| SCS/GFO/81/CR-1 | Hoque, Md. M. The growing of food organisms for fish hatcheries in Bangladesh |
| SCS/GFO/81/CR-2 | Soe Tun. Work on growing of food organisms for hatcheries at the Research Department, People's Pearl and Fishery Corporation, Rangoon, Burma |
| SCS/GFO/81/CR-3 | Kwong, V. Programme on growing food organisms in Fiji |
| SCS/GFO/81/CR-4 | Bhaskaran, M. Scope of aquaculture development in India with special reference to food organisms for fish hatcheries |
| SCS/GFO/81/CR-5a | Titania, N. Work on the growing of food organisms at Sukabumi Freshwater Aquaculture Development Centre and other freshwater stations in Indonesia |
| SCS/GFO/81/CR-5b | Djunaidah, In. S. Work on growing food organisms for fish hatcheries in Indonesia with emphasis on work at the Jepara Brackishwater Aquaculture Development Centre |
| SCS/GFO/81/CR-6 | Khars, Abdul Mohsen H. Al. The growing of food organisms for fish hatcheries in Kuwait |
| SCS/GFO/81/CR-7 | Hamid, Shahima Bt. Abdul. Freshwater farming in the states of Perak, Malaysia with notes on the growing of food organisms |
| SCS/GFO/81/CR-8a | Bautista, C.G. Work on growing food organisms for hatcheries at Kitcharao Freshwater Fish Farm and Nursery, Region X, Philippines |
| SCS/GFO/81/CR-8b | Dasal, R.C. A programme on growing food organisms for Leyte Freshwater Fish Hatchery, Babatngon, Leyte, Philippines |
| SCS/GFO/81/CR-8c | Oandasan, Jr., C.A. The growing of food organisms for fish hatcheries in the Philippines |
| SCS/GFO/81/CR-8d | Palaspas, M. Growing food organisms for fish hatcheries at the A.R. Apacible School of Fisheries, Nasubgu, Batangas, Philippines |
| SCS/GFO/81/CR-8e | Pador, E. The Artemia project in the Philippines under the Bureau of Fisheries and Aquatic Resources - Food and Agriculture Organization/United Nations Development Programme Brackishwater Aquaculture Development and Training Project |
| SCS/GFO/81/CR-8f | Pamposa, L.B. Work on the growing of food organisms for the Tacurong Freshwater Demonstration Fish Farm and Nursery in Region XII, Philippines |
| SCS/GFO/81/CR-9 | Tan Kay Heok. The growing of food organism for fish hatcheries in Singapore |
| SCS/GFO/81/CR-10 | Jayasuriya, P.M.A. Status of programme on growing food organisms for fish hatcheries in Sri Lanka |
| SCS/GFO/81/CR-11 | Haemaprasit, Niphond. Brackishwater aquaculture and growing food organisms for fish and shrimp hatcheries in Thailand |
| SCS/GFO/81/CR-12a | Le Vien Chi. Some results on the culture of Chlorella algae in Vietnam (Abstract) |
| SCS/GFO/81/CR-12b | Vu Thi Tam. Some results of Scenedesmus culture in the laboratory at the Nhatrang Fisheries University, Nhatrang Phukhanh province, Vietnam |
| SCS/GFO/81/CR-12c | Notes on growing diatoms for feeding shrimp larvae at the Aquaculture Research Institute in Vietnam |
| SCS/GFO/81-CR-12c | Vu Van Dung. Notes on growing diatoms for feeding shrimp larvae at the Aquaculture Research Institute in Vietnam |
SCS/GFO/81/CR-1
THE GROWING OF FOOD ORGANISMS FOR FISH HATCHERIES
IN BANGLADESH
by
Md. Mahbobul Hoque1
1. INTRODUCTION
The fisheries of Bangladesh, a country with more than 1.58 million hectares of perennial and about 2.83 million hectares of seasonal inland waters along with the extensive marine resources area, is a vital part of the national economy. People have come to believe that this gift of nature will always be as bountiful as ever.
But the man-made and natural ecological alterations, overfishing and resource use conflicts occurring over the last two or three decades have belied this belief. Fish has not remained as bountiful as in the past. This has disturbed the nation as many have started realizing the fact that like any other natural resource, fish also need scientific management, care and tending if it is to be sustained and utilized in the years to come. However, the elements of fish culture have been introduced more recently and the country is in the process of slowly changing the nature of fish production from a hunting to an aquacultural pursuit.
To transfer the technical know-how of fish culture, supply of fry and training of fish culturist/rural unemployed youths have been boosted in Bangladesh. The Government has started the fisheries extension service with the assistance of UNICEF. The author is working as farm manager under the said scheme.
