Oysters are found everywhere in the world except in the north and south poles. The vertical and horizontal distribution and dispersion of oysters are linked to the planktonic-stage period which is during one to four weeks after spawning, and also by the artificial transplantation of young oyster, For instance, oyster larvae settle in shallow coastal waters and in deeper areas in the open sea. The distribution of C. gigas has been extended to the Pacific coast of America through transplantation from its original habitats in the Korean and Japanese coasts. Distribution of oysters is wide, as shown in Table 3-1.
1. Ostrea edulis (European flat oyster) occurs widely in European coastal areas, particularly in France, England, Denmark, Norway and Italy.
2. Ostrea lurida occurs along the Pacific coast of America.
3. Crassostrea angulata occurs in Portugal, Spain, France and England.
4. Crassostrea virginica (American oyster) occurs widely in the Atlantic from south Canada to the USA. A small amount is found in certain Pacific regions. This species adapts to widely varying environmental conditions.
5. Crassostrea commercialis (Sydney oyster) is oviparous and occurs widely from east to soutwest Australia. The majority of this species live in shallow water, but sometimes in deeper areas.
The species and distribution of Korean oysters are as follows:
6. Crassostrea gigas occurs in Korea, Japan and China. Through transplantation of oyster, its distribution has been extended to America, Australia and Europe.
7. Crassostrea rivularis is oviparous and occurs at the mouth of Nakdong River in Korea.
8. Crassostrea nippona is an oviparous species which occurs from the southern part of the east coast to the south coast of Korea.
9. Crassostrea echinata occurs from the south to the west coast of Korea, and in the coasts of Japan, Australia and Indonesia.
10. Ostrea denselamellosa occurs in the southern part of the east, south and west coast of Korea.
| Species | Distribution area |
| Ostrea edulis | 40 – 60°N |
| European flat oyster | Europe : England and coastal area from the Mediterranean to Scandinavia |
| O. lurida | 33 – 50°N |
| Olympia oyster | Pacific coast of America |
| Crassostrea angulata | 37 – 45°N |
| Portuguese oyster | Portugal, Spain, France |
| C. virzinica | 27 – 47°N |
| Virginia oyster | Atlantic coast of America |
| American oyster | |
| Eastern oyster | |
| C. commercialis | 22 – 28°S |
| Sydney oyster | Australia |
| Australian oyster | |
| C. gigas | 30 – 45°N |
| Pacific oyster | Korea, Japan, China |
Oyster species could be subdivided further according to their distribution in water of different salinities and temperature from shallow areas to the open sea. Figure 3-1 shows the salinity range tolerance of some oyster species. Generally, Ostrea species are distributed in areas with full strength salinities. Those belonging to the genus Crassostrea tend to be more euryhaline (they can survive and develop in fresh to saline waters) and they are found in shallow water with fluctuating salinity levels. Figure 3-2 shows the vertical distribution of several oyster species. Generally, oyster species belonging to the genera Ostrea and Pycnodonta are found in tidal zones and Crassostrea species in the intertidal zones.
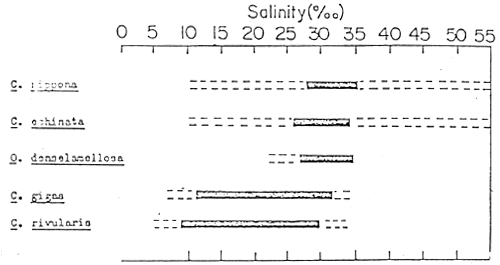
Figure 3-1. Tolerable salinity ranges for various oyster species.
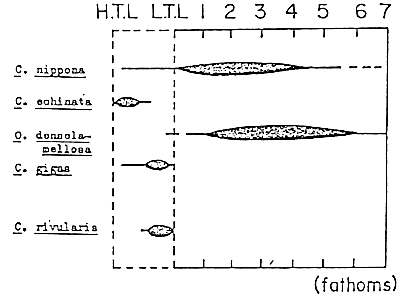
Figure 3-2. Vertical distribution of several oyster species.
H.T.L. - High tide level.
L.T.L. - Low tide level.
Oyster growth is indicated by shell size. Oyster shell is composed of calcium carbonate as with other shellfish, and conchiolin, a kind of protein.
The shell is excreted by the mantle which absorbs calcium ion from sea water (0.4g/l). Absorption is accomplished by the outer layer of the mantle. Thus shells grow without food, except when the level of calcium ion in the seawater is below 20 percent. The growth rate of shell varies from one site to another and is influenced by environmental conditions.
Water temperature has a great influence on shell growth. Water temperature in winter are often too low for shell growth but growth could be stimulated in warmer water bodies. Water temperature may also cause differential growth on different parts of the shell. The growth of soft part is poor during summer and early autumn mainly due to spawning. The severity, however, depends on the abundance of nutrients.
