The Kingdom of Thailand has an area of 514 000 square kilometers and is located in the middle of the Southeast Asian peninsula. Geographically, Thailand can be divided into five separate regions as follows: the Northern Region, the Central Region, the Northeastern Region, the Southeastern Region, and the Southern Region. The population of Thailand is about 45 million.
Thailand has a coastline of about 2 600 km bordering two large oceans, the Pacific Ocean and the Indian Ocean. The coastline can be broadly classified into four regions according to Vibulsresth et al. (1975).
Region 1. This region includes the coastline of the five provinces on the eastern part of the Gulf of Thailand: Trat, Chantaburi, Rayong, Cholburi, and Chachoengsao (see Fig. 1). The coastline is approximately 515 km and includes about 470 km2 of mangrove area. The annual precipitation is about 2 660 mm. The mean temperature is about 27.8°C. The relative humidity lies between 80–85%.
Region 2. This region is located at the uppermost part of the Gulf of Thailand. It includes four provinces: Samut Prakarn, Samut Sakorn, Samut Songkram, and a part of Bangkok. The length of the coastline is about 116 km and has a mangrove forest of 273 km2. The climate of this region is comparatively dry; the annual precipitation is approximately 1 850 mm, and the mean temperature is 28.8°C. The relative humidity is about 75–80%.
Region 3. This region covers the coastline on the western side of the Gulf of Thailand. The bordered provinces are: Petchaburi, Prachuap Khirikhan, Chumporn, Surathani, Nakorn Sri Thamarat, Songkhla, Pattani, and Naratiwat. The coastline is about 1 250 km long with mangrove forests of about 458 km2. The annual rainfall in this region is about 2 600 mm. The mean annual temperature is 27.2°C with an average relative humidity of about 83%.
Region 4. This region is the coast of the Andaman Sea (part of the Indian Ocean) with a length of about 700 km. The provinces of Ranong, Phuket, Krabi, Trang, and Satul bordered by this coastline have a mangrove forest area of 1 917 km2. The mean annual precipitation is about 4 015 mm, the mean annual temperature is 27.5°C, and the relative humidity lies between 80 and 85%.
Figure 1 indicates the distribution of mangrove forests and mudflats in Thailand. It is an interpretation of the LANDSAT 1 and 2 imagery, which gave distinction between forests and mudflats. The imagery on which the interpretation is based dates, however, back to 1973.
The distribution of the mangrove forests in Thailand, based on the data from Vibulsresth et al. (1975), is as follows: about 80% of the mangrove forests and situated on the Andaman coastline and the rest along the Gulf of Thailand. The mangrove forest in Phang Nga province is the largest single area and the one in Prachuap Khirikhan is the smallest. The distribution by province is given in Table 1.
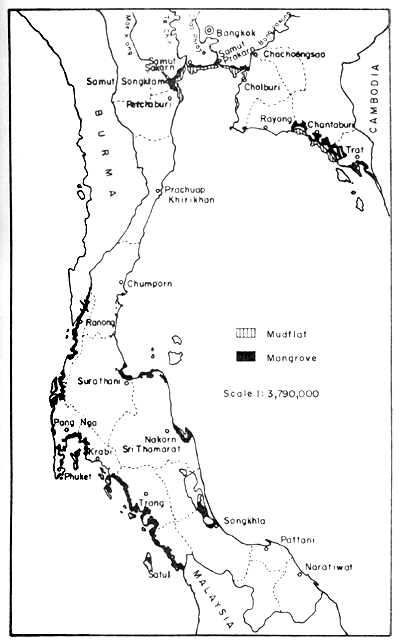
Fig. 1. Distribution of mangrove forests and extended mudflats in Thailand. Gleaned from a map (1:1000 000) showing interpretation from LANDSAT 1 and 2 imagery, provided by Thailand National Remote Sensing Programme, NRC/ASRCT in 1978.
Table 1. Total area of the mangrove forest in Thailand (Vibulsresth et al., 1975)
| Provinces | Total area of mangrove forest* | |
| Rai** | Km2 | |
| Chumporn | 46 000 | 74 |
| Ranong | 151 000 | 242 |
| Phang Nga | 391 438 | 511 |
| Phuket | 19 177 | 31 |
| Kabi | 206 150 | 330 |
| Trang | 212 700 | 340 |
| Satul | 289 475 | 463 |
| Pattani | 6 500 | 11 |
| Songkhla | 37 000 | 59 |
| Patthalung | 11 750 | 19 |
| Nakorn Sri Thamarat | 96 750 | 155 |
| Surathani | 23 000 | 37 |
| Prachuap Khirikhan | 2 250 | 4 |
| Rayong | 34 200 | 55 |
| Chantaburi | 163 325 | 261 |
| Trat | 66 500 | 106 |
| Samut Prakarn | 4 000 | 6 |
| Chachoengsao | 19 000 | 30 |
| Cholburi | 23 750 | 38 |
| Samut Sakorn | 115 750 | 185 |
| Samut Songkram | 51 500 | 82 |
| Petchaburi | 55 250 | 88 |
Total | 1 954 465 | 3 127 |
* Data from satellite survey including mudflats
** 1 Rai = 0.16 ha
Smitinand (1976) made a survey of the mangrove flora of the various regions in Thailand. The species he reported are listed in Table 2. This comparative study indicates that there is no marked difference between the composition of the forests along the southeast coast (Chantaburi - Trat) and the western coast (Indian Ocean).
Table 2. Distribution of main mangrove species in Thailand (Smitinand, 1976)
| Species | Southeast | East | West |
| Acanthus ebracteatus | X | X | X |
| A. ilicifolius | - | - | X |
| A. volubilis | - | - | X |
| Acrostichum aureum | X | X | X |
| A. speciosum | X | - | X |
| Aegiceras corniculata | - | - | X |
| Allophylus cobbe | |||
| Avicennia alba | X | X | X |
| A. marina | X | X | X |
| A. officinalis | X | X | X |
| Bruguiera cylindrica | X | X | X |
| B. gymnorrhiza | X | X | X |
| B. hainesii | X | X | ? |
| B. parvifolia | - | X | X |
| B. sexangula | X | X | X |
| Caesalpinia nuga | X | X | X |
| Calamus aquatilis | - | X | X |
| Ceriops decandra | X | X | X |
| C. tagal | X | X | ? |
| Cleroden enerme | X | X | X |
| Deamonorop leptopus | - | - | X |
| Derris trifoliata | X | X | X |
| Excoecaria agallocha | X | X | X |
| Heritiera littoralis | X | X | X |
| Heritiera sp. nov | X | - | - |
| Hibiscus tiliaceus | X | X | X |
| Intsia bijuga | X | X | X |
| Kandelia candel | ? | X | X |
| Lumnitzera littoralis | X | X | X |
| L. racemosa | X | X | X |
| Melaleuca leucadendra | X | X | X |
| Melastoma villosa | - | - | X |
| Nypa fruticans | X | X | X |
| Oncosperma tigillarium | - | X | X |
| Phoenix paludosa | X | X | X |
| Pramna integerrima | X | X | X |
| Rapanea porteriana | X | - | X |
| Rhizophora apiculata | X | X | X |
| R. mucronata | X | X | X |
| Scolopia macrocarna | - | X | - |
| Scyphiphora hydrophyllacea | - | - | X |
| Sonneratia alba | - | X | X |
| S. calcaclaris | X | X | X |
| S. griffithii | - | - | X |
| S. ovata | X | X | X |
| Thespesia populnea | X | X | X |
| Xylocarpus obovatus | X | X | X |
| X. malaccensis | X | X | X |
A taxonomic study and description on the species of the family Rhizophoraceae was carried out by Sripen and Thamathaworn (1976). They described nine species in this family as shown in Table 3.
Table 3. Nine species of the family Rhizophoraceae studied and described by Sripen and Thamathaworn, 1976
| Scientific name | Thai common name | |
| Rhizophora | mucronata Poir | Kong-Kang Bai Yai, Pangka Baiyai |
| R. | apiculata Bl. | Kong-kang Bai-Lek, Pangka Bailek |
| Pangka Tray | ||
| Bruguiera | gymnorhiza (L.) Luk | Pangka Huasum, Pangka |
| R. | sexangula Lour, Piir | Prasak, Pangka Huasum-Dok Kae., Klak |
| B. | cylindrica (L.) Bl. | Rui, Taodam, Proy |
| B. | parviflora (Roxb.) W. and Alex Griff | Toakae, Rangkra toa Lung Katai |
| Ceriops | tagal (Perr) C.B. Rob. | Prongdaeng, Prong-Yai |
| Prung, Phong, Samae | ||
| C. | decandra (Griff.) Ding How | Prongkae, Prongnu |
| Kapulong, Pulong | ||
| Prongkae, Samae | ||
| Kandelia | condel (L.) Druce. | RangKata, Lui |
| Taunang Choy. | ||
Among the plants of the mangrove forests in Thailand, Excoecaria agallocha (L.) of the family Euphorbiaceae is considered one of the most important economic trees. The wood of this species is soft wood, and the foresters recently requested the Department of Forestry to protect this species.
Epyphytic flowering plants in mangrove forests of Songkhla, Satul, Phuket, Phang Nga, and Chantaburi have been studied by Sahavachrin and Boonkerd (1976). They reported three families (Asclopiadaceae, Loranthaceae, and Orchidaceae), 13 genera, and 18 species (Table 4). Two species in the family Loranthacae were considered to be semi-parasitic epiphytes.
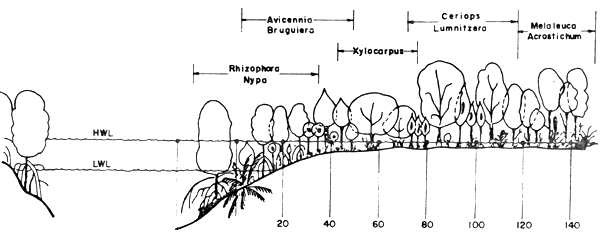
The plant zonations of the mangrove forest in Thailand are classified differently by various authors. At Chantaburi for example, Aksornkoae et al. (1979) classified the zones into Rhizophora-Nypa zone, Avicennia - Bruguiera zone, Xylocarpus zone, Ceriops - Lumnitzera zone, and Melaleuca - Acrostichum zone, as shown in Figure 2.