1 Farm Manager, Fish Seed Multiplication Farm, Narsingdi (Baghata) District, Dacca, Bangladesh
2. BACKGROUND INFORMATION
There are two freshwater and one marine research stations in the country under the Directorate of Fisheries. Research on growing food organisms has been started in one of the freshwater research stations recently. Besides this, some basic research has been carried out at Dacca University, in Dacca, and Chittagong University in Chittagong. The Bangladesh Council of Scientific and Industrial Research in Dacca (BCSIR) has completed a research project entitled “Pure Culture of Chlorella vulgaris Beijerinek in Bangladesh” which has been published in the Bangladesh Journal of Dacca. The Chittagong University has also published in scientific journals at home and abroad. At present, the following institutes and research stations are engaged in research on locally-available natural food organisms:
All the above institutes and research stations are getting much priority to strengthen their research activities on growing food organisms to increase production of fish in the country. At present though, there is no largescale or commercial production of natural food organisms.
3. CULTURE OF CHLORELLA VULGARIS BEIJERINEK
The research stations and academic institutes of the country have undertaken both basic and applied research on locally available species of natural food organisms. Some of the available information on “Pure culture of Chlorella in Bangladesh” is presented here. Chlorella vulgaris is abundant in ponds, lakes, rivers and other water systems of Bangladesh. The species is being utilized as food by major Indian carps and other local fish. The species can tolerate a wide-range of environmental conditions.
The study was done experimentally in the laboratory of the Bangladesh Council of Scientific and Industrial Research in Dacca.
3.1 Procedures and results
With the available instruments and facilities, proper procedures were followed to identify the local strain of Chlorella, determine the effect of inoculum size, incubation period, outdoor and indoor sunlight, growth rate of different methods of a year, depth of culture medium and culture method on its growth.
Data representing the effect of different inoculum size on the growth of a local strain of Chlorella vulgaris in non-sterile culture medium are presented in Table 1.
Table 1. Effect of inoculum size 0.1 ml - 5 ml/l on the growth rate of Chlorella vulgaris cells in non-sterile culture medium
| Experiment Number | Date of experiment | Inoculum size Packed cell | Incubation period (days) | Microscopic observation A. V. of 9 | Growth (%) |
| 1 | 2 | 3 | 4 | 5 | 6 |
| 1 | 12.11.74 | 1 ml/l | 7 | Chlorella cells normal contamination slight | 733 |
| " | " | 14 | " | ||
| " | " | 21 | " | ||
| " | 2 ml/l | 7 | " | ||
| " | " | 14 | " | 467 | |
| " | " | 21 | " | ||
| " | 3 ml/l | 7 | " | 347 | |
| " | " | 14 | " | ||
| " | " | 21 | " | ||
| " | 4 ml/l | 7 | " | 258 | |
| " | " | 14 | " | ||
| " | " | 21 | " | ||
| " | 5 ml/l | 7 | " | ||
| " | " | 14 | " | 165 | |
| " | " | 21 | " | ||
| 2 | 23.4 | ||||
| 31.5.75 | 0.1 ml/l | 7 | " | 1678 | |
| " | " | 14 | " | ||
| " | 0.25 ml/l | 7 | " | ||
| " | " | 14 | " | 800 | |
| " | 0.5 ml/l | 7 | " | ||
| " | " | 14 | " | 533 | |
| " | 1 ml/l | 7 | " | ||
| " | " | 14 | " | 219 |
The smaller inoculum sizes supported better growth of Chlorella cells than the large ones (Table 1). In the first experiments, the smallest inoculum size 1 ml/l yielded the highest amount of Chlorella (733%) whereas the largest inoculum size of 5 ml/l yielded the lowest amount of Chlorella cells (165%). In the second experiment, also the smallest inoculum size 0.1 ml/l was found to be most favourable (1678%) followed by 0.25 ml/l (880%), 0.5 ml/l (533%) and then 1 ml/l (219%).
Inoculum size 0.1 ml/l was recommended for growing Chlorella in non-sterile conditions as it was done in aseptic condition.
Data representing the growth of Chlorella cells in different months of a year are presented in Table 2. The temperature and humidity during the period are also recorded in Table 2.
According to the growth record, the Chlorella strains included in this study can be growth all throughout the year, since the growth is neither totally stopped nor totally reduced in any month (Table 2).
