Fish farmers in Singapore obtain their seed stocks mainly from overseas and as such rely heavily on good packing conditions covering 8–12 hours transportation time to maximise fish survival and quality. For example, a loss of 50% of the stock would immediately double the price of the remaining live ones and this adversely affects the economics of production. While fish may be harvested and sold fresh dead, farmers prefer to transport marketsize fish live from their farms to landing points where the fish are picked up by lorries fitted with live tanks. The produce is then transferred to restaurants where live fish command higher prices.
The transportation of live fish involves the transfer of large numbers (or biomass) of fish in a small volume of water. During transportation, fish are subjected to handling stress and may die, or worse, survive to provide a stunted, marketable crop. The principles governing packaging, handling and transportation of live fish are essentially to minimise stress.
3.1 Dissolved oxygen (DO)
The presence of dissolved oxygen does not presuppose an absence of stress as other adverse factors can still exist with high DO, eg. high water temperature, pH changes.
Fish demand for dissolved oxygen by fish depends on water temperature, fish density (numbers and size), time of last feeding (level of starvation) and transportation time. It is therefore important to keep the transport water cool and fish biomass at an optimum, with due consideration for possible delays in transportation and the need for additional oxygen by the fish. Starving the fish prior to packing would also slow down ammonia accumulation and minimise unnecessary uptake of dissolved oxygen.
3.2 Ammonia (NH3)
Ammonia is excreted by fish and is reported to be toxic at low concentrations of 0.6 ppm. Ammonia excretion by fish decreases as its concentration in water increases, resulting in high blood ammonia. High blood ammonia elevates blood pH which affects enzyme-catalysed reactions affecting metabolism. Starvation and lowered temperatures reduce ammonia excretion.
3.3 Carbon dioxide
Fish become distressed when carbon dioxide (from respiration) accumulates rapidly in water since the blood is unable to carry oxygen under these conditions. Low levels of carbon dioxide (3–6 ppm) may be beneficial since it prevents the buildup of unionised ammonia. Carbon dioxide is also a mild anaesthetic and may be considered in alleviating stress during transportation.
3.4 Handling
Stress during handling and packing may be so severe as to cause chronic and acute mortalities. Poor handling and packing procedure may also cause osmoregulatory and metabolic disfunctions. Therefore it is important to proceed gently and quickly.
3.5 Water temperature (heat and cold)
Water temperatures greater than 28°C accompanied by declining dissolved oxygen and increasing ammonia, create a hostile environment. This is the likely situation if fish are over-packed or transportation is delayed under tropical conditions. Temperatures that are too low (<18°C) can cause thermal shock, especially in young fish. Stressed fish usually succumb to diseases after 1–2 weeks, if not already dead on arrival.
4.1 Reducing transport water temperature
This prevents thermal stress and improves oxygen stability. Ice should be used in the correct quantities and this depends on fish species and size and also the transportation period. Alternatively, cooled water (18°C) can also be used by lightly sedating the fish in 18°C water prior to packing, and then using water at the same temperature for transport. Under air freighting conditions, this temperature increases by about 1–4°C after 12–14 hours, and fish are usually alive.
4.2 Insulation
The use of insulated conmtainers like styrofoam boxes, newspaper lagging helps to maintain the temperature of transport water, being poor heat conductors. They also reduce vibration.
4.3 Anaesthesia
Anaesthesia prevents fish hyperactivity. The oxygen consumption of newly-packed fish elevates for 30–60 minutes and declines as fish acclimate to the new environment. The first 30–60 minutes after packing is therefore important. Some anaesthetics used are MS-222, carbonic acid, benzocaine and phenoxyethanol. However the use of certain chemicals for anaesthesising food fish is not to be recommended.
5.1 Fish are transported live as:
5.1.1 Imports and exports (eg. food fish fry and fingerlings, aquarium fish) where
live fish are transported by air, road or overseas source to the local farm site for culture.
live aquarium fish from overseas or local sources are transported by road or air to packing sites (eg. aquarium fish for export or re-export.
live food fish supplies from local waters are received and exported by sea or air.