Table 4. Epiphytic flowering plants in the mangrove forest (Sahavachrin and Boonkerd, 1976)
| Family | Genus | Species | Selected Thai common name |
| Asclipiadaceae | Hoya | parasitics (Roxb.) | Nom Pikud-Daeng |
| Wall. ex. Wight | Nom Tam Rai | ||
| Nom Pichit | |||
| Hoya | lacunosa Blume | Tao-Nom Meae | |
| Dischidia | rafflesiana Wall | Juk Rohinee | |
| Taopung-Pla | |||
| Klay-Mu-lung | |||
| Loranthaceae | Dendrophthoe | pentadra (L.) Miq. | Ka-Phak-Ma-Maung |
| Viscum | ovalifolium D.C. | Ka-Phak-Mai-Tatum | |
| Ka-Phak-Che-Kohin | |||
| Ka-Phak-Mai-Huad | |||
| Orchidaceae | Bullophyllum | parpuracens T. & B. | |
| Bullophylum | dixoni Roffe | ||
| Dendrobium | crumenatum Sw. | Wai Ta-Moy | |
| Dendrobium | indivisum (Bl.) Miq. | Bau-Klang Haw | |
| Dendrobium | subulatum (Bl.) Ldl. | ||
| Eria | albido-tomentosa (Bl.) Ldl. | ||
| Eria | ornata Ldl. | ||
| Eulophia | keithii Ridl. | ||
| Luisia | zollingeri Rehl. f. | ||
| Paphiopedilum | exul (O'Brien) Pfitz | Rong Tao Naree-Krabee | |
| Pholidota | imbricata (Roxb.) Ldl. | ||
| Pomatocalpa | siamensis (Rolfe. ex. Downie) Summ | Eong-Sae-Paew | |
| Trichoglottis | misera (Ridl.) Roltt. |
| Open | Forest | ||||||||
| Water Properties: | area | margin | Distance, m | ||||||
Temperature, °C | 29.5 | 29.1 | 28.7 | 82.2 | 27.9 | 27.8 | 27.6 | 27.5 | 27.6 |
pH | 7.2 | 7.0 | 6.9 | 6.8 | 6.7 | 6.7 | 6.7 | 6.6 | 6.6 |
Salinity, ‰ | 22.21 | 22.04 | 21.46 | 21.18 | 20.96 | 20.67 | 19.87 | 18.62 | 19.49 |
Dissolved oxygen, ml/l | 4.40 | 3.37 | 2.99 | 2.68 | 2.36 | 2.15 | 1.99 | 1.55 | 1.70 |
COD, mg/l | 16.00 | 17.91 | 18.88 | 17.73 | 16.48 | 16.82 | 19.88 | 19.82 | 20.62 |
PO4, Ug-at P/l | 1.179 | 1.290 | 0.967 | 0.966 | 0.823 | 0.838 | 0.869 | 0.834 | 0.859 |
NO3, Ug-at N/l | 2.025 | 2.220 | 2.388 | 2.533 | 2.418 | 2.365 | 2.461 | 2.407 | 2.590 |
SiO2, Ug-at Si/l | 87.57 | 94.33 | 94.73 | 91.61 | 99.72 | 92.46 | 103.31 | 100.82 | 107.84 |
Fig. 2 Average values of water properties at different locations from forest margin to the land in a mangrove forest (from Aksornkoae et al., 1979)
Piyakarnchana et al. (1979) who studied the mangrove forest at Samut Songkram Province near the eastern side of the Mae Klong river mouth (see Fig. 1), divided the zones into: Nipa fruticans zone, Rhizophora apiculata zone, and Rhizophora mucronata zone.
In the first or Nipa fruticans zone, the dominant species of plants are of course Nipa fruticans. Other plants in the zone are Xylocarpus granatum, Rhizophora apiculata, Thespesia populanea, Soungratia caseolaris, Xylocarpus molucensis; Ceriops tagal, and Wedalia biflora. There are also climbing plants notable; Derris trigoliata.
In the second or Rhizophora apiculata zone, the most dominant plant is Rhizophora apiculata, with Xylocarpus granatum and Nipa fruticans as secondary ones. These plants are scattered throughout the Rhizophora apiculata zone.
In the third or Rhizophora mucronata zone, there are Rhizophora mucronata, Avicennia officinalis, Avicennia alba, Rhizophora apiculata, Nipa fruticans, Bruguiera cylindrica, and Sessurium portulacastrum. In addition Sueaeda maritima can be found in some spots.
On the west coast of the river mouth, which has largely been transformed into cultivated forest, the zonation of species composition is Nipa fruticans zone; Nipa and Rhizophora zone, and cultivated Rhizophora zone. However, the species composition of the plants in all zones of the west side of the river mouth is the same as those found on the east side.
Lewmanomont (1976) studied the algal flora of mangrove swamps in various parts of Thailand. She divided those algae into 2 types based on their habitats. One group consists of those that are sedentary on the trunks or roots of mangrove, the other comprised those that grew on the mudflat or sandy mud areas.
The mangrove roots support red algae of the genera Catenella, Bostrychia, and Murrayella. Many others of red algae found in the mangrove forests of Thailand belong to the genera Laurencia, Gracilaria, Hypnea, Centoceros, and Polysiphonia. The muddy areas are the habitats of many kinds of algae. Among them are recorded green algae of the genera Enteromorpha, Cladophora, Rhizoclomium, Bryopsis, Ulva, Avrainvilles, Halimeda, and Caulerpa. The genera of brown algae are Ectocarpus, Dictyota and Padina. Blue green algae belong to at least 2 genera. Lyngbya and Symploca, which are found on the mudflats.
Lewmanomont also reported 3 genera of submarine flowering plants that are commonly found in the mangrove forests: Halophila, Enhalus, and Cymodocea
The algae specles of the mangrove swamps in Thailand are listed in Tables 5 and 6.
Table 5. Red algae in the mangrove forests in Thailand (Lewmanomont, 1976)
| Phylum | Family | Genera | Species | Habitats |
| Rhodophyta | Rhabdoniaceae | Catenella | nipae | roots, trunks |
| Catenella | repens | roots, trunks | ||
| Rhodomelaceae | Bostrychia | tenella | roots, trunks | |
| Bostrychia | binderi | rocks of creeks nearby | ||
| Bostrychia | radicans | roots, trunks | ||
| Murrayella | periclados | roots (rare species) |
Table 6. Marine algae commonly found on the mud flats near the mangrove forests (Lewmanomont, 1976)
| Phylum | Family | Genus | Species |
| Oscillatoriaceae | Lyngbya, Symploca | - | |
| Phormidium | - | ||
| Rivulariaceae | Calothrix | - | |
| Chlorophyta | Ulvaceae | Ulva | reticulata |
| Enteromorpha | flexuosa | ||
| Enteromorpha | intestinalis | ||
| Cladophora | fasciculoris | ||
| Cladophora | sociolis | ||
| Cladophoraceae | Rhizocionium | - | |
| Dasycladaceae | Acetabuloria | major | |
| Codiaceae | Avrainuillea | erecta | |
| Halimeda | incrassata | ||
| Bryopsidaceae | Bryopsis | - | |
| Caulerpaceae | Caulerpa | racemosa | |
| Caulerpa | verticillata | ||
| Caulerpa | peltata | ||
| Phaeophyta | Ectocorpaceae | Ectocarpus | - |
| Dictyotaceae | Dictyota | dichotoma | |
| Dictyota | cervicornis | ||
| Padina | boryana | ||
| Padina | gymnospora | ||
| Padina | tetrastromatica | ||
| Sargassaceae | Sargassum | - | |
| Turbinaria | conioides | ||
| Turbinaria | ornata | ||
| Turbinaria | decurrens | ||
| Rhodophyta | Gracilariaceae | Gracilaria | verrucosa |
| Gracilaria | crassa | ||
| Gracilaria | salicornis | ||
| Hypneaoeae | Hypnea | ospori | |
| Hypnea | cervicornis | ||
| Hypnea | musciformis | ||
| Rhodomelaceae | Laurencia | papillosa | |
| Laurencia | pavipapillata | ||
| Laurencia | obtusa | ||
| Herposiphonia | - | ||
| Acanthophora | specifera | ||
| Ceramiaceae | Centoceros | clavulatum | |
| Spyridia | filamentosa |
Records from the Forest Department during 1968 to 1975, indicate that the amount of wood extracted from mangrove forest in 17 provinces of Thailand fluctuates from year to year. In 1973 for example, the amount was ca. 867 000 cubic meters; in 1975, however, it decreased to ca. 751 000 cubic meters (Tongma and Kongsaengchai, 1979).
Christensen's study (1978) on the biomass production of Rhizophora apiculata results in estimation of a total net production in a mangrove forest in southern Thailand of 27 t dry matter/ha/a (or 6.9 g ash free dry matter/d). The production is also studied in mangrove plantations by Aksornkoae (1975). He estimated the biomass of 11 to 14 years old mangrove plants to be approximately 155 t dry matter/ha/a. The increment would then be 24 t/ha/a.
Few studies on the animal communities in the mangrove forests have been done in Thailand. Frith et al. (1976) was the first group who reported on the zonation of macrofauna (epifauna, infauna, and mangrove tree fauna) on a mangrove shore in Phuket province. They recorded 139 species, and the composition of these organisms are: 59 species of crustaceans, 42 species of molluscs, 22 species of polychaetes, 6 species of fish, 4 species of ceolenterates, 1 species of nemertenes, 2 species of sipunculids, 2 species of echinoderms, 1 species of platyhelmintes, and 1 species of brachyopods (Table 7). They concluded that ecological factors notably the substratum and the exposure period at low tide, were the most important factors limiting the distribution of these animals. Some well-known ecological factors, such as the temperature, the salinity, and the pH were considered to be of minor importance.
Piyakarnchana (1976) studied the intertidal mudflat community at Ang Sila, Cholburi province during 1966 to 1971. This study showed that there are seasonal differences among the composition of various species and an abundance of intertidal organisms. The highest values of total biomass were found during December and January.
A survey on insects in a mangrove forest at Bangpoo was carried out by Vaivanijkul (1976). Another one was done by Sittilert et al. (1976) on the vertebrate animals (excluding fishes) in Bang Bane (Ranong), Ban Chan (Chantaburi), Bangpoo, and Samutprakarn.
Naiyanat (1979) reported on the distribution of 11 species of Uca crabs along the coastal zones of Thailand. There are 8 species of Uca found at Phuket province alone. Piyakarnchana et al. (1979) studied the distribution and density of animals in the three mangrove zones of the polluted estuary of the Mae Klong River.
In zone 1 or the Nipa fruticans zone, the animals recorded were Uca spp., Sesarma spp., Cerithium spp., Assiminea brevicula, Alpheus spp., Periopthalmus spp., and the species of snake Cerberus rhychops. Among these animals, the gastropod molluscs Cerithium spp. and Assiminea brevicula are the most abundant organisms.
In zone 2 or the Rhizophora apiculata zone, the animals noted were similar to those in zone 1. However, in this zone they found horseshoe crab (Carcinoscorpius rotundicauda) on the mudflat and Balanus spp. on the mangrove roots. Only one species of the gastropod mollusc, namely Cerithium spp. is abundant in this zone.
The animals in zone 3 or Rhizophora mucronata zone were Sesarma spp., Uca spp., a gastropod species, two crab species and Assiminea brevicula.