Table 2. Study on growth rate of a local strain of Chlorella vulgaris in different months of a year
| Serial No. | Date of experiment | Inoculum size packed cell volume> (ml/l) | Date of harvest | Incubation period (days) | Growth (%)* | Temperature (°C) | Humidity (%) |
| 1 | 3/1/74 | 0.5 | 21/1/74 | 18 | 230 | 17 | 78.66 |
| 2 | 22/1/74 | 0.5 | 7/2/74 | 16 | 230 | 18 | 78.00 |
| 3 | 12/2/74 | 0.5 | 4/3/74 | 20 | 200 | 19 | 70.66 |
| 4 | 9/3/74 | 0.5 | 28/3/74 | 19 | 170 | 29 | 73.66 |
| 5 | 4/4/74 | 0.5 | 24/4/74 | 20 | 200 | 28 | 90.33 |
| 6 | 26/4/74 | 0.5 | 15/5/74 | 19 | 185 | 30 | 82.33 |
| 7 | 18/5/74 | 0.5 | 7/6/74 | 20 | 200 | 27 | 80.66 |
| 8 | 12/6/74 | 0.5 | 3/7/74 | 20 | 180 | 31 | 87.00 |
| 9 | 8/7/74 | 0.5 | 29/7/74 | 21 | 200 | 28 | 89.66 |
| 10 | 3/8/74 | 0.5 | 24/8/74 | 21 | 200 | 29 | 85.66 |
| 11 | 4/9/74 | 0.5 | 20/9/74 | 16 | 200 | 29 | 83.66 |
| 12 | 29/9/74 | 0.5 | 17/10/74 | 19 | 200 | 29 | 89.00 |
| 13 | 1/11/74 | 0.5 | 20/11/74 | 19 | 200 | 27 | 80.00 |
| 14 | 29/11/74 | 0.5 | 17/12/74 | 19 | 210 | 24 | 91.66 |
| 15 | 19/12/74 | 0.5 | 4/1/75 | 16 | 220 | 21 | 82.00 |
However, growth of Chlorella was maximum when temperature was lower (January). Good growth, however, was also recorded in higher temperature accompanied with high humidity (April-October). On the other hand, low temperature in combination with low humidity supported less growth than higher temperature and higher humidity (February recorded less growth than November and December).
Since growth in all the months has been found to be above 100% the strain included in this study may be recommended for growing all throughout the year.
4. EFFECT OF DEPTH OF CULTURE MEDIUM ON GROWTH OF CHLORELLA CELLS
Data representing the growth of Chlorella cells in different depths of culture medium are presented in Table 3.
Table 3. Study of the effect of depth of culture medium on the growth of Chlorella
| Serial No. | Date of experiment | Inoculum size packed cell volume (ml/l) | Depth of culture medium (cm) | Date of harvest | Incubation period (days) | Average growth packed cell volume (ml)* | Growth (%)* |
| 1 | 10/10/76 | 0.1 | 5 | 15/11/76 | 30 | 5 | 900 |
| 2 | 10/10/76 | 0.1 | 10 | 15/11/76 | 30 | 10 | 1900 |
| 3 | 10/10/76 | 0.1 | 15 | 15/11/76 | 30 | 12 | 2300 |
| 4 | 10/10/76 | 0.1 | 20 | 15/11/76 | 30 | 15 | 2900 |
| 5 | 10/10/76 | 0.1 | 25 | 15/11/76 | 30 | 10 | 1900 |
Growth of Chlorella cells increased with increase in depth of culture medium up to a certain level (20 cm depth). Further increase in depth of culture medium, however, reduced the growth of Chlorella (23 cm depth). Probably, temperature and light intensity at 20 cm depth was optimum for the growth of Chlorella. In Japan, cultures were set in media with depths ranging from 5–20 cm; however, 10 cm depth was selected for the pilot plant studies. The depth, 6–15 cm, has been adopted by some.
4.1 Effect of culture methods on pure culture of Chlorella cells
Data representing the effects of different culture methods on pure culture of Chlorella are presented in Table 4.
Fresh Chlorella cells, grown in fresh culture medium, yielded pure Chlorella cells. The other techniques employed in this study were not found to be suitable for pure culture, since these cultures were more or less infected by blue-green filaments and bacteria. These cultures probably got infected due to repeated exposures to open air (while adding fresh medium every 15 days of harvesting and inoculation of the used medium every 15 days, and addition of chemicals every 15 days). Therefore, under this climate condition, in order to have a pure culture, or unialgal culture, it is essential that sterile conditions should be maintained throughout the culture period, including use of fresh culture in fresh sterile culture medium.
Table 4. Effect of culture technique on pure culture of Chlorella
| Experiment No. | Inoculum size packed cell volume (ml/l) | Culture technique | Total growth in 1/2 month packed cell volume (ml)* | Average growth (%)* |
| 1 | 0.1 | Fresh Chlorella cells cultured in fresh medium for 15 days, cells harvested and fresh culture set up again for another 15 days. Total duration of experiment: 1-1/2 months (i.e., harvest) | 11.34 | 3.780 |
| 2 | 0.1 | Addition of fresh medium to the same Chlorella at an interval of 15 days. Total duration of the experiment: 1-1/2 months | Culture infected hence rejected | - |
| 3 | 0.1 | Chlorella cells harvested every 15 days and fresh culture set up in the used medium. The same medium used for 1-1/2 months | -do- | - |
| 4 | 0.1 | Chlorella cells cultured in the same medium for 1-1/2 months, but fresh chemicals added every 15 days | -do- | - |
6. CONCLUSION
The strain of Chlorella vulgaris included in this study has been found to be suitable for culturing all throughout the year. The optimum depth of culture medium for growing Chlorella was found to be 20 cm and the technique involving the use of fresh culture in fresh sterile medium, under sterile condition was found to be superior to the others for pure culture of Chlorella.