(a)-(b) are usually by the “plastic bag” method whose advantage is that only cheap materials and equipment like plastic bags, rubber bands, compressed oxygen, cardboard or styrofoam boxes are needed.
Tables IX/1 & 2 below give some of the actual packing conditions for live fish imported into and exported from Singapore. Aquarium fish are packed singly or in small numbers, with several bags in one carton, while food fish fingerlings are packed at about 500 per bag at two bags per carton. It can be generally observed that as fish size increases, the numbers packed per bag decreases. However, a higher biomass is tolerated by larger fish.
| Fish species | Packing details | Country of origin/ destination | Fish mean wt (g) or total length (cm) | Volume of water (1/bag) | Biomass (g/l) | No. bags per box | Transporttation time (hr) |
| 1 Food fish fingerlings (imports) | |||||||
| 1.1 Srouper | Philippines | 15–45 g | 13 | 10–170 | 1–2 | 10–12 | |
| 1.2 Seabass | Thailand | 5–105 g | 6 | 70–520 | 1–2 | 6–8 | |
| 2
Marine aquarius fish (exports) | Europe & ASEAN | <5 cm | 0.15 | - | 70–105 | 12–40 | |
| 5–8 cm | 0.2–0.25 | - | 50–75 | 12–40 | |||
| 10–13 cm | 0.6–0.7 | - | 8–16 | 12–40 | |||
| 0.9 | - | 4 | 40 | ||||
| Packing conditions | Fish mean wt (g) | No./beg | Biomass | Transport water temp. (°C) | |
| g/l | no./l | ||||
| Fish species | |||||
| 1 Grouper | 8–11 | 100 | 89–100 | 7–9 | 28–29 |
| 11–14 | 50 | 102–128 | 4–5 | 28–29 | |
| 11–17 | 50 | 147 | 4–5 | 28–29 | |
| 2 Seabass | 4.5–5 | 70–100 | 50–100 | 11–20 | 27–28 |
| >5–10 | 60–100 | 93–148 | 14–35 | 25–28 | |
| >10–15 | 50–100 | 94–180 | 8–16 | 27–28 | |
| >15–20 | 50 | 133 | 8 | 27–28 | |
| >20–30 | 35–60 | 200–300 | 7–10 | 27–28 | |
| >30–40 | 35–40 | 222–267 | 6–7 | 27–28 | |
| >40–50 | 35 | 292 | 6 | 27–28 | |
| >50–70 | 25 | 267 | 4 | 27–28 | |
| 105 | 20–25 | 525 | 5 | 27–28 | |
5.1.2 Local produce (transportation of larger-sized fish within Singapore
Transfer of live marketsize fish from farm to landing point (by boat in tanks).
Transportation of live marketsize fish from landing point to restaurant (in tanks on lorries).
Transfer of live fish from farm to farm (by boat, in tanks).
Table IX/3 gives some of the local transport conditions of market-sized food fish from fish farms. the above mentioned circumstances. Since fish transportation period in open containers is short (<1 hr by boat) stocking biomass can be as high as 1 kg/1 (0.14–1.06 kg/1) by sea transport and 0.1 kg/1 by road (0.5–1.5 hr).
| Transport conditions | Fish mean wt (g) | Container | Volume of seawater (1) | Fish biomass (g/1) | Transportation time (hr) |