Table 7 Macrofauna found on the mangrove shore at Ao Nam Bor, Phuket, Thailand (after Frith et al., 1976)
| Actinozoa | Capitellidae |
Heteromastus similis Southern | |
Sea anemone sp. A (not identified) | Paraheteromastus tenuis Monro |
Sea anemone sp. B (not identified) | |
Sea anemone sp. C (not identified) | Maldanidae |
Sea anemone sp. D (not identified) | Euclymene annandalei Southern |
| Annelida | Ampharetidae |
Melinna aberrane Fauvel | |
Polychaeta-Errantia | M. cristata (Sars) |
Aphroditidae | Platyhelminthes |
Lepidonotus kumari Rullier | |
Flatworm sp. (not identified) | |
Iospilidae | |
Pariospilus sp. nov | Nemertea |
Nereidae | Nemertine sp. A (not identified) |
Ceratonereis erythraeensis Fauvel | Nemertine sp. B (not identified) |
Dendronereis arborifera Peters | Nemertine sp. C (not identified) |
Nereis falsa Quatrefages | Nemertine sp. D (not identified) |
Perinereis aibuhitensis Grube | |
Perinereis nuntia (Savigny) | Sipuncula |
Perinereis vancaurica (Ehlers) | |
Phasolemma lucro (Solenka & de Man) | |
Eunicidae | Sipunulus sp. |
Arabella iricolar (Nontagh) | |
Diopatra monroi Day | Brachiopoda |
D. neopolitana Delle Chiaje | |
Drilonereis filum Claparede | Lingula unguis (L.) |
Lumbriconereis impatiens Claparede | |
Marphysa mossambica (Peters) | Echinodermata |
Onuphis sp. | |
Holothuridae | |
Polychaeta-Sedentaria | Holothuria parva Lampert |
Orbiniidae | Ophiodermatidae |
Scoloplos armiger (Muller) | |
Brittle star sp. (not identified) | |
| Mollusca | Amphiboridae |
Salinator sp. | |
Gastropoda | |
Lamellibranchia | |
Neritidae | |
Nerita birmanica Philippi (T) | Arcidae |
N. chamaeleon L. | Anadara granosa L. |
Theodoxus oulaniensis (Lesson) | |
Mytilidae | |
Littorinidae | Brachidontes rostratus Duncket (T) |
Littorina carinifera Menke (T) | |
L. scabra L. (T) | Isognomonidae |
Isognomon ephippium (L.) (T) | |
Assimineidae | |
Assiminia brevicula Pfeiffer | Anomidae |
Enigmonia aenigmatica (Sowerby) (T) | |
Potamidae | |
Cerithidea cingulata (Gmelin) | Ostreidae |
Cerithidea obtusa Lamarck | Crassostrea cucullata (Born) (T) |
Telescopium telescopium (L.) | |
Cerithium breve Quoy & Gaimard | Trapeziidae |
C. coralium Kiener | Trapezium sublaevigatum Lamarck |
C. patulum Sowerby | |
Ungulinidae | |
Capulidae | Diplodonta cumingii Sowerby |
Capulus sp. | |
Veneridae | |
Naticidae | Gafrarium tumidum (Roding) |
Polinices mamilla (L.) | Geloina ceylonica (Lamarck) |
Katelysia striata (Gmelin) | |
Muricidae | |
Murex capucinus (Lamarck) | Mesodesmatidae |
Caecella sp. | |
Nassaridae | |
Nassarius globosus Quoy & Gaimard | Tellinidae |
N. jacksonianus (Quoy & Gaimard) | Tellina sp. |
N. olivaceous Brugiere | |
N. thersites (Brugiere) | Glauconomidae |
Glauconome virens (L.) | |
Haminoeidae | |
Haminoeia sp. | Solenidae |
Solen delesserti Chemnitz | |
Onchidiidae | Sinonovacula constricta (Lamarck) |
Onchidium sp. | |
Pholadidae | |
Ellobiidae | Xylophaga sp. |
Cassidula aurisfelis Brugiere | |
Ellobium sp. | Teredinidae |
Teredo sp. | |
Laternulidae | Decapoda-Brachyura (I) |
Laternula truncata (Lamarck) | |
Leucosiidae | |
| Crustacea | Ebalia malefactris Kemp |
Cirripedia | Portunidae |
Thalamita crenata (Latreille) | |
Balanidae | |
Balanus amphitrite Darwin | Xanthidae |
Eurycarcinus sp. | |
Chthamalidae | Heteropanope glabra Stimpson |
Chthamalus withersii Pilsbry | Typhlocarcinodes sp. |
Amphipoda | Ocypodidae |
Amphipod sp. (not identified) | Camptandrium hexdentatum Stimpson |
Dotilla myctiroides (H. Milne Edwards) | |
Isopoda | Ilyoplax delsmani (de Man) |
L. lingulatus (Rathbun) | |
Sphaeromidae | L. obliguus Tweedie |
Sphaeroma walkeri (Stebbing) | L. punctatus Tweedie |
Leipocten sordidulum Kemp. | |
Ligidae | Macrophthalmus bosci Audouin & Savigny |
Ligia sp. | M. brevis (Herbst) |
M. definitus Adams & White | |
Decapoda-Anomura | Paracleistostoma microcheirum Tweedie |
Paracleistostoma sp. | |
Callianassidae | Tylodiplax tetratylophora de Man |
Laomedia astacina (de Haan) | Uca angustifrons de Man |
Thalassina anomala (Herbst) | U. annulipes (H. Milne Edwards) |
Upogebia sp. | U. mani Rathbun |
U. triangularis (H. Milne Edwards) | |
Coenobitidae | U. vocans (L.) |
Coenobita cavipes Stimpson | |
Grapsidae | |
Paguridae | |
Clibanarius padavensis de Man | Chiromanthes darwinensis Campvell |
Diogenes avarus Heller | C. dussumieri (H. Milne Edwards) |
C. eumolpe (de Man) | |
Porcellanidae | C. indiarum (de Man) |
Petrolisthes sp. | C. semperi Burger |
Chiromanthes sp. | |
Penaeidae | Clistocoeloma merguiensis de Man |
Metapenaeus ensis (de Haan) | Metaplax crenulata (Gerstaecker) |
Penaeus latisulcatus Kishinouye | M. distinctus (H. Milne Edwards) |
M. elegans de man | |
Synalpheidae | Nenosesarma batavicum (Moreira) |
Alpheus euphrosyne de Man | Neopisesarma mederi (H. Milne Edwards) |
N. versicolor (Tweedie) | |
Parasesarma plicatum (Latreille) | |
Sarmatium crassum Dana | |
S. germani (H. Milne Edwards) | |
Stomatopoda | Gobiidae |
Taenioides caeculus (Schneider) | |
Chlorida rotundicauda (Miers) | T. gracilis (Valenciennes) |
Taenioides sp. | |
| Pisces | |
Periophthalmidae | |
Electeridae | Periophthalmus koelreuteri (Pallas) |
Scartelaos viridis (H. Buchanan) | |
Undescribed sp. |
The soil type and the chemical compositions of the soil in the mangrove forests and the tidal mudflat on the north and eastern coastline of the Gulf of Thailand were studied by Tandatemiya (1976) and Aksornkoae et al., 1979.
Tandatemiya (1976) classified the coastal soils in the area between Chao Phraya River and Mae Klong River to belong to the soil series of Tachin and Samut Prakarn types. The characteristics of those soils are classified by the water, logging conditions, and high salinity.
The detailed chemical composition of the mangrove forests at Chantaburi was studied by Aksornkoae et al. (1979). The details of their findings are shown in Figure 2. It is interesting to note that certain chemical properties of water in the mangrove forest such as pH, dissolved oxygen, phosphate were lower in the open water at the edge of the forest than in the dense forest. In contrary, other chemical properties such as the water salinity, the chemical oxygen demand (COD), nitrate and silicate were found higher at the outer edge of the forest than in the forest itself.
A further study about the variability of the chemical properties of the water between two locations close to each other, and through the year cycle was reported by Vuthisathirapinyo (1976). He compared the chemical properties of the two mangrove forests.
The chemical properties of the soil in the mangrove forest at Phang Nga province and their relationship to the kinds of vegetation was studied by Kongsaangchai (1976). His findings showed that the amount of sand increased landward. The other properties such as the amount of clay, organic matter content, cation exchange capacity and soil salinity were gradually increased seaward.
The coasts of Thailand are exposed to three different types of tides: the Andaman Sea coast is exposed to a semi-diurnal tide, i.e. two high and low tides a day, the northern part of the west coast of the Gulf to a diurnal tide, high and low tide once a day, and at the rest of the coastline receives mixed types of tides, but prevailingly diurnal (Fig. 3).
The regional current systems for the Southeast Asian Seas between Australia, Japan and India are shown in Figures 4a and 4b. The current on the Andaman Coast form part of the current system in the Bay of Bengal. During the months from June to November, it is more pronounced setting south entering also the Straits of Malacca. According to Wyrtki (1962), this lasts only till August/September. After a transition period the current from the Malacca Strait becomes much stronger setting north along the Thai coast. January/February seems again to be the transition period reversing the current direction.
The current system in the Gulf seems much more complicated. Wyrtki (1962) drew a current pattern of the outer Gulf, based on two observation periods in August and September (Figs. 5a and 5b). This is of course not sufficient to explain the transport mechanisms of pollutants for example. But it shows clearly that one must distinguish between currents at various depths, and that zones of upwelling and downwelling change relatively quickly. The characteristics in the Upper or Inner Gulf was studied by the Division of Oceanography (Pongsapipat, 1977).