7. FUTURE PROGRAMME ON GROWING FOOD ORGANISMS
The Fisheries Department of the Government of the People's Republic of Bangladesh is fully aware of the vast potential of aquaculture in the country and has adopted the following projects in the ongoing second and five-year Development Plan (1980–1981 to 1984–1985):
Development of brackish shrimp culture in Bangladesh. Under the scheme, two shrimp hatcheries will be established with facilities for growing natural food organisms.
Intensification of fisheries extension programme. Establishment of a small limnological laboratory in each farm under the scheme to analyze soil and water, and production of natural food organisms.
Marine Fisheries Exploitation and Biological Research Station
at Cox's Bazar.
To conduct applied research on exploitation of marine fisheries
and biology of commercial important species of fish and shrimp
to develop brackishwater aquaculture. Facilities will be available
to culture natural food organisms in the station.
Establishment of a fisheries technological research station
at Chittagong.
Facilities will also be available to conduct research on growing
of natural food organisms.
8. CONCLUSION AND RECOMMENDATION
At present, there is no commercial scale production unit on growing food organisms in Bangladesh. Growing of natural foods organisms is still in the experimental stage. The country is advancing slowly towards the utilization of her available aquatic resources. However, the management and production of growing food organisms are very important as it is the key to success of fish and shrimp hatcheries. But the achievement of the desired goal depends on the trained and experienced personnel who are now short in the country.
So the relevant authorities and organizations are requested to extend their kind cooperation to assist Bangladesh to utilize her potential resources in fisheries to usher a bright future for the economy of Bangladesh.
SCS/GFO/81/CR-2
WORK ON GROWING OF FOOD ORGANISMS FOR HATCHERIES AT THE
RESEARCH DEPARTMENT, PEOPLES' PEARL AND FISHERY CORPORATION
RANGOON, BURMA
by
Soe Htun1
1. INTRODUCTION
In Burma, the Research Department of the Peoples' Pearl and Fishery Corporation, established in 1974, is conducting pilot-scale production of postlarvae of the giant prawn Macrobrachium rosenbergii. Pond construction is underway for the culture of penaeid shrimps. The Marine Biology Department of the Moulmein College is also conducting taxonomic and other studies of some cultivable species. Research activities on the culture of edible frog, earthworms, turtle, fish and freshwater prawn are carried out by the Research Department which is situated in Thaketa. Live nauplii obtained from Artemia cysts are used as food for the larvae of the giant freshwater prawns. For the culture of prawns and fish, the Live Food Culture Section of the Research Department was opened in 1980 with the following objectives:
To provide the required phytoplankton, zooplankton and Artemia stocks for the hatcheries.
To experiment on the isolation of different species of phytoplankton that can be used as larval feed and to maintain stock cultures.
To work on the production of Artemia in solar salt beds.
1 Research Officer, People's Pearl and Fishery Corporation, Rangoon, Burma
2. STATUS ON PHYTOPLANKTON CULTURE
Culture of phytoplankton has been carried out since November 1979. Both freshwater and marine cultures of some species were maintained. Some freshwater species of phytoplankton were isolated from the lakes in the Rangoon area. Isolation was carried out through the micropillary method and agar plate method. Clonal cultures were maintained in the laboratory. Species isolated were Scenedesmus sp. and Selenastrum sp. The cultures were maintained in Erdschreiber's and Chu's media. Chlorella sp. was also isolated and maintained in Sato's media. Another marine species. Chaetoceros calcitrans which was introduced from Thailand was also maintained in Sato's media. Maximum cell densities were attained on the third day.
3. STATUS OF BRINE SHRIMP (ARTEMIA SALINA) CULTURE
Culture of Artemia was initiated in July 1980 when a packet of cyst was received. Laboratory scale cultures were carried out to understand the general biology of the brine shrimp Artemia salina. Growth measurements were recorded. Gradually, the volume of the cultures were scaled up to 1 120 liters. Cultures were maintained in brick ponds. Fine rice bran was suspended and given as food for the Artemia. Salinity was raised by adding coarsa salt. It was found that the San Franciso Bay strain is zygogenetic whereas Thailand's strain is parthenogenetic.
3.1 Inoculation of the brine shrimp Artemia salina in man-made salt ponds
Inoculation of salt ponds was initiated with the following objectives:
The Peoples' Pearl and Fishery Corporation, with the cooperation of the Salt Industries Corporation was able to experiment on 2 acres of salt pond area in Paung, near Moulmein. The site is 7 miles from Paung and 2 miles away from the sea.
In the experiment, seawater intake was from Bi-laung creek. Pond preparation was done according to Anand (1977). Seawater with a salinity of 80 0/00 was pumped in from the other ponds. Artemia cysts from Thailand were used. Hatching was performed in cone-shape plastic bags for 48 hours with 30 percent seawater. Differences in growth of Artemia salina in ponds were observed at different salinities. Ponds with lower salinity resulted in faster growth. The phytoplankton observed were Pleurosigma, Navicula, Oscillatoria, Achnanthes and Monas species. Mass mortalities occurred in two of the inoculated ponds. Due to the onset of monsoon, the station was closed in May and 1.05 kg of cysts were harvested. The bi-phase floatation method was used for cyst collection (Sorgeloos et al., 1978). It was estimated that 100 kg of Artemia biomass were left in the ponds when the work ended.