The weight variations of the soft part is related to the rate of gonad development against glycogen; accumulation during a one-year cycle. Follicular cells dispersed in connective tissue develop rapidly during spring to summer. After that, the central part of soft body is filled with mature eggs or sperms except the outer layer of follicle cell wall.
After spawning, the connective tissue increases rapidly and the remnant of gonads is absorbed. A thick connective tissue containing glycogen is formed during winter.
The weight increase in the soft part of the organism due to the above mentioned process is generally known as “full up.” Filling up of the soft part varies from season to season. This phenomenon proceeds as follows:
The degree of glycogen accumulation varies from one place to another. However, the process begins generally towards the end of September and the flesh becomes distinctively white in appearance. According to the increment of accumulated glycogen, the style sac becomes white by November, and the white part is extended to the whole part except the gills. Flesh becomes thick.
The economic value of the soft part varies with the degree of fill up. The degree of fill up is the rate of dry weight of the soft part versus the volume of the shell multiplied by 1,000. This is called “condition index.”
The factors that influence oyster flesh growth are water temperature, food quantity, feeding rate, spawning and population density. The most important one is food quantity which is influenced by water circulation and such climatic conditions as rainfall, wind velocity and tide.
Oysters suffering from malnutrition grow slowly or not at all. Generally, high stocking density could be the reason,. Oysters from a nursery with low water velocity or from high density population or at the center of a culture structure, such as raft or line, slowly fill up so that the product is comparatively leaner than those placed in favorable conditions. The soft part of cultured oyster under poor nutrition and high population density becomes transparent; the vacuum left by spawning is filled with water rather than glycogen. The style sac is clearly visible.
Generally, oyster do not fill up during summer and autumn because of the spawning process. Quantitative aspects of spawning such as duration, amount of seed, etc. influence the rate of the next fill up.
Maturation of oysters mainly depends on water temperature. Table 3-2 shows the number of days required for the maturation and spawning of cultured C. virginica in relation to different water temperatures (Loosanoft, 1945).
Spawning can be induced by rapid rise of temperature. Orton (1926) and Korringa (1947) reported that a full moon induced spawning. However, recent observations have attributed induced spawning to salinity, temperature and water pressure rather than to lunar and tidal cycles. Salinity induces oyster to spawn with the rapid rise of temperature.
On the other hand, spawning induced by the use of diluted sperm medium was reported by Galsoff (1938). The physiological responses of oyster to various forms of physico-chemical stimuli have not been fully understood, however.
Environmental factors influencing fertilization development and growth of juveniles are mainly water temperature and salinity. Fertilization occurs in a wide range of temperature, but development up to the D-stage is limited within a certain range. At low temperatures, growth is slow and morphological variations, e.g. projection of umbo, do not occur easily. Therefore, settling time of larvae cultured at low temperature is longer than that at high temperature, and so is the planktonic period.
| Habitat | Water temperature(°C) | |||
| 12 | 21 | 24 | 27 | |
| Long Island Sound | 68 | 15(18) | 5 | 5.5 |
| Now Jorney | - | 55(78) | 32 | 22.5 |
( ) : Time required in days for spawning
- : Immature
The planktonic period of C. gigas larvae differs with water temperature. It is usually more than three weeks at 19–20 degrees Celsius and ten days at about 27 degrees Celsius (Yoo et. al., 1974).
Salinity has a strong influence on the rate of development of larvae. Optimal salinities of some species have been determined in experiments as shown in Table 3–3. Optimal salinities for juveniles are similar to those for adult specimens.
Salinity tolerable to C. gigas and to the Portuguese oyster (C. angulata) ranges between 14 to 37 ‰
The time required for the embryonic development stage of the Pacific oyster is shown in Table 3–4. The shape of eggs before and after spawning, and its developing stages, are shown in Figure 3–3. Prior to spawning, the shape of the eggs in the gonad is irregular; after spawning it becomes spherical with a 50 μm diameter.
| Species | Optimum Salinity (‰) | Remark |
| C. gigas | 20–26 | Amemiya (1928) |
| 23.3–28.5 | Seno et al. (1926) | |
| 12–23 | Ranson (1948) | |
| C. angulata | 26–35 | Amemiya (1928) |
| 18–23 | Ranson (1948) |
| Developmental stage | Time required |
| 1st polar body releasing | 50 minute - 1 hour 10 minute |
| 2nd polar body releasing | 1 hour 00 minute - 1 hour 20 minute |
| 1st division | 1 hour 20 minute - 1 hour 40 minute |
| 2nd division | 2 hour 00 minute - 2 hour 20 minute |
| Morula stage | 3 hour 5 minute - 3 hour 25 minute |
| Blastula stage | |
| Rotary movement | 5 hour 20 minute - 5 hour 50 minute |
| Free swimming | 5 hour 30 minute - 6 hour 00 minute |
| Gastrula stage | 6 hour 10 minute - 6 hour 30 minute |
| Trochophore | 9 hour 40 minute - 10 hour 00 minute |
| D-shaped larva | 15 hour 00 minute - 28 hour 00 minute |
Newly spawned eggs are in the first oocyte stage before maturation division occurs. The germinal vesicle does not disappear until this stage.