The analysis of the measurements (Figs. 6a-6f) showed that the main factor governing the movement of current in the Gulf was the tidal force from the lunar gravitational attraction while the other factors usually such as wind stress and density difference which should generate the current according to dynamical principle were at minimum. The current component induced by wind was not more than 0.5 knot. Pongsapipat concluded that the influential force of tide is the sole factor predominating the flow of stream in the Upper Gulf of Thailand with the mean velocity of around 1.5 to 2.0 knots.
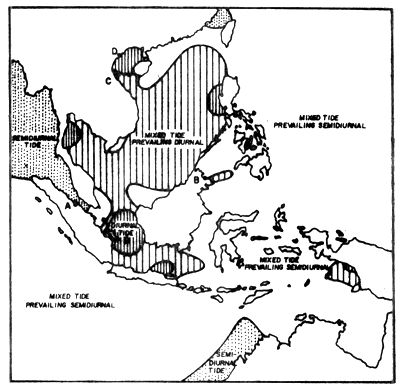
Fig. 3 Geographical distribution of tidal types in Southeast Asia (from Wyrtki, 1961)
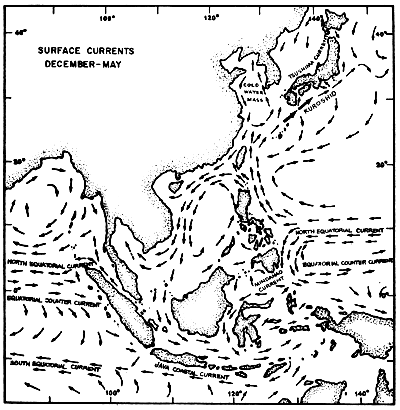
Fig. 4a Surface current pattern of the Indonesian and adjacent waters during the northwest monsoon, December-May (after Soegiarto, in press)
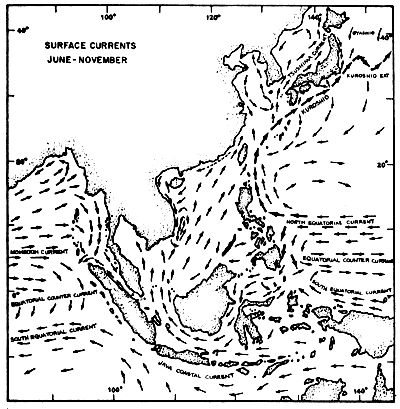
Fig. 4b Surface current pattern of the Indonesian and adjacent waters during the southeast monsoon, June-November (after Soegiarto, in press)
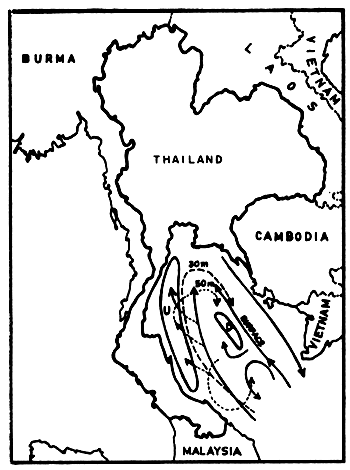
Fig. 5a Current observations in the Gulf of Thailand, 2–14 August 1959, southwest monsoon (Wyrtki, 1962) U = upwelling, D = downwelling, dashed and dotted lines indicate currents at given depths
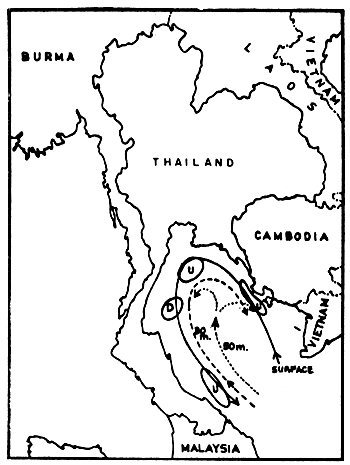
Fig. 5b Current observations in the Gulf of Thailand, 19–30 September 1959, transient between southwest and northeast monsoon (McGarry et al., undated) U = upwelling, D = downwelling, dashed and dotted lines indicate currents at given depths
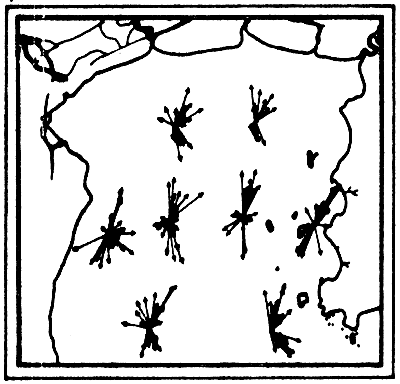
a) Current vectors at one-hour intervals for one-day cycle
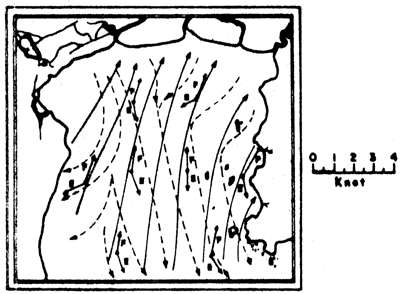
b) Summary flood and ebb tide current vectors. E = ebb tide; F = flood tide
Figs. 6a, b. Tidal current characteristics in the Upper Gulf of Thailand (from Pongeapipat, 1977)
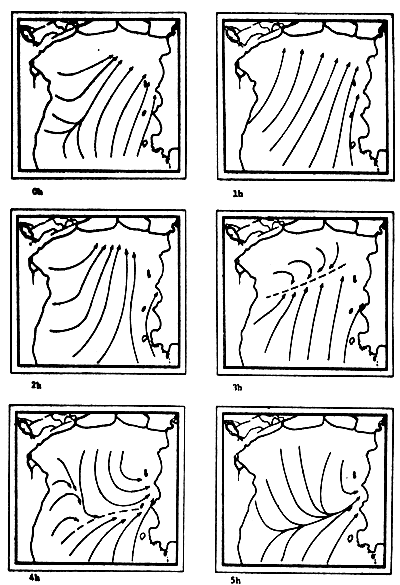
Fig. 6c-f Tidal current pattern for one day cycle at one hour intervals c) 0 - 5h; d) 6 - 11h; e) 12 - 17h; f) 18 - 23h (from Pongsapipat, 1977)
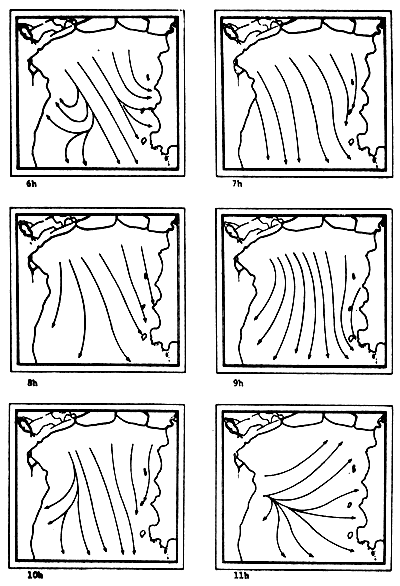
Fig. 6d
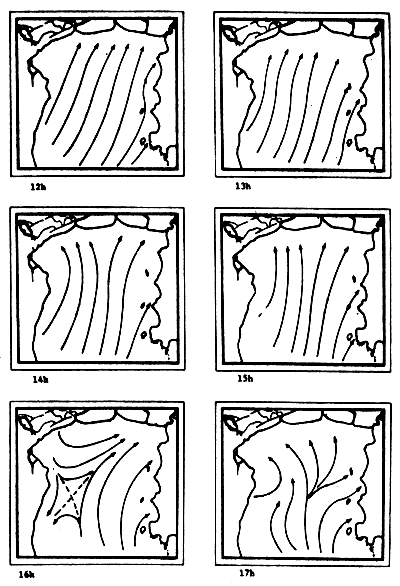
Fig. 6e
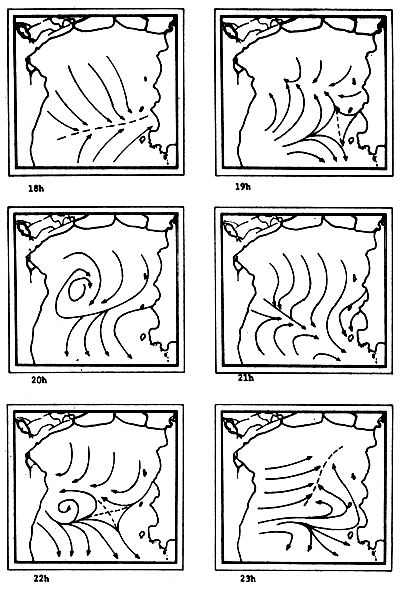
Fig. 6f
Salinity is the predominant factor in changing of water density, and it clearly shows the seasonal effects on the water mass in the upper part of the Gulf of Thailand. In the dry season, the water body is homogeneous from the surface to the bottom, occasionally it is slightly stratified. In the rainy season and at consequent immense river runoff from three sides, the distribution of water is highly stratified with a very strong halocline (Fig. 7). The average salinity is 29‰ and the difference of salinity between maximum and minimum is 3.8‰. The NE monsoon and SW monsoon play an important role in the water temperature distribution. The average of water temperature is 29.2°C, and the difference of water temperature between maximum and minimum is 3.3°C. Average transparency is 6.5 meters and water colour is ranging from code 10 to 3. Maps of general distribution of salinity and temperature in the waters of Thailand and adjacent seas are also shown in E. Gomez - Synoptical Report of this series.
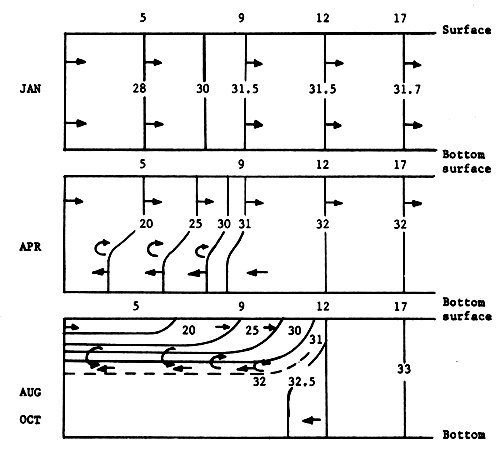
Fig. 7. Isohalines along a central N-S section through Stations 5, 9, 12, and 17 in the Upper Gulf of Thailand (for locations of stations, refer to Fig. 19) (from Charusombat, 1977)
It has been estimated by Sabhasri (1979) that about 30% of the mangrove forests in Thailand have been destroyed during the last decade. The four major causes of the destruction are: charcoal factories, human settlement, mariculture, and tin ore mining.
The agricultural land of the coastal zone in Thailand is used as rice-fields, coconut plantations, nipa plantations, casuarina plantations, and Rhizophora plantations. The Coastal Zone Development Projects of the Ministry of Agriculture and Cooperatives showed that there are more than 28 species of fruit trees and vegetables which could be grown in the saline soil of the coastline (Ruangchotivit, 1979). Data on the agricultural production of this land are not available at the present time. However, it is felt that the development of coastal land for agricultural use in Thailand is a slow process. This is due to the fact that the agricultural activities in the coastal zone are only a supplement for those who are engaged in other jobs, e.g. fisheries. Rice growing is frequently obstructed by the intrusion of saline water into the ricefields.
In many provinces of Thailand, the fishermen fish in the mangrove swamps and also on the mudflats nearby. Gillnets surround the selected sites at high tide and the fish are collected at low tide. It has been found that many young fishes and shrimps are killed, and it is illegal to use this fishing method.
There is no scientific report in Thailand which confirme the claim that the mangrove forests are used as a spawning ground for some marine species. De la Cruz (1978), quoting Green and Chuensri, states that the freshwater prawn Macrobrachium rosenbergii spawns in the brackishwaters of mangrove areas and the larvae feed there for about a month before returning to the freshwater habitat. In Thailand, a species of horseshoe crab, Carcinoscorpius rotundicauda is found to spawn in the mudflat area at Cholburi province. The fry of seabass (Lates calcarifer), greasy grouper (Epinephelus tauvina), the milkfish (Chanos chanos), and larvae of shrimps are reported to abound in mangrove forests on the Andaman coast and in Prachuab Kirikhan province (Phatia, 1976; Suntarotok et al., 1976; Chuensri et al., 1976; Sukwongs et al., 1979).
It is estimated by the Department of Fisheries that the total coastal area usable for shrimp farming is about 84 440 ha. This figure includes about 18 360 ha of the area approximately 2 km from the shore. This is suitable for shrimp pen/cage culture, and about 62 880 ha of the mangrove area are available for shrimp culture. It is also projected that if the shrimp culture area expands as planned, the annual shrimp production will be over 15 000 t assuming the yield is 187 kg ha-1.
In the field of mollusc culture, Rabanal et al. (1977) cited data from the Department of Fisheries saying that Thailand had cultivation areas for 4 species of molluscs (green mussel, horse mussel, cockle and oyster) in 22 coastal provinces, covering 4 856 ha, and that the potential area for development was in the order of 64 000 ha Figs. 8, 9, 10 and 11).
It has also been shown that the mollusc production from natural waters in some coastal areas, e.g. Samut Songkhram province has declined abruptly (Table 8).
Table 8. Production of green mussel, horse mussel, and cockles in Samut Songkhram province. Second line shows change in % to previous years (adapted from Rabanal et al., 1977)
| Green mussel (t) | Horse mussel (t) | Cockle (t) | |||||||||
| 1971 | 1972 | 1973 | 1974 | 1971 | 1972 | 1973 | 1974 | 1971 | 1972 | 1973 | 1974 |
| 63 798 | 12 886 | 8 440 | 2 690 | - | 19 | - | - | 285 | 1 260 | 1 890 | 718 |
| - | -79.2 | -34.5 | -68.1 | - | +342 | +1 465 | -620 | ||||
Table 9 shows that the cockle farm areas in the provinces around the Inner Gulf declined or varied but that the total production slowly increased again due to new cultures in Satun and Ranong provinces. The possibilities for cultivation or introduction to the new areas of the above four species are high. This purpose will however accelerate the destruction of the mangrove forest. Parallel with the increase of total farm area for the mentioned provinces the total production of cockles has also increased again, as shown in Table 10.
Table 9. The number total area of cockle farms in four provinces from 1973–1977 (Fishery Statistics, 1977)
| 1973 | 1974 | 1975 | 1976 | 1977 | ||||||
| Province | No. | Area (ha) | No. | Area (ha) | No. | Area (ha) | No. | Area (ha) | No. | Area (ha) |
| Samut Songkram | 35 | 142 | 20 | 85 | 35 | 136 | 33 | 127 | 46 | 166 |
| Petchaburi | 120 | 323 | 106 | 286 | 88 | 210 | 53 | 130 | 82 | 129 |
| Satun | - | - | 1 | 42 | 44 | 202 | 4 | 158 | 5 | 312 |
| Ranong | - | - | - | - | - | - | - | - | 2 | 39 |
| Total | 154 | 465 | 127 | 413 | 127 | 564 | 90 | 401 | 135 | 643 |
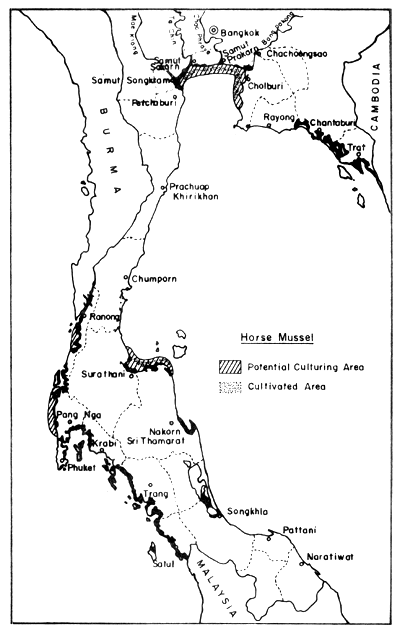
Fig. 8. Distribution of present horse mussel farms (300 ha) and potential culture areas (4 600 ha). Black areas = mangrove swamps
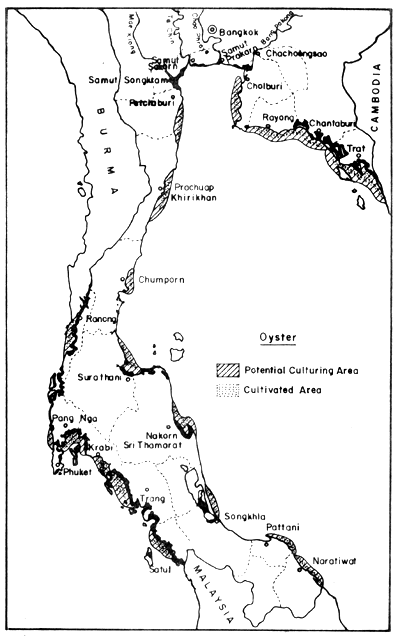
Fig. 9. Distribution of present oyster farms (630 ha), and potential culture areas (13 190 ha). Black areas = mangrove swamps.