Most Burmese like to have fish and shrimp paste for food. Shrimp paste together with some green vegetables is one of the main dishes on a Burmese table. Artemia paste and Artemia flakes were prepared and tasted. Artemia adults seem very promising as food. Artemia is used as food in Libya.
4. SCOPE OF FUTURE WORK
Mass cyst production will be initiated using different strains. Ponds will be prepared earlier for inoculation. An integrated farming system will be tried with the Artemia biomass that was left after cyst collection.
5. REFERENCES
Anand, Tunsutapanich. 1979 Cyst production of Artemia salina in salt ponds in Thailand.
Sorgeloos, P. et al. 1978 The use of Artemia cysts in aquaculture. The concept of hatching efficiency and description of a new method for cyst processing. Proceedings of the World Mariculture Society, 1978.
SCS/GFO/81/CR-3
PROGRAMME ON GROWING FOOD ORGANISMS IN FIJI
by
Virginia Kwong1
1. INTRODUCTION
Although aquaculture has been in Fiji for many years, it is still a novelty to the people in the country. It was only during the last decade that people became really interested in fish culture. With the ever increasing interest in this field, there exists a need for a centre whereby information could be made available and a source where farmers can obtain fish fry and shrimp postlarvae.
The Aquaculture Development Section is at present involved in the preliminary stages of setting the foundation for aquaculture development in Fiji. This base will determine the role aquaculture will have in our economy.
The Aquaculture Development Section is responsible for coordinating research, development and management of all aspects of the culture of aquatic resources. It will also serve as an information centre on the various aspects of aquaculture activities.
Its main objective is to promote both freshwater culture and mariculture in our country. In order to achieve this, a hatchery for finfish, crustaceans and molluscs will be established in the near future.
In addition to the hatchery, France Aquaculture and the Fiji Government will undertake a joint project in the raising of the penaeid P. monodon at Raviravi. This project will begin in September 1981. It consists of three phases:
Pilot programme on raising P. monodon larvae to postlarval stage.
Raising postlarvae in grow-out ponds using artificial feed.
Research on the utilization of local products for pelleted feeds.
1 Technical Officer I, Division of Fisheries, Ministry of Agriculture and Fisheries, Suva, Fiji
2. LIVE FOOD CULTURE
There is no programme on live food culture for hatcheries in Fiji at present, but as would be required, the Aquaculture Development should adopt this activity as part of its work.
One of the most important factors that affects the growth and survival of larvae and fry is the availability of the right type of larval feed. The primary conditions for the culturing of the larval feed include the following:
Water quality - must be free from any form of industrial pollution, sediments and other contaminants.
Culture vessels must be sterilized prior to use.
Adequate aeration must be maintained throughout the culture period.
Appropriate lighting is required to activate optimum growth.
Appropriate temperature, salinity and pH should be provided for optimum growth.
Most important of all the quality and quantity of the food organisms must be maintained.
For the raising of P. monodon larvae from zoea to early mysis, the larvae will probably be fed on Chaetoceros calcitrans and Tetraselmis. The rotifer Brachionus plicatilis can be fed to the laten mysis stage up to the postlarva stage. This will be supplemented with Artemia (brine shrimp).
2.1 Phytoplankton culture
Phytoplankton are cultured initially under controlled laboratory conditions and later transferred to grow-out tanks. Pure strains of Chlorella and Tetraselmis and Chaetoceros must be used. Chlorella and Tetraselmis are cultured as feed for the Brachionus. Sterilized seawater, enriched with fertilizer is the medium used. For mass culture, the algal culture is transferred to grow-out tanks using the same enriched medium. Adequate light, temperature, salinity and aeration are essential to activate exponential growth.
2.2 Zooplankton culture
Initial culture of zooplankton is similar to that of phytoplankton. For mass culture, Brachionus is fed with either Chlorella and Tetraselmis. Adequate light and aeration are needed.
2.2.1 Artemia culture
Using the decapsulation/hatching method, the hatching efficiency of the cysts is increased when temperature is maintained at 30°C, pH at 8–9 and high aeration is provided to keep cysts in suspension when hatched. The resulting nauplii are then fed to the shrimp larvae. Artemia may also be cultured using ricebran in an air-water-lift system for feeding at a later date.
3. RESEARCH SECTION
A part of the Food Culture Section should be set aside especially for the algal room. This room should have laboratory facilities, continuous lighting, low temperature (20°C) and a good aeration system for keeping of starter cultures.
In Fiji, research should be carried out on various algal cultures under various conditions of light, salinity, temperature and media and also under ambient conditions. Local strains of plankton should also be tested for their food value under various conditions.
It is through good management of live food culture and research that quality of larval feed can be determined. This in turn will increase the survival and growth rate of the larvae being raised in the hatchery.
