise on the utilization of dietary protein.
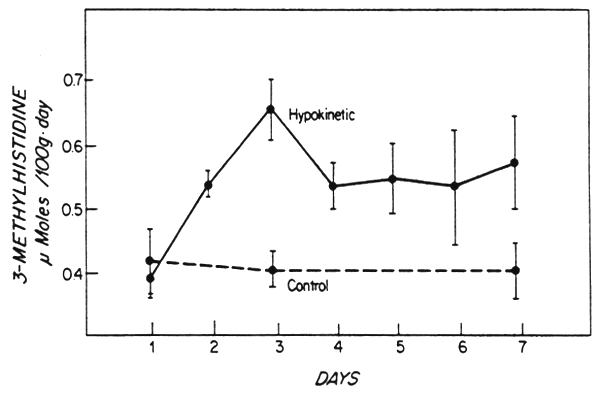
Fig. 5. Change in urinary 3-methylhistidine excretion during 1 week of hypokinesia in young rats (170–190 g body weight). Drawn from Musacchia et al. (1980).
In these studies a group of children, aged 2–4y, under treatment for protein-energy malnutrition, was stimulated to be more physically active through a daily program of games that required mild to moderate levels of energy expenditure. Measurements were made of growth and of body energy and metabolic nitrogen balances and results compared with those obtained in a similar group of children treated in the traditional manner at INCAP. A partial summary of these data is given in Table 13 and the changes in linear growth for the two groups during the course of the study are illustrated in Figure 6. These balance and anthropometric data indicate that the more active children grew better in height and in lean body mass. Thus, the utilization of dietary protein and energy for growth was more efficient in the more active children.From these results it is to be concluded that moderate systemic exercise had a growth-enhancing effect and a favorable impact on the utilization of dietary protein.Furthermore, it seems reasonable to expect that this would also apply to well-nourished subjects, both children and adults.
| Group | ||
|---|---|---|
| Active | Control | |
| Energy Balance (kcal kg-1day-1) | ||
| Net intake | 119 ± 42 | 120 ± 3 |
| Expenditure | 94 ± 9** | 74 ± 8 |
| Retention | 26 ± 9** | 46 ± 8 |
| Retention per | ||
| g weight gain | 5.0 ± 3.3** | 9.3 ± 2.9 |
| Nitrogen Balance (mg kg-1day-1) | ||
| Intake | 398 ± 8 | 402 ± 11 |
| Retention | 145 ± 37 | 139 ± 30 |
| Creatinine (mg/day) | ||
| Increment in six weeks | 41.7 ± 13.4 | 31.0 ± 11.6 |
1 Unpublished data of Torun and Viteri (INCAP, 1981).
2 Mean ± SD:M = 66–67 (active) and 54–63 (control)
** Significantly different from control group (p < 0.01).
CUMULATIVE CHANGES IN HIGHT FOR ACTIVE AND CONTROL CHILDREN RECOVERING FROM PEM
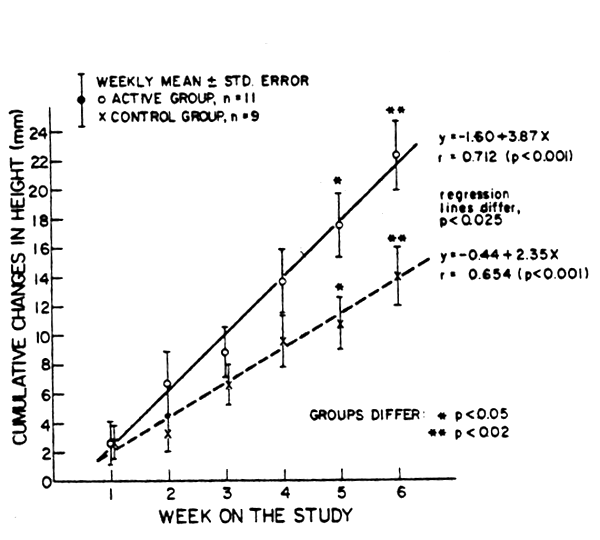
Fig. 6. Linear growth in a group of physically active children, as compared with a control group, during recovery from protein-energy malnutrition (PEM). Unpublished data of B. Torun and F. Viteri (INCAP).