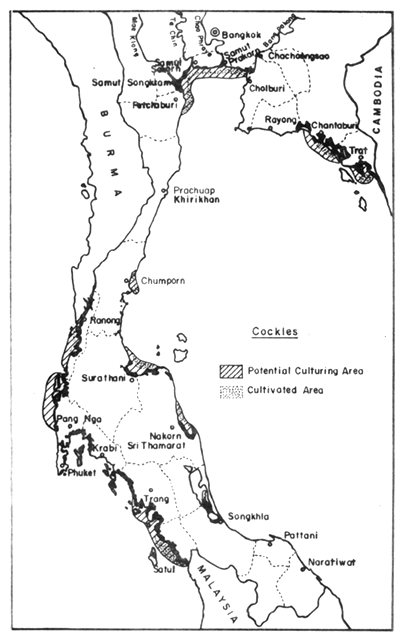
Fig. 10. Distribution of present cockle farms (500 ha), and potential culture areas (11 300 ha). Black areas = mangrove swamps.
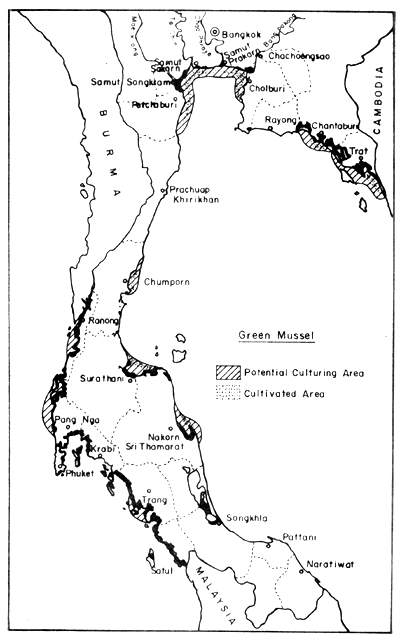
Fig. 11. Distribution of present green mussel farms (2 626 ha), and potential culture areas (34 994 ha). Black areas = mangrove swamps
Table 10. Area of cockle farms and production of cockles at Samut Songkram, Petchaburi and Satul provinces (*converted from figures given in rai, 1 rai = 0.16 ha) (Chomdej and Poocharoen, 1979)
| Year | Area (ha)* | Change (%) | Production (t) | Change (%) | Kg/ha/year* |
| 1973 | 465 | - | 3 920 | - | 8 240 |
| 1974 | 413 | -11.24 | 2 494 | -36.37 | 6 041 |
| 1975 | 564 | +36.51 | 5 566 | +123.17 | 9 877 |
| 1976 | 415 | -26.33 | 12 093 | +117.26 | 29 132 |
| 1977 | 643 | +54.82 | - | - | - |
There are no statistical data on the amount of land in the mangrove forests that has already been reclaimed for plantations, other types of agriculture, industrial projects and housing projects. However, under a recent special cabinet order concerned Provincial Governors and other government agencies with coastal development projects, are instructed to submit their proposals to the National Committee on the Mangrove Forest Development (National Research Council) for the Committee's considerations. In 1979 alone, two provinces, Satul and Nakhon Sithammarat submitted their development projects to the Committee. Satul proposes to use about 288 ha of mangrove forest land for town expansion, 40 ha thereof for the construction of a fishing pier and fish meal production plants.
The total population of Thailand is about 45 million people. The Population and Housing Census in 1970 showed also that at that time, about 9.6 million people lived in the provinces along the coasts of Thailand (Table 11). Half of these, 4.7 million, lived in the provinces around the Upper Gulf
Table 11. Population and number of households in the provinces bordering the coastline of Thailand (National Statistical Office, Population and Housing Census, 1970)
| Province | Population (total) | No. of households |
| Bangkok and Thonburi | 3 077 361 | 500 116 |
| Chanthaburi | 216 344 | 39 404 |
| Chachoengsao | 354 521 | 62 462 |
| Chol Buri | 541 695 | 94 478 |
| Tray | 94 119 | 17 163 |
| Prachuap Khirikhan | 249 202 | 43 018 |
| Petchaburi | 289 719 | 51 540 |
| Rayong | 250 671 | 46 893 |
| Samut Prakarn | 329 404 | 52 638 |
| Samut Songkram | 162 526 | 28 008 |
| Krabi | 149 209 | 25 860 |
| Chumphorn | 235 494 | 43 736 |
| Trang | 326 614 | 55 920 |
| Nakhon Sri Thammarat | 928 520 | 157 110 |
| Naratiwat | 326 633 | 66 043 |
| Pattani | 330 217 | 64 329 |
| Phang Nga | 135 101 | 22 929 |
| Phatthalung | 304 972 | 55 132 |
| Phuket | 100 021 | 16 175 |
| Ranong | 59 147 | 10 416 |
| Songkhla | 621 849 | 118 575 |
| Satun | 117 035 | 21 307 |
| Surathani | 43 437 | 76 716 |
Total | 9 638 135 | 1 670 031 |
The history of modern industry in Thailand can be traced back to the time of the first introduction of the steam engine into this country for the rice mill in 1855. In 1977, there were about 65 000 factories registered with the Department of Industrial Works. Of these about 15 000 are situated in the Greater Bangkok Region. The rest are scattered throughout various provinces of the Kingdom.
Figs. 12 to 15 show the types and distribution of factories along the four major rivers, Mae Klong, Ta-Chin, Chao Phraya, and Bang Pakong, that empty into the Upper Gulf.
Legend to Fig. 12
| Industry Code No. | Main product |
| 1 | Writing and printing paper |
| 2 | Galvanized malleable iron pipe and fittings |
| 3 | Motorcycles |
| 4 | Galvanized iron sheets |
| 5 | Dry cell batteries |
| 6 | Automotive and motorcycles batteries |
| 7 | Galvanized iron sheets |
| 8 | Television, radio receivers |
| 9 | Plywood |
| 10 | Repaired stationary battery plates |
| 11 | Copper wire |
| 12 | Bicycle parts |
| 13 | PVC resins and PVC compound |
| 14 | Automotive and motorcycle batteries |
| 15 | Paint |
| 16 | Caustic soda |
| 17 | Automotive electric equipment |
| 18 | Buses, trucks |
| 19 | Paint |
| 20 | Galvanized iron sheets |
| 21 | Repaired stationary batteries |
| 22 | Automotive and motorcycle batteries |
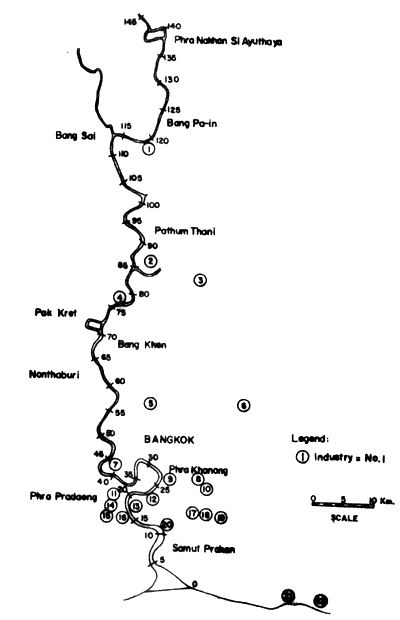
Fig. 12. Locations of selected industries along Chao Phraya river. Small figures indicate distances in km from river mouth (= 0). For legend of industries see table on page 56 (after Polprasert et al., 1979)
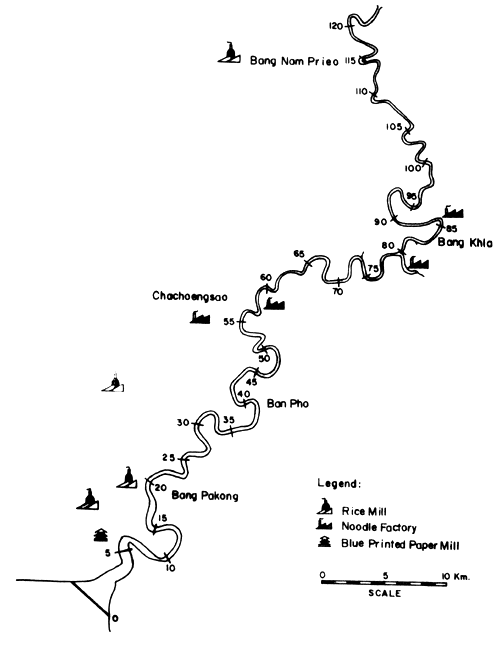
Fig. 13. Locations of various industries along the Bang Pakong river Figure indicates distances from river mouth (= 0) (after Polprasert et al., 1979)
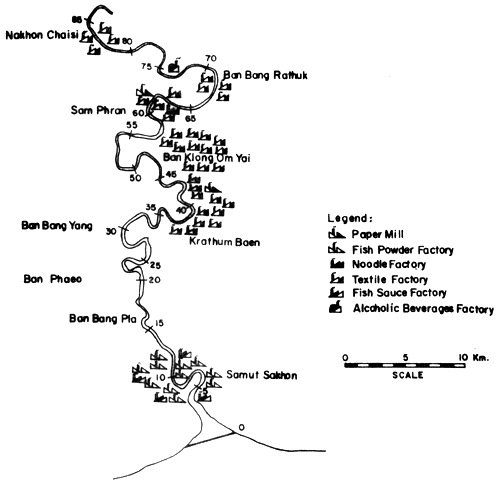
Fig. 14. Locations of various industries along the Ta-Chin river Figure indicates distances in km from river mouth (= 0) (after Polprasert et al., 1979)
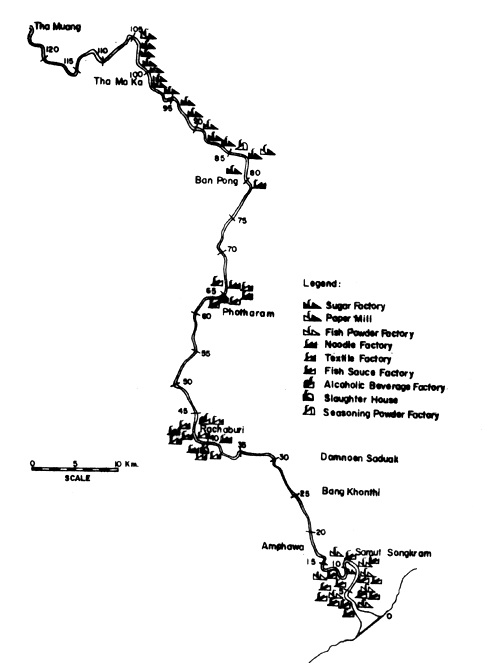
Fig. 15. Locations of various industries along the Mae Klong river. Figure indicates distance in km from river mouth (= 0) (after Polprasert et al., 1979)
Tin mining along the coastline of Thailand has been started more than a century ago. Open pit mining was generally practised. The areas under tin mining concession in four provinces of southern Thailand cover 427 000 ha. Only 21.6% of this area is being mined (Table 12). The value of the tin produced was 410 million Bahts in 1977 (Suthas Na Ayudhaya, 1979).
Chua - Intra (1976) showed that in Ranong, Phang Nga, and Phuket provinces, the area for which mining concessions are sought cover about 37 790 ha of mangrove forest.
Tin mining in the sea and in the mangrove forest along the coastline have caused severe damage of the marine life in some locations of the Andaman Sea coast. At present, there are many offshore dredges in the areas of Phuket Island and Phang Nga Bay. The two offshore dredges in Phang Nga Bay, each of them with an excavating capacity of 77 000 m3/month or 0.77 million m3/a for a 10-month operation period, excavate ca. 1.5 million m3 of marine sediments per year. The washed sediments discharged from the dredges (tailings) and spread into marine habitats are believed to cause severe destruction to marine life, especially to the coral reefs around Phuket Island.