SCS/GFO/81/CR-4
SCOPE OF AQUACULTURE DEVELOPMENT IN INDIA WITH SPECIAL
REFERENCE TO FOOD ORGANISMS FOR FISH HATCHERIES
by
M. Bhaskaran1
1. INTRODUCTION
The estimated population of India is 621 million. The fish production of the country is 1.958 m tons; the per capita consumption of fish is only 2.8 kg/year. In order to reduce the wide gap between fish production and requirement, several measures have to be taken. Capture fisheries alone can not solve the increasing demand. In this context, aquaculture offers the best alternative approach to supplement the requirements.
1.1 Resources
The aquaculture resources of India are as follows:
| Million (ha) | ||
| (a) | Freshwater area presently under culture | 0.6 |
| (b) | Freshwater area readily available for fish culture | 0.4 |
| (c) | Reservoirs | 2.0 |
| (d) | Brackishwater areas | 2.0 |
1.2 Research facilities
Various aspects of freshwater and marine research are handled by the Central Research Institutions viz. Central Inland Fisheries Research Institute, Central Marine Fisheries Research Institute, and the National Institute of Oceanography with substations throughout the country. Besides these institutes, the state governments also handle various research projects of local interest. Established private industries are also now taking interest in supporting and organizing research programmes.
1 Research Officer, Government of Gujarat, India
2. AQUACULTURE
2.1 Freshwater cultivated species
On the basis of complementary feeding habits, six species of carps are cultured together in freshwater ponds known as composite fish culture. There are three Indian major carps viz. Catla catla, a surface zooplankton feeder, Labeo rohita, column dweller feeding on decaying vegetation and Cirrhina mrigala, a bottom dwelling fish utilizing semi-rotten and decaying vegetation. Three exotic carps, the grass carp, a macrovegetative feeder, common carp, an omnivore, and silver carp, a surface phytoplankton feeder, are also available for culture.
The fry and fingerlings of the major Indian carps are available by natural collection as well as by inducedbreeding to meet the present requirements of stocking. One month before stocking, depending upon the nature of the soil, alum at about 200–500 kg/ha/year is mixed with the soil. Before stocking the fingerlings' organic fertilizer at 5–15 tons/ha/year is added. The fertilizer consists of ricebran and oil cake at a ratio of 1:1. Feeding is approximately 1–2 percent of body weight. The feeding is carried out monthly in equal installments. Alternately, inorganic fertilizers are also used. These include urea, ammonium sulfate and super phosphate weighing 200 kg, 450 kg and 250 kg per year/ha, respectively.
The stocking rate is about 5 000/ha. The average annual production is in the range of 3 000–5 000 kg/ha/year.
2.2 Brackishwater cultivable fish and prawns
These include grey mullets viz. Mugil cephalus, M. tade, M. macrolepis and M. parsia. Milkfish (Chanos chanos), pearl spot (Etroplus surantensis), Bedki (Lates calcarifer), thread fin (Polynemus tetradactylus), rock perch (Lutjanus argentimaculatus), and prawns including Penaeus monodon, P. indicus, P. mer- guiensis, Metapenaeus brevicornis, M. dobsoni and M. monoceros. Crassostrea spp. and pearl oyster (Pinctada sp.) are also cultivated in the Gulf of Mannar and Gulf of Kutch in a limited scale.
Brackishwater and mariculture for mullets and prawns are carried out in the traditional way by polyculture. Natural seeds are collected and stabilized in the nurseries, unwanted varieties are removed and then stocked at rates of 40 000 to 50 000/ha. Food supply is mainly the food organisms coming with the incoming water about 8–10 days in a month depending upon the tide. The production of shrimp is about 350 kg/ha/year and fish 1 500 kg/ha/year.
3. CULTURE OF FOOD ORGANISMS
Laboratory experiments for the production and uniculture of phytoplankton and zooplankton are in progress.
4. CONCLUSION
Most of the farm sites and marketing centres are far apart. Majority of the fishermen are below the poverty line. Therefore, a well planned strategy to synchronize the various aspects of development such as water supply, electricity, approachable road, marketing system has to be envisaged. Training of technical manpower, inputs and basic technology also should be looked into. Conventional aquaculture practices have been in existence. New technologies such as the growing of food organisms for the fishmeal production centres should be introduced. What is urgently required is to raise this technology at the level of organized small-scale industries and spread them to all parts of the country.
SCS/GFO/81/CR-5a
WORK ON THE GROWING OF FOOD ORGANISMS AT SUKABUMI
FRESHWATER AQUACULTURE DEVELOPMENT CENTER AND
OTHER FRESHWATER STATIONS IN INDONESIA
by
Nita Titania1
1. INTRODUCTION
This paper gives a short description of some activities and methods of natural or live food culture at the Sukabumi Freshwater Aquaculture Development Center and other freshwater stations in Indonesia. The Freshwater Aquaculture Development Center at Sukabumi, Indonesia and other freshwater stations use natural/live food as supplemental feed for fish larvae and fry. All sorts of live/natural food we have cultured like Infusoria, Daphnia, and Moina are cultured directly in the pond.