SUMMARY AND CONCLUSIONS
In this brief and selective review we have discussed recent observations that concern the relationships between physical activity and protein and amino acid metabolism, with particular reference to the requirement for dietary protein. The major focus of our attention has been given to the effects of infrequent, moderate to heavy, exercise on human protein and amino acid metabolism. The metabolic picture that is now emerging leads to the strong speculation that moderate and heavy exercise in adult subjects results in an increase in the minimum physiologic requirement for specific indispensable amino acids and/or total protein. This contrasts with the prevailing view that physical activity per se does not result in an increased need for dietary protein. Because heavy manual labor is characteristic of many of the populations in the developing regions of the world and also because of the growing interest by the public in the United States and other technically advanced nations in physical exercise as a means of improving health and well-being, it would be prudent to undertake a more careful and comprehensive exploration of the effects of physical activity, of various types, intensity and duration, on the amino acid and protein needs of human subjects of all ages.
REFERENCES
Ahlborg G, Felig P, Hagenfeldt L, Hendler R, Wahren J (1974). Substrate turnover during prolonged exercise in man. Splanchnic and leg metabolism of glucose, free fatty acids, and amino acids. J Clin Invest 53:1080.
Astrand PO (1979). Nutrition and physical performance. In “Nutrition and the World Food Problem”, Basel: Karger Press, p 63.
Bergstrom J, Hultman E (1972). Nutrition for maximal sports performance, J Am Med Assoc 221:999.
Bessman SP, Geiger PJ (1981). Transport of energy in muscle: the phosphorylcreatine shuttle. Science 211:448.
Bloom SR, Johnson RH, Park DM, Rennie MJ, Sulaimon WR (1976). Differences in the metabolic and hormonal response to exercise between racing cyclists and untrained individuals. J Physiol (London) 258:1.
Booth FW (1977). Time course of muscular atrophy during immobilization of hind limbs of rats.J Appl Physiol: Respirat Environ Exercise Physiol 43:656.
Booth FW, Seider MJ (1979). Early change in skeletal muscle protein synthesis after limb immobilization of rats. J Appl Physiol: Respirat Environ Exercise Physiol 47:974.
Boreer KT (1980). Characteristics of growth-inducing exercise. Physiol and Behavior 24:713.
Buse MG, McMaster J, Buse J (1975). The effect of denervation and insulin on protein synthesis in the isolated rat diaphragm.Metab 14:1220.
Chochinov RH, Pelman K, Moorehouse JA (1978). Circulating alanine production and disposal in healthy subjects. Diabetes 22:230.
Costill DL, Fink WJ, Pollack ML (1976). Muscle fiber composition and enzyme activities of elite distance runners. Med Sci Sports 8:96.
Costill DL, Miller JM (1980). Nutrition for endurance sport: carbohydrate and fluid balance. Intrl J Sports Med 1:2.
di Prampero PE (1981). Energetics of muscular exercise. Rev Physiol Biochem Pharmacol 89:143.
Dohm GL, Hecker AL, Brown WE, Klain GL, Puente FR, Askew EW, Beecher GR (1977). Adaptation of protein metabolism to endurance training: increased amino acid oxidation in response to training. Biochem J 164:705.
Dohm GL, Kasperek GJ, Tabscott EB, Beecher GR (1980). Effect of exercise on synthesis and degradation of muscle protein. Biochem J 188:255.
FAO/WHO (1973). “Energy and Protein Requirements”, World Health Organization (WHO) Tech Rept Ser No 522, Geneva: World Health Organization.
Felig P, Wahren J (1979). Role of insulin and glucagon in the regulation of hepatic glucose production during exercise. Diabetes 28:Suppl 1,71.
Felig P, Wahren J (1975). Fuel homeostasis in exercise. N Engl J Med 293:1078.
Felig P, Wahren J (1971). Amino acid metabolism in exercising man. J Clin Invest 50:2703.
Goldberg AL (1971). Relationship between hormones and muscular work in determining muscle size. In Alpert NR (ed): “Cardiac Hypertrophy”, New York: Academic Press, p 39.
Goldberg AL (1969). Protein turnover in skeletal muscle. I and II. J Biol Chem 244:3217.
Goldberg AL, Chang TW (1978). Regulation and significance of amino acid metabolism in skeletal muscle. Fed Proc 37:2307.
Goldberg AL, Etlinger JD, Goldspink DF, Jablecki C (1975). Mechanisms of work-induced hypertrophy of skeletal muscle. Med Sci Sports 7:185.
Goldberg AL, Jablecki CM Li JB (1974). Effects of use and disease on amino acid transport and protein turnover in skeletal muscle. Ann NY Acad Sci 228:190.
Goldspink DF (1978). The influence of passive stretch on the growth and protein turnover of the denervated extensor digitorum longus muscle. Biochem J 174:595.
Goldspink DF (1977). The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J Physiol 264:267.
Gollnick PD, Armstrong RB, Saubert CW, Piehl K, Saltin B (1972). Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J Appl Physiol 33:321.