Table 12. Tin mines and mangrove forest of four provinces on Andaman Sea coastline (adapted from Siripong, unpublished data)
| Province | Present mining concession area (km2) | Mangrove forest covered (km2) | Mining operation (km2) |
| Ranong | 2 642.16 | 242 | 684.64 |
| Phang Nga | 1 542.96 | 511 | 184.32 |
| Phuket | 81.92 | 31 | 56.8 |
| Krabi | - | - | - |
| Total | 4 267.04 | 1 114 | 925.76 |
Salt production from sea water also uses a certain amount of land especially in the mudflats or mangrove forests. The salt farms in Thailand are mostly located on the coast of the upper portion of the Gulf. Six provinces with ca. 10 500 ha of salt farms form the major salt production centres of the country (Table 13).
Table 13. The area of salt farms in 1972–1973 (Department of Land Development, 1979), *converted from figures given in rai (1 rai = 0.16 ha)
| Province | Area* (ha) | No. of operating families (units) |
| Samut Sakorn | 5 788 | 965 |
| Samut Prakarn | 2 197 | 378 |
| Pethaburi | 1 182 | 68 |
| Samut Songkram | 1 024 | 194 |
| Chachoengsao | 198 | 35 |
| Cholburi | 164 | 24 |
| Total | 10 553 | 1 734 |
Sedimentation from land erosion and tin mining are one of the most important factors which cause harmful effect to the mangrove forest ecosystem. Figure 16 shows the river flow and the amount of sediment which was carried into the Gulf of Thailand by the Chao Phraya River. No detailed study on the effect of this sediment to the benthic organisms in the Gulf region has yet been made. (Sedimentation from tin mining is mentioned in Section 1.4.2).
Lohwongwatana (1979) listed the number of large-scale factories producing larger quantities of waste water (Table 14).
The waste discharge from these factories is carried into the Inner Gulf of Thailand. The amounts of BOD produced by industries in 1975 and 1979 indicate that, except for the two large rivers, Chao Phraya and Ta-Chin, the organic load decreased (Table 15). It is claimed that this is due to the restriction control of the industrial waste effluents by the Department of Industrial Works (Ministry of Industry). In the case of Chao Phraya River, it is claimed that the deterioration is due to the municipal wastes such as raw sewage, septic tank effluents, and seepage. Dumping of solid wastes into the river is one of the main causes of the Chao Phraya pollution. It is estimated that about 70–75% of the municipal wastes go directly into the Chao Phraya River. The effect on the dissolved oxygen regime in the Chao Phraya River is illustrated through the two curves for the lower part of the river and the estuary. The curve for the lower river part show a steady decrease of the minima from 1970 to 1977, and an increase of the length of period with low values of dissolved oxygen (Fig. 17).
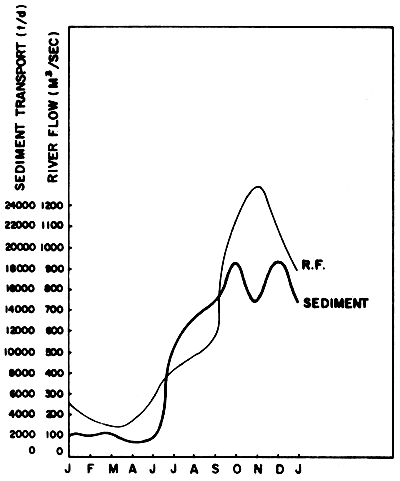
Fig. 16 Average amount of freshwater and sediments which were carried into the mouth of Chao Phraya River in 1970–1976 (Source: Silpipat, 1979)
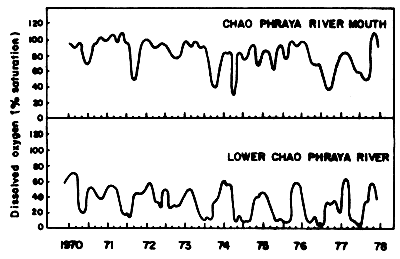
Fig. 17 Amount of dissolved oxygen in lower layer of water Chao Phraya River from 1970–1978 (Silpipat, 1979)
Table 14. Factories producing large amount of waste water (Lohwongwatana, 1979)
| Categories | No. of factories |
| White sugar factories | 47 |
| Paper mills | 35 |
| Distilleries | 52 |
| Breweries | 2 |
| Tapioca starch factories | 145 |
| Tanneries | 96 |
Table 15. Industrial wastes (BOD t/d) discharged into the six large rivers and east coast waters (Lohwongwatana, 1979)
| Sources | 1975* | 1979 | Remarks |
| Chao Phraya River | 10.3 | 21.04 | *Reported by Ludwig and Tongkasame, 1978 |
| Maeklong River | 47.4 | 2.40 | |
| Ta-Chin River | 4.5 | 7.90 | |
| Bang Pakong River | 0.5 | 0.22 | |
| Pranburi River | 4.8 | 0.02 | |
| Pethaburi River | n.a. | 0.62 | |
| East Coast | 157.7 | 107.7 | |
| Total | 225.2 | 139.9** | **Exclude 32 tons/day of distillery slops which are transported by boats and discharged into the Inner Gulf directly |
In the case of the Ta-Chin River pollution, it is alleged to be caused by the wastes from livestock and by the low efficiency of the water treatment plants in most of the ca. 130 factories along the banks of this river.
In the estuarine regions with Upper Gulf of Thailand, most of the pesticides originate also from agricultural areas further upsteam (Fig. 18). However, only part of the areas with pesticide application drain into the Upper Gulf as indicated by the approximate water shed. The imported quantities of pesticides increased from 15.1 to 20.4 thousand tons from the year 1977 to 1978 (Table 16). The amount distributed to the farmers (and used in agriculture) (Table 17) is, however, not equal with the annually imported amounts, but even though other than agriculturally used pesticides reach the environment.
It is also notable that the import of DDT in 1977 was 13.5% of all pesticides and decreased to 2.2% in 1978. The increased use of other pesticides inevitably resulted in more pollutional loads to the river basins. To give an idea of the contamination by pesticides the concentration of long living DDT and some of its derivatives in upstream tributaries are shown in Table 18.
A comparison of the pesticide contamination in the estuaries of the four big rivers has been given by Polprasert et al., 1979 (Tables 19a–19d), analyses of coastal waters were also done by the latter and Hungspreug and Wattayakorn, 1977 (Tables 20 and 21). All authors did not find any DDT in the water at the river mouths or coastal, or mid gulf waters. PCB levels were also “not detectable” in the water samples. The expected accumulation of DDT in the sediments was detectable and ranged from 1 to 8 μg/kg dry sediment in the river mouths, 1 to 51 μg/kg at the coastal stations, and 4 to 17 μg/kg at the mid gulf stations; PCB's again were not detectable.
Contamination of the biota was measured in some instances. Polprasert et al. (1979) reported 10 ug DDT/kg (wet weight) in fish from Ta-Chin estuary, 34 μg/kg (wet weight) from Ban Pakong estuary and 20 μg/kg (wet weight) in fish from coastal waters. “Nekton” samples from the whole Upper Gulf analysed by Hungspreugs and Wattayakorn (1977) ranged even from 88 to 112 μg/kg.
The Andaman sea receives much less land drainage waters than the Gulf. Again there was no detectable DDT in the water samples (Hungspreugs and Wattayakorn, 1977), and levels in sediments (Table 22) and biota were much lower. Both averaged 1.9 μg/kg (dry weight for sediment, wet weight for biota).
As a basis for coastal water pollution it is quite interesting to have an idea about the magnitude of heavy metal discharge into the rivers. Polprasert et al.; 1979 in their comprehensive study give figures on analyses of the 22 selected factory effluents into Chao Phraya rivers. (see Fig. 12). Based on repeated analyses and the discharge flow, the average river load for 6 metals is calculated. The figures for the discharge per year is a careful calculation with only 300 days (Table 23). The total amount including other sources than the monitored factories lies certainly higher.
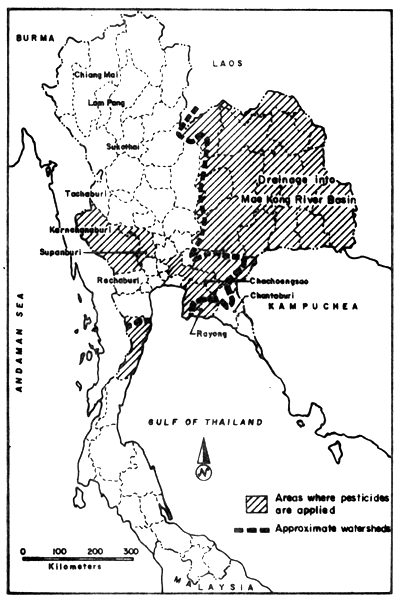
Fig. 18. Total agricultural areas where pesticides are applied (adapted from Polprasert et al., 1979)
Table 16. Data of imported pesticides in Thailand in 1977 and 1978 (Foreign Trade Statistics of Thailand, Department of Customs, Bangkok, from Polprasert et al., 1979)
| Import Code No. | P r o d u c t | Quantity in 1977 (kg) | Quantity in 1978 (kg) |
| 381111 | Insecticides put up in aerosol tins | 53 403 | 11 135 |
| 381112 | Fungicides put up in aerosol tins | 207 115 | 222 655 |
| 381113 | Weed killers put up in aerosol tins | 134 659 | 47 972 |
| 381114 | Pesticides put up in aerosol tins | 21 040 | 240 668 |
| 381121 | DDT, preparations put up in aerosol tins | 2 045 110 | 444 910 |
| 381122 | BHC preparations put up in aerosol tins | 58 002 | 12 000 |
| 381123 | Diolefin based insecticides | 20 091 | 41 453 |
| 381124 | The organic-phosphorus insecticides | 517 187 | 97 472 |
| 381125 | The carbamate insecticides | 10 000 | 67 497 |
| 381126 | Other insecticides | 4 512 800 | 7 071 889 |
| 381127 | Fungicides | 1 179 501 | 2 371 072 |
| 381128 | Weed killers plant hormones | 3 238 646 | 6 044 180 |
| 381129 | Pesticides | 536 772 | 3 266 130 |
| 381131 | Rat poisons | 58 007 | 45 373 |
| 381132 | Disinfectants | 197 568 | 165 893 |
| 381144 | Insect repellants | 3 201 | 40 122 |
| 381149 | Other similar products of disinfectants, insecticides, etc. for sale by retail | 286 031 | 226 052 |
| T O T A L | 15 079 133 | 20 416 473 |
Table 17. Quantity of fungicides, pesticides, and herbicides distributed to the farmers during period October 1977-July 1980. (Ministry of Agriculture and Cooperatives, Thailand, 1973, from Polprasert et al., 1979)
| Date | Powder (kg) | Solution (liter) | |
| October | 1977 | 207 495 | 35 268 |
| November | 1977 | 50 401 | 31 124 |
| December | 1977 | 206 | 16 433 |
| January | 1978 | 224 894 | 43 108 |
| February | 1978 | 93 367 | 46 944 |
| March | 1978 | 12 270 | 4 802 |
| April | 1978 | 83 552 | 7 254 |
| May | 1978 | 31 633 | - |
| June | 1978 | 14 641 | 3 672 |
| July | 1978 | 20 634 | 17 447 |
| T O T A L | 739 102 | 206 052 | |
Table 18. Amount of DDT and its derivatives in some rivers in Thailand (ug/1) (adapted from Chaiyaraj, 1976)
| River (Province) | p-p'-DDT | p-p'-TDE | p-p'-DDE | Dieldrin |
| Northern provinces | ||||
| Yom River (Sukothai) | 3.2 | - | - | - |
| Wang R. (Lam Pang) | 2.2 | - | - | - |
| Ping R. (Chiang Mai) | 2.1 | - | - | - |
| Central province | ||||
| Ta-Chin River (Supanburi) | 3.1 | - | - | - |
| Kwai River (Karnchanaburi) | - | - | - | - |
| Mae Klong River (Tachaburi) | 1.7 | - | - | - |
| Damnern Sadeuk River (Rachaburi) | 3.4 | - | 0.8 | 4.1 |
| Eastern provinces | ||||
| Bang Pakong River (Chachoengsao) | 2.1 | 0.6 | - | - |
| Rayong River (Rayong) | 1.1 | - | - | - |
| Chantaburi River (Chantaburi) | 0.1 | - | - | - |
| Krasae River (Rayong) | 2.5 | - | - | - |
NOTE: The rivers analysed lie partly in areas not comprised under “areas where pesticides are applied” (see Fig. 18), i.e. they are probably applied in minor quantities.