| Fish species | |||||
| 1 Sea transport+1 (commercial) Transported with aeration and ice | |||||
| Grouper & seabass | 630–760 | Styrofoas box | 36 | 140–310 | 0.25–0.5 |
| Plastic drum | 96 | 810 | 0.25–0.5 | ||
| Plastic bin | 25 | 1060 | 0.25–0.5 | ||
| 2 Sea transport (experimental) | |||||
| Grouper & seabass | 106–135 | Live fish tank+2 | 1400 | 30 | 2.5 |
| 170 | Plastic bin | 40 | 642 | 0.25–0.5 | |
| 2226–352 | Live fish tank+2 | 1400 | 66 | 1–2 | |
| 3 Road transport+3 (commercial) | |||||
| Grouper & seabass | 630–760 | Fibreglass tank | 300 | 100 | 0.25–0.5 |
+1 From farm to landing point
+2 With through-flowing seawater
+3 From landing point to restaurant
6.1 The results of packing trials with marketsize grouper using a variety of light sedations are shown in Table IX/4.
6.2 It was found that the light sedation using cooled water (18°C) was the most convenient, economical and effective to use.
6.3 As a general rule it is advisable to avoid the chemicals with animals meant for human consumption. Although the use of MS 222 is allowed in the United States for example, they require that no drug is used for 21 days prior to sacrifice.
| Treatment (anaesthesia) | Mean wt (g) | Oxygen:SW volume | Dead : Live | Transport Water | Final dissolved | |||
| (Fish: SW weight = 1:3) | fish ratio | Temperature (°C) | pH | oxygen (ppm) | ||||
| Initial | Final | Initial | Final | |||||
| Fish biomass 333 g/l | ||||||||
| Ice @ 75 g/L | 500–800 | 2.4–5:1 | 1:5 | 20–21 | 19–20 | 5–8 | 6 | 7–13 |
| Cooled water | 600–1000 | 2.4:1 | 1:3 | 19 | 21 | 8 | 5.5–6 | 4.4–17.2 |
| (18°C) | ||||||||
| Carbonic acid | 500–900 | 2.4:1 | 8:2 | 19–21 | 19–22 | 6–8 | 7 | 5–17 |
| MS 222 | 500 | 3.0:1 | 0:1 | 20.6 | 23 | 8 | 5.4 | 8 |
| MS 222 + ice @ | 700 | 2–3:1 | 0:2 | 20.6 | 22–23 | 7 | 6 | 5–6.5 |
| 75 g/L | ||||||||
| Fish biomass 143 g/l | ||||||||
| Cooled water | 550- | 4:1 | 0:3 | 18 | 21–22 | 7 | 6 | 10–13 |
| (18°C). | 600 | 1:1 | 0:2 | 18 | 19–20 | 7.1 | 6.2 | 20 |
| Cooled water | 570- | 4–5:1 | 0:3 | 18 | 20–23 | 7 | 6.3 | 9–15 |
| (18°C) | 670 | 1:1 | 2:0 | 18 | 18.5 | 7.3 | 6.8–7.3 | 17–18 |
| + ice @ 25g/l | ||||||||
1 Objective(s)
1.1 To compare the effect of two packing densities. on seabass fingerling tolerance and survival over 8 hours under simulated air cargo conditions.
1.2 To compare (1.1) with the response of larger-sized seabass fingerlings packed under similar conditions.
2 Plan
| Fish mean wt (g) | Packing g/l | density fish/l*1 | sw vol/bag (l) | Fish/bag | Replicate | Group |
| 5 | 100 | 20 | 7 | 140 | 2 | 1 |
| 5 | 200 | 40 | 7 | 280 | 2 | 2 |
| 10 | 100 | 10 | 7 | 70 | 2 | 3 |
| 10 | 200 | 20 | 7 | 140 | 2 | 4 |
*1 Numbers are adjusted if fish mean weight is not exactlyas stated.
3 Materials
3.1 Filtered seawater for fish packing (30–50 l).
3.2 Plastic bags 90 cm × 51 cm.
3.3 Dissolved oxygen meter with temperature sensor, pH meter, salinity refractometer.
3.4 500–1000 seabass fingerlings of about 5g (total length 3.5 cm) and 10g (total length 7.5–10 cm).
3.5 Measuring board, weighing balance, containers and tanks, aerators, scoop nets, oxygen cylinder, pressure gauge and air tubes, measuring cylinder, rubber bands, styrofoam boxes.