2. INFUSORIA CULTURE
Infusoria are useful as natural food of fish larvae after the reserve food from the yolk has been consumed.
For Infusoria culture, we use cabbage leaf slices at 5 g/1 with adequate aeration and water circulation. We collect Infusoria after 6–8 days.
3. DAPHNIA CULTURE
Daphnia is usually fed live to fish fry and fingerlings and cultured in the ponds. For Daphnia culture, we use chicken dung at 200 g/m 3. Inorganic fertilizers (Urea and TSP) at 20 g/m3 are also applied. After a week, the Daphnia population reaches as much as 5 000–10 000 individuals per m3.
4. MOINA CULTURE
In Moina culture using fiberglass tanks, chicken dung in a sack is applied at 200 g/1. Water is allowed to stand for 2 days in the tanks before fertilizer is applied. After a week, Moina begin to appear and the population is maintained at 30 000–50 000/m3.
1 Aquaculturist, Jalan, Bhajanghasa No. 110, Sukabumi, Indonesia
5. FEEDING SYSTEM PERIOD FOR FRESHWATER FISH CULTURE
| DAY 0 | 3 | 7 | 21 | 45 | 76 |
| Egg yolk Infusoria | D a p h n i a P e l l e t c r u m b l e | P e l l e t | |||
| M o i n a R i c e b r a n S o y b e a n m e a l | |||||
SCS/GFO/81/CR-5b
WORK ON GROWING FOOD ORGANISMS FOR FISH HATCHERIES
IN INDONESIA WITH EMPHASIS ON WORK AT THE JEPARA
BRACKISHWATER AQUACULTURE DEVELOPMENT CENTRE
by
Iin S. Djunaidah1
1. INTRODUCTION
To facilitate the development of fresh and brackishwater pond culture of fish and prawn, universities and some research institutes in Indonesia carry out research in the culture of live food organisms for feeding the larval stages of fish and prawn. For example, research in the culture of Infusoria sp. and Skeletonema sp. is being carried out in the Bogor Agricultural University. On the other hand, the Inland Fisheries Research Institute and the Freshwater Aquaculture Development Centre in Sukabumi, have been engaged in the culture of live food organisms for the rearing of freshwater and fish shrimp larvae. These include Infusoria sp., Daphnia sp. and Moina sp., the common carp (Cyprinus carpio) and Brachionus sp. for the giant prawn (Macrobrachium rosenbergii de Man). Further, the Marine Fisheries Research Institute and the Brackishwater Aquaculture Development Centre in Jepara is engaged in the culture of Skeletonema costatum, Tetraselmis chuii, Chaetoceros sp., Isochrysis sp., Brachionus plicatilis and Artemia salina for the larval rearing of Penaeus monodon as well as Brachionus sp. and Artemia salina for the culture of Macrobrachium larvae.
This paper describes some of the activities at the Jepara Brackishwater Aquaculture Development Centre with emphasis on the growing of food organisms for the rearing of larval prawn. The main activities are: (a) hatchery of P. monodon and M. rosenbergii; (b) culture of Chanos chanos, P. monodon, P. merguiensis and Siganus sp.; (c) preparation of artificial feed and live food organisms.
2. WORK ON GROWING FOOD ORGANISMS AS FEEDS FOR LARVAL PRAWN AT JEPARA BRACKISHWATER AQUACULTURE DEVELOPMENT CENTRE
In hatcheries, artificial feed and live food organisms are used including Skeletonema costatum, Tetraselmis chuii, Chaetoceros sp., Isochrysis sp., Brachionus plicatilis and Artemia salina.
1 Food Technologist, Pemandian Kartini St., Jepara, Indonesia
2.1 Skeletonema costatum
Skeletonema costatum is fed to larvae of P. monodon at the zoea stage.
Culture method in 1 liter:
The equipment is sterilized.
To 1 liter seawater the following are added:
2 ml KNO3 solution (20.2 g/100 ml distilled water)
2 ml Na2HPO4 solution (2.0 g/100 ml distilled water)
2 ml Na2Sio3 solution (1.0 g/100 ml distilled water)
8 drops FeCL3 solution (1.0 g/20 ml distilled water)
Inoculation with Skeletonema starter
After 3–4 days, the population density is about 6 million cells/ml.
2.2 Tetraselmis chuii
Just like Skeletonema costatum, Tetraselmis chuii is given to penaeid larval at the zoea stage.
Culture method in 1 gallon:
To 3 liters of seawater add the following:
urea (46–0–0): 60–100 ppm
TSP (0–46–0): 20–50 ppm
Inoculation with Tetraselmis chuii
After 4 or 5 days, the population density is about 4.5 million cells/ml.
2.3 Chlorella sp.
Chlorella sp. is used both in P. monodon and M. rosenbergii hatcheries. This functions as food for Brachionus sp. and maintains water quality.
Culture method in 1 gallon:
To 3 liters of seawater (12–15 ppt) add Allen Miquel fertilizer as:
6 ml solution A: KNO3 -20.2 g/100 ml distilled water
3 ml solution B: Na2H2O - 4 g
CaCl2.6H2O - 2.0 ml in 80 ml distilled water
FeCl3 - 2.0 ml
Inoculation with Chlorella starter
After 5 days, the population density reaches 30 million cells/ml.