As the sperm enters the egg, the fertilization membrane forms on the surface of the egg and the first polar body is released through the maturation division (Figure 3-3c). This stage takes about 50 to 70 minutes. The egg nucleus is at first fertilized and the egg then undergoes cleavage (Figure 3-3d, 3-de, 3-3f). Cleavage continues to give rise to the morula and blastula stages. At the blastula stage, the egg is ciliated at the surface and begins to rotate.
| Salinity (‰) | No. of settled larva (Mean No./disk) |
| 26–27 | 141 |
| 25.0 | 325 |
| 22.5 | 146 |
| 20.0 | 76 |
| 17.5 | 15 |
| 15.0 | 2 |
| 12.5 | 0 |
| 10.0 | 0 |
| 7.5 | 0 |
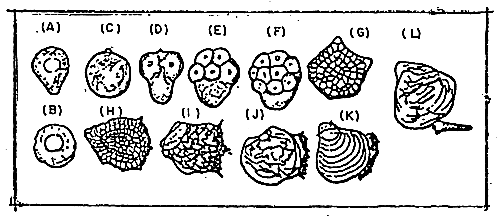
Figure 3-3. Developmental stages of C. gigas. A, egg prior to spawning; B, newly-liberated egg; C, fertilized egg; D, 3-celled stage; E, 8-celled stage; F, 16-celled stage; G, multi-celled stage; H, ciliated gastrula stage; I, trocophore stage; J, D-shaped larvae; K, early umbonate larvae; L, mature pediveliger larvae.
Following the blastula stage is gastrulation at which stage the blastopore depressions become evident. It takes six and one-half hours to develop until this stage. After that, the larvae becomes trochophore with cilia ring.
The velum then develops and the larva becomes D-shaped with shell. To develop until this stage, it takes 25 to 28 hours. The larva at this stage is known as veliger.
The time it takes for D-shaped juvenile to settle depends mainly on water temperature. Settlement of the juveniles begins with the appearance of the foot gland, foot and eyespot. Movements are aided by the velum which enables the larvae to creep until it finds a suitable place for permanent attachment.
Settling of juvenile is also affected by salinity. In flat oysters, the optimal salinity range is 22.5 to 25.0‰; settling drastically decreases as shown in Table 3–5. In the case of American oyster, settling time also varies with salinity, and greatest rate occurs at 15–20‰ (Figure 3–4).
Spat settling also depends on water current and concentration of copper ion. Settling does not occur where water current is more than 5 to 7 cm/sec.
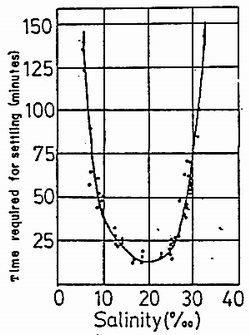
Figure 3–4. Larval setting time in relation to salinity levels.
It has been observed that there is plenty of spatting where the speed of water velocities is slowed down by a barrier (Korringa, 1941; Thomson, 1950). Clean substances are preferred to muddy substances (Needler, 1941) and the concentration of settled spats is generally higher on the lower part of the cultches (Knight, 1951; Wilson, 1941), mainly believed to be due to the absence of sediments and seaweeds (Korringa, 1941).
The density of settled spats is directly related to the density of the planktonic stage. Theoretically spats settle in a wide range of depth. However, this range is restricted by certain environmental factors that could induce mass mortality at the early stages of spat development. For instance, newly-settled spats cannot withstand three to four hours of direct sunshine in summer, and cannot settle on muddy surface or in turbid areas. Consequently, oyster settling is determined by these and other phenomena. However, the settling layer varies somewhat with depth, depending on species.
After settling, foot, velum and eye spot degenerate, gill and adduction muscles develop and the chitin shell is replaced with calcium.
The density of planktonic larvae indicates the degree of future settling levels (Lambert, 1946).
The number of spats per million spawned eggs is estimated to be in the range of one percent. In order to improve the collection of natural spat, further studies are needed on the developing processes of the larvae, and on the environmental conditions that influence the movement of the larvae in the water.