Table 19a, b. Concentrations (arithmetic means) of heavy metals and pesticides in water, sediment, and biota at the mouths of Mae Klong (a) and Ta-Chin River (b) (from Polprasert et al., 1979) (-. non available, N D. non detectable, *; one sample only)
(a)
| Mae Klong | |||||
| Parameter | Mean of 4 Samplings Water (μg/1) | Mean of 2 Samplings (μg/g) | |||
| Sediment | Phytoplankton | Zooplankton | Fish | ||
| Cd | 44.93 | 0.71 | 7.60 | 3.10* | - |
| Cu | 43.25 | 8.61 | 86.67 | 254.48 | - |
| Cr | 114.70 | 26 62 | 73.32 | 62.77 | - |
| Pb | 287.43 | 84 20 | 113 49 | 126.26 | - |
| Zn | 46.25 | 33 78 | 184.38 | 116.88 | - |
| Total Hg | 3 460 | 0.083 | 0.242 | 0.457 | - |
| Free Hg | 0 275 | 0 056 | - | - | - |
| Total DDT | N.D. | N.D. | - | N.D. | - |
| PCBs | N.D. | N.D. | - | N.D. | - |
(b)
| Ta-Chin | |||||
| Parameter | Mean of 4 Samplings Water (μg/l) | Mean of 2 Samplings (μg/g) | |||
| Sediment | Phytoplankton | Zooplankton | Fish | ||
| Cd | 44.10 | 0.69 | 29.34 | 14.63 | 0.32 |
| Cu | 48.40 | 21.00 | 79.78 | 90.02 | 2.38 |
| Cr | 102.14 | 49.42 | 23.84 | 18.76 | 18.83 |
| Pb | 227.08 | 50.33 | 467.59 | 174.84 | 8.74 |
| Zn | 48.44 | 24.91 | 202.55 | 268.91 | 36.57 |
| Total Hg | 2.450 | 0.124 | 0.267 | 0.625 | 0.044 |
| Free Hg | 0.106 | 0.018 | - | - | N.D. |
| Total DDT | N.D. | 0.001 | N.D. | N.D. | 0.010 |
| PCBs | N.D. | N.D. | N.D. | N.D. | N.D. |
Tables 19c, d. Concentrations (arithmetic means) of heavy metals and pesticides in water, sediment, and biota at the mouths of Chao Phraya (c) and Bang Pakong (d) (from Polprasert et al., 1979) (-: non available, N.D.: non detectable, *: one sample only)
(c)
| Chao Phraya | |||||
| Parameter | Mean of 5 Samplings Water (μg/1) | Mean of 2 Samplings (μg/g) | |||
| Sediment | Phytoplankton | Zooplankton | Fish | ||
| Cd | 66.02 | 3.09 | 12.85 | 15.81* | 1.01 |
| Cu | 43.84 | 22.44 | 69.18 | 42.69 | 3.58 |
| Cr | 62.78 | 20.36 | 20.45 | 41.90 | 17.36 |
| Pb | 187.74 | 96.92 | 246.01 | 300.40 | 15.68 |
| Zn | 29.80 | 105.15 | 188.30 | 190.51 | 52.98 |
| Total Hg | 3.792 | 0.198 | 1.348 | 0.049 | 0.170 |
| Free Hg | 0.132 | 0.030 | - | - | 0.040 |
| Total DDT | N.D. | 0.008 | N.D. | N.D. | 0.049 |
| PCBs | N.D. | N.D. | N.D. | N.D. | N.D. |
(d)
| Bang Pakong | |||||
| Parameter | Mean of 5 Samplings Water (μg/1) | Mean of 2 Samplings (μg/g) | |||
| Sediment | Phytoplankton | Zooplankton | Fish | ||
| Cd | 47.26 | 1.14 | 5.59* | 1.76* | 0.80 |
| Cu | 40.50 | 19.69 | 53.63 | 135.82 | 7.22 |
| Cr | 117.02 | 26.88 | 25.79 | 16.85 | 10.61 |
| Pb | 342.40 | 74.22 | 199.60 | 32.70 | 15.08 |
| Zn | 42.12 | 36.69 | 333.33 | 71.93 | 63.77 |
| Total Hg | 4.810 | 0.151 | 0.401 | 0.494 | 0.059 |
| Free Hg | 0.278 | 0.031 | - | - | 0.025 |
| Total DDT | N.D. | 0.005 | - | - | 0.034 |
| PCBs | N.D. | N.D. | - | - | N.D. |
Table 20. Concentration of heavy metals and pesticides in coastal waters of Upper Gulf of Thailand at coastal stations off the river mouths (from Polprasert et al., 1979)
| Parameter | Mean of 4 samplings (water μg/1) | Mean of 8 samplings (μg/g) | |||
| Sediment | Phytoplankton | Zooplankton | Fish | ||
| Cd | 77.3 | 4.14 | 4.76 | 3.91 | 3.64 |
| Cu | 59.5 | 6.65 | 55.35 | 46.75 | 3.80 |
| Cr | 252.8 | 12.57 | 42.41 | 32.86 | 0.13 |
| Pb | 463.8 | 59.17 | 117.91 | 199.63 | 30.48 |
| Zn | 74.9 | 28.60 | 112.00 | 103.56 | 47.06 |
| Total Hg | 3.264 | 0.102 | 0.843 | 0.184 | 0.073 |
| Free Hg | 0.12 | 0.030 | - | - | 0.018 |
| Total DDT | - | 0.008 | - | - | 0.020 |
| PCBs | - | N.D. | - | - | N.D. |
Table 21. DDT and its metabolites in dry sediments (μg/kg) of Upper Gulf from 1974–1976. For locations of stations, see Fig. 19. CS = Coastal Stations off river mouths; MG = Mid-Gulf Stations (from Hungspreugs and Wattayakorn, 1977)
| Station | Oct '74 | Jan '75 | Apr '75 | Jul '75 | Mar '76 | May '76 | Sept '76 |
| CS 1 | 2.5 | 2.3 | 45.5 | 0.1 | - | - | 9.1 |
| CS 2 | 4.4 | 4.5 | 75.5 | - | 31.9 | - | 14.7 |
| CS 3 | 7.5 | 7.4 | 68.5 | 0.9 | 22.9 | 20.2 | 24.8 |
| CS 4 | 15.3 | 40.0 | 19.0 | 2.5 | 7.6 | 6.0 | 26.5 |
| CS 5 | 1.6 | 7.4 | 139.6 | 0.7 | 50.8 | 16.7 | 4.7 |
| CS 6 | 1.5 | 8.1 | 8.8 | 1.8 | 29.5 | 9.9 | 3.9 |
| CS 7 | 12.1 | - | 5.4 | - | 12.3 | 33.4 | 6.7 |
| 8 | 1.1 | 9.1 | 6.4 | 0.1 | 5.1 | 21.9 | 11.5 |
| MG 9 | 11.4 | 3.9 | 2.4 | 1.3 | - | 6.8 | 6.1 |
| 10 | 4.1 | 3.3 | 4.3 | 0.7 | 20.7 | 6.4 | 17.8 |
| 11 | 30.4 | 11.3 | - | - | 2.5 | 21.5 | 8.0 |
| MG 12 | 2.1 | 3.3 | 4.1 | 0.6 | 11.4 | 14.2 | 7.3 |
| 13 | 1.6 | 4.9 | 5.5 | - | 4.4 | 21.8 | 25.3 |
| 14 | 4.0 | 2.6 | 3.0 | 0.1 | 4.4 | 36.0 | 7.1 |
| 15 | 8.1 | - | 4.5 | 0.3 | 7.1 | 13.1 | 11.2 |
| 16 | 8.9 | 2.5 | - | - | 3.6 | 6.8 | 10.3 |
| MG 17 | 4.5 | 3.3 | 4.9 | 0.3 | 4.7 | 30.5 | 11.5 |
| 18 | 3.4 | 3.3 | - | 0.6 | 86.0 | 12.2 | 0.9 |
| Total average | 7.0 | 7.5 | 26.3 | 0.7 | 19.2 | 16.8 | 11.8 |
| Average Coastal Stations | 6.4 | 11.6 | 51.8 | 1.2 | 25.8 | 17.2 | 12.9 |
| Average Mid-Gulf Stations | 6.0 | 3.5 | 3.8 | 0.7 | 8.1 | 17.2 | 8.3 |
Table 22. DDT and its metabolities in dry sediments (μg/kg) of Andaman Sea coast, March 22–29, 1976. For location of stations see Fig. 19 (Hungspreugs and Wattayakorn, 1977)
| Station | Type of Sediment | DDT μg/kg dry weight |
| A 1 | Sandy mud with some shells | 2.4 |
| A 2 | Sandy mud with some shells | 2.4 |
| A 3 | Sandy mud with some shells | - |
| A 4 | Sandy mud with some shells | - |
| A 5 | Sandy mud with some shells | 1.7 |
| A 6 | Sand and shells | 3.6 |
| A 7 | Sandy mud and shells | 1.5 |
| A 8 | Sandy mud and shells | 0.9 |
| A 9 | Sandy mud | - |
| A 10 | Sandy mud and shells | 0.7 |
| A 11 | Sandy mud and shells | 0.6 |
| A 12 | Sandy mud and shells | 1.0 |
| A 13 | Sand and shells | 0.9 |
| A 14 | Sandy mud and shells | 0.7 |
| A 15 | Sandy mud | - |
| A 16 | Sandy mud and shells | - |
| A 17 | Sand and shells | 1.8 |
| Total Average | 1.9 |
Table 23. Average daily and annual discharge of some heavy metals into Chao Phraya River from 22 selected factories (based on data from Polprasert et al., 1979)
| Element | kg/day | kg/year |
| Cd | 0.071 | 21 |
| Cu | 0.760 | 228 |
| Cr | 5.141 | 1 542 |
| Pb | 2.550 | 765 |
| Zn | 16.034 | 4 810 |
| Total Hg | 0.075 | 22.3 |
| Free Hg | 0.004 | 1.2 |
For the situation at the river mouths of the 4 main rivers Polprasert et al., 1979 give a comparison (Table 19a-d). The concentration of the 6 heavy metals monitored lies at the same magnitude for each river: Cd at 44–66 μg/1, Cu at 40–48 μg/1, Cr at 62–117 μg/1, Pb at 187–342 μg/1, Zn at 29–48 μg/1, total Hg at 2.4–4.8 μg/1 and free Hg at 0.1–0.3 μg/1.
For the coastal stations (off the river mouths) Polprasert et al., 1979 found values higher than those at the river mouths' stations, while Idthikasem (1978) and NRCT (1973–1977) reported concentrations ranges of up to 100 fold lower values but these ranges include values from coastal station as well as Mid Gulf and Lower Gulf stations (Table 24).
The accumulation in the sediments is quite obvious. While Polprasert et al. (1979) give a good comparison again for the river mouths situation, NRCT (1973–1977) and Idthikasem (1978) give a list of data from the whole Gulf (Table 19a-d, and Table 25). The average concentrations at the 4 river mouths reached 3 μg/g for Cd, 22 μg/g for Cu, 49 μg/g for Cr, 96 μg/g for Pb, 105 μg/g for Zn, 0.15 μg/g for total Hg and 0.06 μg/g for free Hg.