4 Procedure
4.1 Acclimation and sedation of fish using cooled water
In all treatments, acclimate and sedate the fish (about 30–40 g/l density) slowly in a tank to 18°C water temperature.
This can be done by placing 4 kg ice in plastic bottles gradually into the water containing the fish, and constantly monitoring the temperature drop.
Remove or add in ice bottles according to whether water temperature is declining too rapidly or remaining stagnant for too long. Observe fish behaviour.
Aerate the water throughout.
Record dissolved oxygen, pH and temperature in Table IX/5
Cool the filtered seawater to 18°C in a separate container, according to the method outlined in (b) and (c). Aerate vigorously.
Record dissolved oxygen, pH and temperature and take a sample of the filtered seawater for ammonia determination, (Table IX/5).
4.2 Fish packing in oxygenated plastic bags
Measure 7 litres of the cooled filtered water and pour into a double-layered plastic bag (about 15 cm height).
Count and transfer the required numbers of fish into the plastic bag.
Insert the delivery tube from the oxygen cylinder well into the water and oxygenate slowly, twisting the plastic bag round the tube to prevent oxygen loss.
Gradually inflate the bag so that oxygen occupies about twice the volume of the seawater.
Secure the bag with rubber bands, leaving the outer bag free. Make sure the bag is firmly inflated and not flaccid.
Secure the outer bag with more rubber bands.
Place the packed bag of fish into a styrofoam box lined with newspaper (lagging to prevent rapid temperature change).
Store the box in an air-conditioned room (19–22°C) to simulate air cargo temperatures.
4.3 Observations
Observe the fish over 8 hours, noting any signs of stress.
If fish appear stressed during the period, unpack and release. Measure final water temperature, pH and dissolved oxygen and take a water sample for ammonia-nitrogen analysis (Table IX/2).
If fish appear normal, allow the trial to run for 8 hours. Take final readings as described.
Record results in (Table IX/2).
5 Exercises
5.1 Does fish size appear to have an effect on survival and water quality parameters, at the same packing density?
5.2 What factor(s) do you think influenced fish survival most in this observation?
5.3 What would the approximate optimal packing density be for smaller and larger fingerlings?
5.4 How would dead fish affect the transport water and the remaining live fish?
5.5 What problems could arise if acclimation and packing were not done properly?
Note
More replications are required and a larger range of fish sizes should be used for more reliable results.
| Name: |
| Country: |
| Date: |
Sample description | Water quality parameters | |||
| Temperature | pH | Dissolved oxygen | NH3-N | |
| (°C) | (ppm) | |||
| 1. Acclimation and sedation water sample | ||||
| Group 1 | ||||
| Group 2 | ||||
| Group 3 | ||||
| Group 4 | ||||
| 2. Filtered seawater prior to fish packing | ||||
| Group 1 | ||||
| Group 2 | ||||
| Group 3 | ||||
| Group 4 | ||||
| 3. Final transport water samples | ||||
| Group 1 | ||||
| Group 2 | ||||
| Group 3 | ||||
| Group 4 | ||||
1 Objective(s)
1.1 To acclimate marketable seabass to sedation water temperature of 18°C.
1.2 To pack sedated marketable seabass in 18°C and iced transport water.
2 Plan
| Groups 1 & 2 | n = 4 fish | Sedation at 18°C | Packing in 18°C filtered seawater. |
| Groups 3 & 4 | n = 4 fish | Sedation at 18°C | Packing in 18°C filtered seawater + ice at 25 g/l. |
3 Materials
3.1 8 marketable seabass were brought from fish farm and stocked in indoor tanks to stabilise for 4 days.
3.2 Packing facilities and materials
Oxygen cylinder and pressure gauge, air tubing, plastic bags (90cm × 51cm), rubber bands, styrofoam boxes, weighing balance, measuring cylinder, aerators, containers.