Gontzea I, Sutzescu R, Dumitrache S (1975). The influence of adaptation to physical effort on nitrogen balance in man. Nutr Rept Intl 11:231.
Gontzea I, Sutzescu P, Dumitrache S (1974). The influence of muscular activity on nitrogen balance and on the need of man for proteins. Nutr Rept Intl 10:35.
Gyntelberg F, Rennie MJ, Hickson RC, Holloszy JO (1977). Effect of training on the response of plasma glucagon to exercise. J Appl Physiol: Respirat Environ Exercise physiol 43:302.
Homosh M, Lesch M, Baron J, Kaufman S (1967). Enhanced protein synthesis in a cell-free system from hypertrophied skeletal muscle. Science 157:935.
Hartley LH, Mason JW, Hogan RP, Jones LG, Kotchen, TA, Maugey EH, Wherry FE, Pennington LL, Ricketts PT (1972a). Multiple hormonal responses to prolonged exercise in relation to physical training. J Appl Physiol 33:607.
Hartley LH, Mason JW, Hogan RP, Jones LG, Kotchen, TA, Maugey EH, Wherry FE, Pennington LL Ricketts PT (1972b). Multiple hormonal responses to graded exercise in relation to physical training. J Appl Physiol 33:602.
Holloszy JO, Booth FW (1976). Biochemical adaptations to endurance exercise in muscle. Ann Rev Physiol 56:273.
Hutson SM, Cree TC, Harper AE (1978). Regulation of leucine and α-ketoisocaproate metabolism in skeletal muscle. J Biol Chem 253:8126.
Hutzler J, Dancis J (1975). Lysine-ketoglytarate reductase in human tissues. Biochem Biophys Acta 377:42.
Khatra BS, Chawla RK, Swell CW, Rudman D (1977). Distribution of branched-chain α-keto acid dehydrogenases in primate tissues.J Clin Invest 59:558.
Laurent GJ, Sparrow MP (1977). Changes in RNA, DNA and protein content and the rates of protein synthesis and degradation during hypertrophy of the anterior latissimus dorsi muscle of the adult fowl (Gallus Domesticus). Growth 41:249.
Laurent GJ, Sparrow MP, Millward DJ (1978). Turnover of muscle protein in the fowl. Changes in rates of protein synthesis and breakdown during hypertrophy of the anterior and posterior latissimus dorsi muscles. Biochem J 176:407.
Lemon PWR, Mullin JP (1980). Effect of initial muscle glycogen levels on protein catabolism during exercise. J Appl Physiol: Respirat Environ Exercise Physiol 48:624.
Lemon PWR, Nagle FJ (1981). Effects of exercise on protein and amino acid metabolism. Med Sci Sport Exercise 13:141.
Lund P (1981). Precursors of urea synthesis. In Waterlow JC, Stephen JML (eds): “Nitrogen Metabolism in Man”, London: Applied Science Publishers. (In press).
MacDougal JD, Ward GR, Sale DG, Sutton JR (1977). Biochemical adaptation of human skeletal muscle to heavy resistance training and immobilization. J Appl Physiol: Respirat Environ Exercise Physiol 43:700.
Miller LL (1962). The role of the liver and the non-hepatic tissues in the regulation of free amino acid levels in the blood. In Holden J (ed) “Amino Acid Pools: Distribution, Formation and Function of Amino Acids”, Amsterdam: Elsevier Press, p 708.
Millward DJ (1980). Protein turnover in skeletal and cardiac muscle during normal growth and hypertrophy. In Wildenthal K (ed) “Degradative processes in heart and skeletal muscle”, New York: Elsevier/North-Holland Biomedical Press, p 161.
Millward DJ, D vies CTM, Halliday D, Wolman SL, Matthews D, Rennie M (1981). The effect of exercise on protein metabolism in man as explored with stable isotopes. Fed Proc (In press).
Motil KJ, Matthews DW, Bier DM, Burke JF, Munro HN, Young VR (1981). Whole body leucine and lysine metabolism: response to dietary protein intake in young men. Am J Physiol 240:E712.
Munro HN (1964). General aspects of the regulation of protein metabolism by diet and by hormones. In Munro HN and Allison JB (eds) “Mammalian Protein Metabolism”, New York: Academic Press, p 381.
Musacchia XJ, Deavers DR, Meininger GA, Davies TP (1980). A model for hypokinesia; effects on muscle atrophy in the rat. J Appl Physiol: Respirat Environ Exercise Physiol 48:479.