The concentration gained from the station net in the Gulf cover these values, but it is notable that even for the Lower Gulf concentrations are still quite high.
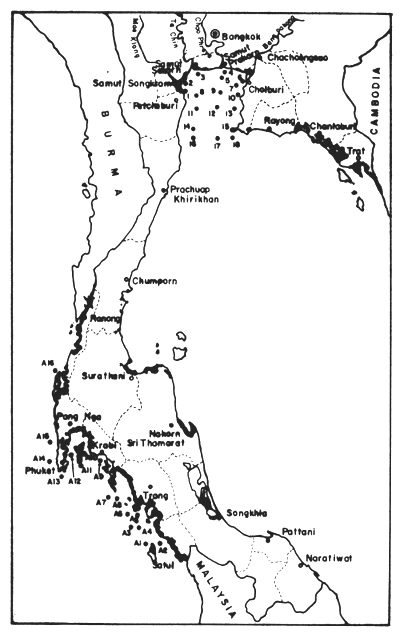
Fig. 19 Locations of sampling stations for pollution analyses by Hungspreugs and Wattayakorn (1977) (see Table 20, 21)
Table 24. Concentrations of heavy metals in water samples from Gulf of Thailand (after NRCT (1973–1977) and Idtikhasem, 1978)
| Source and Sampling Time | Heavy metal concentration (μg/l) | |||||
| Cd | Cu | Cr | Fb | Zn | Total Hg | |
| Gulf of Thailand October, 1973 | 0.02–0.03 | 16.5 | - | 7.0 | 10–15 | 0.5–4.0 |
| April, 1974 | 0.04–0.05 | 1–2 | - | 3–6 | 10–20 | 0.17–4.0 |
| Oct./Nov. 1974 | 0.03 | 5–25 | - | 0.01–0.13 | 2–11 | 0.01–0.09 |
| January 1975 | 0.03–0.05 | 0.40–5.70 | - | 0.33–1.33 | 7.1–20.8 | 0.02–0.11 |
| April, 1975 | 0.01–0.05 | 0.70–3.40 | - | 2–4 | 5–7.5 | 0.02–0.07 |
| July, 1975 | 0.02–0.05 | 0.25–3.75 | - | 2–4 | 6.8–11 | 0.01–0.05 |
| October, 1975 | 0.03–0.05 | 0.18–0.91 | - | 0.67–4.00 | 0.3–2 | 0.01–0.04 |
| March, 1976 | 0.02–0.05 | 0.10–0.50 | - | 0.71–3.57 | 0.1–2 | 0.01 |
| March, 1976 | 0.01–0.04 | 0.14–0.67 | - | 1.00–3.50 | 0.15–10.00 | 0.01 |
| May, 1976 | 0.01–0.04 | 0.48–1.43 | - | 0.63–1.88 | 4.75–7.75 | 0.002–0.007 |
| September, 1976 | 0.01–0.02 | 1.09–5.17 | - | 0.25–1.88 | 4.21–8.16 | 0.01–0.08 |
| May, 1977 | 0.11–0.40 | 1.55–7.75 | - | 25–48 | 6–43 | 0.30–0.87 |
| June, 1977 | 0.14–0.71 | 1–22 | - | 14–50 | 8–28 | 0.28–0.86 |
| September, 1977 | 1.10–20.00 | 3.3–12.0 | - | 10–53 | 11–27 | 0.02–0.07 |
| Lower Gulf of Thailand March/April, 1977 | 0.36–1.01 | 1.3–1.8 | - | 15–65 | 11–20 | 0.29–3.14 |
| Chao Phraya coastal station (depth 6.3 m) | 0.05 | 1.6 | - | 3.0 | 20 | 1.0 |
| Chao Phraya coastal station (depth 4.0 m) | 0.05 | 2.0 | - | 3.0 | 20 | 4.0 |
Table 25. Concentrations of heavy metals in sediment samples from Gulf of Thailand (after NRCT (1973–1977) and Idtikhasem, 1978)
| Source and Sampling Time | Heavy metal concentration (μg/g) | |||||
| Cd | Cu | Cr | Pb | Zn | Total Hg | |
| Gulf of Thailand October, 1973 | nil-1.50 | 14.8–21.9 | - | nil-0.5 | nil-0.6 | nil-43.9 |
| April, 1974 | 0.05–8.05 | 2.3–13.6 | - | 0.15–6.17 | nil-12 | nil-16.2 |
| Oct./Nov., 1974 | 0.07–0.23 | 7.3–31.0 | - | 0.20–8.7 | 0.4–4.0 | 0.3–0.6 |
| January, 1975 | 0.03–0.05 | 0.3–2.8 | - | 0.9–4.9 | 0.15–0.33 | 0.01–0.29 |
| April, 1975 | 0.01–0.07 | 0.04–4.44 | - | 0.06–2.8 | 0.22–1.32 | 0.01–0.07 |
| July, 1975 | 0.01–0.06 | 0.14–1.16 | - | 0.16–3.26 | 0.21–6.98 | 0.01–0.13 |
| October, 1975 | 0.01–0.05 | 0.064–3.93 | - | 0.4–4.8 | 0.08–4.8 | 0.01–0.07 |
| March, 1976 | 0.01–0.02 | 0.47–4.32 | - | 0.16–3.47 | 0.16–3.47 | 0.001–0.003 |
| March, 1976 | 0.01 | 0.4–4.0 | - | 0.14–2.05 | 0.14–2.05 | 0.002–0.004 |
| May, 1976 | 0.001–0.02 | 0.58–29.1 | - | 0.05–3.04 | 0.05–3.04 | 0.15–0.33 |
| September, 1976 | 0.01–0.02 | 1.09–5.17 | - | 0.38–1.83 | 0.38–1.83 | 0.001–0.006 |
| May, 1977 | 0.2–0.61 | 5–53 | - | 15–44 | 15–44 | 0.03–0.14 |
| June, 1977 | 0.2–1.0 | 4–124 | - | 8–141 | 8–141 | 0.02–0.67 |
| Lower Gulf of Thailand March/April, 1977 | 0.19–0.56 | 7–42 | - | 83–117 | 23–43 | 0.04–37 |
Table 26. Concentrations of heavy metals in marine animals from Gulf of Thailand (if no range given, this means average values)
| Source and Sampling Time | Type of Samples | Heavy Metal Concentration (μg/g) | References | |||||
| Cd | Cu | Cr | Pb | Zn | Total Hg | |||
| Upper Gulf of Thailand October/November, 1974 | Fish and other marine organisms | - | - | - | - | - | 0.001–0.086 | NRCT (1973–1977) and Idtikhasem (1978) |
| January, 1975 | " | - | - | - | - | - | 0.002–0.075 | NRCT (1977) |
| April, 1975 | " | - | - | - | - | - | ||
| July 1975 | " | - | - | - | - | - | 0.003–0.107 | " |
| 1977 | Fish only | - | - | - | - | - | Total 0.002–0.65 mean-0.041 Or 0.006–0.013 | Cheevaparanapivat (1979) |
| 1976 | Fish | 0.42 | 3.52 | - | 3.55 | 48.11 | Chanpongsang (1978) | |
| 1976 | Cuttle-fish | 0.72 | 19.96 | - | 3.47 | 89,15 | ||
| Mantis shrimp | 42.8 | 62.83 | - | 3.68 | 124.22 | |||
| Swimming crab | 9.88 | 52.96 | - | 3.38 | 159.97 | |||
| Scallop | 45.8 | 7.55 | - | 3.52 | 55.37 | |||
While the accumulation of the monitored metals in phyto- and zoo-plankton is very high at the river mouths, the concentrations measured in fish (this includes some macro invertebrates) lie much lower (Tables 19a-19d, 20 and 26). Extremely high value are reported by Chanponsang (1978) for Cd in mantis shrimp, swimming crab, and scallop. The latter showed 46 ppm Cd.
Limpasaychol (unpublished) reported on some heavy metal (Cu, Zn, Pb, Cd and Cr) levels in coastal and mangrove areas, Phuket Province. His finding indicated that these heavy metals were accumulated in high concentration in the mud, especially in the mangrove forest at Ao Nam Bor.
Menasveta (1980) reported that the problem of oil pollution does exist in Thai waters but is still relatively small. An accidental oil spill occurred in April 1974 when the 5 000 ton coastal vessel, “Visahakit” collided with another ship about 8 km from the mouth of Chao Phraya river and spilled 9 000 barrels of oil into the coastal waters. The slick drifted towards Bang Rang Chau Village and was washed ashore into a mangrove swamp, shrimp ponds, and salt pans.
A study on the deposition of tarballs on the sandy beaches contiguous to Thai waters was carried out by the sub-committee on the Quality of Waters and Marine Living Resources in Thai Waters under the Marine Science National Committee during 1974–1976. Three institutions have participated, namely: the Department of Marine Science (Chulalongkorn University, Bangkok), the Science Faculty (Prince of Songkhla University, Songkhla Province) and the Phuket Marine Biological Center (Department of Fisheries, Phuket Province) during the period of September 1974 until February 1976. The amount of tar-balls however, varied according to the location of the beaches and the season of the year. On the coasts around the Gulf of Thailand, for example, high tarball values were found during the summer months (March-April). On the Andaman Sea high amounts were found during the latter period of the rainy season (September-October). The highest accumulation of tarballs was found at Songkhla Beach and the lowest maximum of a station on a beach of Phuket Island (Figs. 20 and 21).
The amounts of tarballs on Songkhla beach are surprisingly high (0.19–715 g/m2) and the average amounts in April-July 1975, and in February, 1976 were higher than those on the east coast of the Gulf. It is interesting to note that on Songkhla beach tarballs were found at all twenty nine sampling dates.
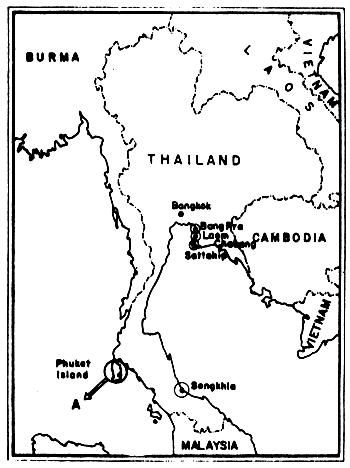
Fig. 20 Beach tar survey locations in Thailand A: see enlargement in Fig. 21 (after Piyakarnchana et al., 1979)
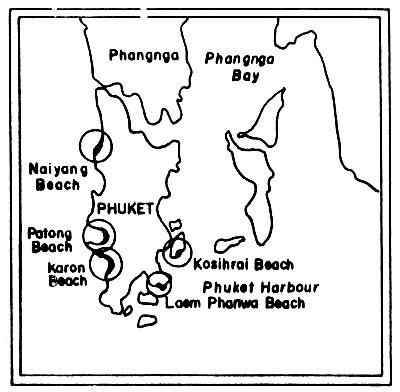
Fig. 21 Beach tar survey locations on Phuket Island, Thailand (Andaman Sea) (after Piyakarnchana, 1977)
In view of the prevalent south and southwest winds and also the existing currents yielding intrusion of the South China Sea waters into the gulf, the considerable amounts of oil discharged from tankers in the South China Sea might be accumulated on the coasts of the Gulf of Thailand.
Fig. 21 shows the sampling site on Phuket Island. At Laem-Phanwa beach the amount of tarballs ranges from 0.1 to 63.8 g/m2, and the highest value was observed in December 1975. The amounts increased gradually from November and reached their highest peak in December.
On Karon Beach, located on the less protected coast, the results showed higher amounts of tarballs than at Laem-Phanwa Beach. They ranged from 0.1 – 180.4 g/m2. On this beach the amount gradually increased from August onwards.
Tarballs were also recorded from Nai-Yang Beach and Patong Beach. However, no tarballs were detected on Ko Sihrai Beach during the course of this survey (Piyakarnchana et al., 1977).