3.3 Dissolved oxygen meter with temperature sensor, pH meter, salinity refractometer, sample bottles.
4 Procedure
4.1 Acclimation and sedation of fish to 18°C
Weigh the fish.
Transfer into a container of aerated, filtered seawater.
Add plastic bottles of ice gradually; monitor temperature drop.
Remove or add in ice bottles according to whether water temperature is declining too rapidly or remaining stagnant for too long. Observe fish behaviour.
Record dissolved oxygen, pH and temperature and take a sample for ammonia-nitrogen analysis (Table IX/6).
Cool filtered seawater to 18°C in a separate container according to the method outlined in (c) and (d). Aerate vigorously.
4.2 Fish packing in oxygenated plastic bags
Groups 1 & 2
Measure about 3 times the fish weight of filtered seawater (eg. if fish is 600g, measure about 1.8 litres of seawater) and pour into a plastic bag.
Transfer the fish gently into the bag.
Measure the height of water in the bag.
Insert the delivery tube from the oxygen cylinder well into the water and oxygenate slowly, twisting the plastic bag round the tube to prevent oxygen loss.
Gradaully inflate the bag so that oxygen occupies about 4 times the water volume.
Secure the bag with rubber bands, leaving the outer bag free. Make sure the bag is firmly inflated and not flaccid.
Secure the outer bag with more rubber bands.
Place the packed bag of fish into a styrofoam box lined with newspaper (lagging to prevent rapid temperature change).
Store the box in an air-conditioned room (19–22°C) for 12 hours to simulate our cargo temperatures and transport.
Repeat with 3 more marketable fish.
Groups 3 & 4
Follow through 2(a)-(b) of Groups 1 & 2 instructions.
Calculate the volume of cooled (18°C) filtered seawater required to cover the fish (weight of seawater = 3 × wt. of fish).
Pour the water into the plastic bag.
Calculate the weight of ice required (25g ice/l seawater) and weigh the ice out in a small plastic bag. Secure the bag of ice and place it in the plastic bag of water.
Transfer the fish gently into the bag.
Follow through 2(c)-(j) of Groups 1 & 2 instructions.
4.3 Observations
Open the bags the next day, after 12 hours.
Measure and record dissolved oxygen, pH, temperature and take a sample for ammonia-nitrogen analysis (Table IV/6).
Observe fish condition.
5 Exercises
5.1 What was the percentage fish survival in both treatments?
5.2 Is there an advantage in using ice?
5.3 Can you expect the ratio of fish weight: transport seawater (1:3) to apply to other fish sizes/species? Why not?
5.4 How long does it take for marketable seabass to be sedated with ice? How quickly will the fish recover?
5.5 Calculate how long it took to lower 1 litre of seawater at ambient temperature to 18°C.
Note
More replications are required and a larger range of fish sizes should be used to make positive conclusions.
| Name: |
| Country: |
| Date: |
| Sample description | Water quality parameters | |||||||||||||||||||
| Temperature 0 | Dissolved | |||||||||||||||||||
| (°C) | pH | oxygen(ppm) | NH3-N | |||||||||||||||||
| 3 | ||||||||||||||||||||
| Fish No. | 1 | 2 | 3 | 4 | 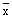 | 1 | 2 | 3 | 4 | 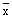 | 1 | 2 | 3 | 4 | 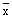 | 1 | 2 | 3 | 4 | 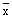 |
| 1. Acclimation and sedation water sample | ||||||||||||||||||||
| Groups 1 & 2 | ||||||||||||||||||||
| Groups 3 & 4 | ||||||||||||||||||||
| 2. Filtered seawater prior to fish packing | ||||||||||||||||||||
| Groups 1 & 2 | ||||||||||||||||||||
| Groups 3 & 4 | ||||||||||||||||||||
| 3. Final transport water sample | ||||||||||||||||||||
| Groups 1 & 2 | ||||||||||||||||||||
| Groups 3 & 4 | ||||||||||||||||||||
D43 naca-9