Phinney SD, Horton ES, Sims EAH, Hanson JS, Danforth E Jr., LaGrange BM (1980). Capacity for moderate exercise in obese subjects after adaptation to a hypocaloric, ketogenic diet. J Clin Invest 66:1152.
Refsum HE, Stromme SB (1974). Urea and creatinine excretion in urine during and after prolonged heavy exercise. Scand J Clin Lab Invest 33:247.
Rennie MJ, Halliday D, Davies CTM, Edwards RHT, Krywawych S, Millward DJ, Matthews DE (1981). Exercise induced increase in leucine oxidation in man and the effect of glucose. In Walser M, Williamson JR (eds): “Metabolism and Clinical Implications of Branched Chain Amino and Ketoacids”, New York: Elsevier/North-Holland, p 361.
Saltin B (1973). Metabolic fundamentals in exercise. Med Sci Sports 5:137.
Sobel BE, Kaufman S (1970). Enhanced RNA polymerase activity in skeletal muscle undergoing hypertrophy. Arch Biochem Biophys 137:469.
Sutton JR (1978). Hormonal and metabolic responses to exercise in subjects of high and low work capacities. Med Sci Sports 10:1.
Torun B, Scrimshaw NS, Young VR (1977). Effect of isometric exercises on body potassium and dietary protein requirements of young men. Am J Clin Nutr 30:1983.
Turner LV, Garlick PJ (1974). The effect of unilateral phrenicectomy on the rate of protein synthesis in rat diaphragm in vivo. Biochim Biophys Acta 349:109.
Turner LV, Manchester KL (1973). Effects of denervation hypertrophy in rat diaphragm muscle on the activity of ribosomes and sap factors in protein synthesis. Biochim Biophys Acta 299:612.
US/FNB (1980). “Recommended Dietary Allowances”, 9th edition, Washington: National Academy of Sciences, p 185.
Wahren J, Felig P, Ahlborg G, Jahrfeldt L (1971). Glucose metabolism during leg exercise in man. J Clin Invest 50:2715.
Waterlow JC (1967). Lysine turnover in man measured by intravenous infusion of L-[U-14C] lysine. Clin Sci 83:507.
Waterlow JC, Garlick PJ, Millward DJ (1978). “Protein turnover in mammalian tissues and in the whole body”, New York: Elsevier/North Holland.
White TP, Brooks GA (1981). [U-14C]glucose, - alanine and -leucine oxidation in rats at rest and two intensities of running. Am J Physiol 240:E155.
Wirth A, Diehm C, Mayer H, Mörl H, Vogel I, Bjorntorp P, Schlierf G (1981). Plasma C-peptide and insulin in trained and untrained subjects. J. Appl Physiol: Respirat Environ Exercise Physiol 50:71.
Yoshimura H, Inoue T, Yamada T, Shiraki K (1980). Aneamia during hard physical training (sports anaemia) and its causal mechanism with special reference to protein nutrition. World Rev Nutr Diet 35:1.
Young VR (1981). Skeletal muscle and whole body protein metabolism in relation to exercise. In Poortmans J, Niset G (eds): “Biochemistry of Exercise: Exercise and Hormone Regulations, Baltimore: University Park Press, p 59.
Young VR (1970). The role of skeletal and cardiac muscle in the regulation of protein metabolism. In Munro HN (ed): “Mammalian Protein Metabolism”, Vol 4 Chpt 40, New York: Academic Press.
Young VR, Bier DM (1981a). Stable isotopes (13C and 15N) in the study of human protein and amino acid metabolism and requirements. In Beers RF, Bassett EG (eds): “Nutritional Factors: Modulating Effects on Metabolic Processes”, New York: Raven Press, p 267.
Young VR, Bier DM (1981b). Protein metabolism and nutritional state in man. Proc Nutr Soc (In press).
Young VR, Meguid M, Meredith C, Matthews D, Bier D (1981b). Recent developments in knowledge of human amino acid requirements. In Waterlow JC, Stephen JML (eds): “Nitrogen Metabolism in Man”, London: Applied Science Publishers, (In press).
Young VR, Munro HN (1978). NT -methylhistidine (3-methyl-histidine) and muscle protein turnover: an overview. Fed Proc 37:2991.
Young VR, Robert JJ, Motil K, Matthews D, Bier DM (1981a). Protein and energy intake in relation to protein turnover in man. In waterlow JC, Stephen JML (eds): “Nitrogen Metabolism in man”, London: Applied Science Publishers, (In press).
Zinman B, Vranic M, Albisser AM, Leibel BS, Marliss EB (1979). The role of insulin in the metabolic response to exercise in diabetic man. Diabetes 28:Suppl I:76.