2.4 Isochrysis sp.
Isochrysis sp. is given to larvae of P. monodon.
Culture method in 1 liter:
| (a) | To 1 liter seawater add: | KNO3 - 100 ppm |
| K2HPO4 - 10 ppm | ||
| Na2SiO3 - 5 ppm | ||
| Na EDTA - 10 ppm | ||
| (b) | Inoculate with Isochrysis starter. | |
| (c) | Incubate for 4 days. | |
2.5 Brachionus plicatilis
Culture method:
Prepare “green water” in the tank for media of Brachionus
starter using inoculation from the pond.
After several days, Brachionus will be available in the tank.
Fill the other tank with seawater.
Inoculate with Brachionus starter with density of about one individual/ml.
After 3–5 days, population density is about 300 individuals/ml.
2.6 Artemia salina
Nauplii of Artemia salina are used as food for the larval stages of P. monodon and M. rosenbergii.
Producing nauplii of Artemia salina:
Old method
Artemia cysts are hatched in seawater (2 g/1) for 24–48 hours.
New method
Artemia cysts are decapsulated for the removal of the outer membrane by dissolution in hypochlorite. Non-hatched cysts and thin shells can not be digested by shrimp.
After decapsulation, Artemia cysts can be incubated for 24–48 hrs (5 g/1 seawater).
SCS/GFO/81/CR-6
THE GROWING OF FOOD ORGANISMS FOR FISH
HATCHERIES IN KUWAIT
by
Abdul Mohsen H. Al Khars1
1. INTRODUCTION
The Mariculture and Fisheries Department in the Kuwait Institute for Scientific Research is engaged in two major activities - fisheries management and mariculture.
The fisheries management staff is responsible for collecting data either from the sea or out of the sea with detailed knowledge of the size frequency and species composition in the market, studies on the distribution and migration of fish, shrimp and zooplankton in the sea and to evaluate these data and provide these to the Government to help production of natural marine resources.
Two ways by which the mariculture project have sought to increase supplies of fish and shrimp are: (i) by restocking the declining shrimp population with juveniles grown to viable sizes from eggs hatched in the laboratory, and (ii) by growing young fish and shrimp to marketable size with the aim of eventually establishing a commercial fish farming industry.
Two major projects are being undertaken: the fish culture and shrimp culture projects, respectively. Besides these, there are three subprojects which provide the fish and shrimp nutrition, fish health and food production organisms.
The various projects of the Department require a wide-range of food organisms as feed. These feeds can be provided either in live or dried forms which may be included as a raw material in the production of formulated feeds. The project meets these requirements by culturing:
1 Research Assistant, 9 Home, 36 Street Part 3, Andalia, Kuwait
Single-cell marine micro-organisms, including the mass production of marine yeast by the batch or continuous system.
This system is used in the culture of zooplankton and shrimp larvae. Different feeds have been used as a source of carbohydrates, including glucose, sugar, mollasses and date extract. Four strains of marine yeast have been isolated from local seawater which has successfully produced mass cultures.
Scheme for mass culture production of marine yeast using the batch culture system
Marine phytoplankton including the mass culture production of Chlorella sp. by using autotrophic outdoor culture technique.
Chlorella is used in zooplankton cultures and also by the fish culture project to maintain water quality.
Wind-blown mixed diatoms grow in the shrimp larvae tanks by direct fertilization of the culture tank.
Marine zooplankton for fish and shrimp feed including the production of rotifers (Brachionus plicatilis) using the batch culture system, continuous culture system and outdoor culture system.
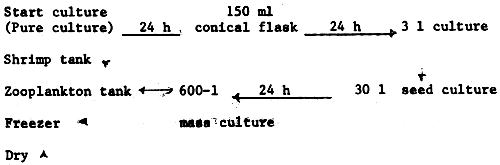
(a) Batch culture system - it is a Japanese system that uses culture 500-1 plastic tank as culture units. Two types of food organisms are used in this system: Chlorella cells which are considered the main food of rotifers and bread yeast, a food organism that is related directly to the density of rotifers in the culture. The density reaches up to 600 individuals/ml. The disadvantages of this system are the high labour requirements and the need for large amounts of Chlorella.
(b) Continuous culture - the culture unit used is 1 000-1 metal tank. The yeast is considered the only food used in the system. The culture is done over one month with daily harvest of 1/10 of the total culture volume with about 600 individuals/ml. But in this system, the rotifer feeds only on one kind of food yeast which is nutritionally inadequate for the fish larvae.
(c) Outdoor culture system - this is the most favourable system used in Kuwait mariculture which satisfies the rotifer demand in the hatcheries. The culture is supported by strong aeration and a heating system to keep the water temperature between 25–28°C and to avoid the variation in the surrounding temperature during a day which may sometimes be over 10°C. The productivity of the culture is 14 × 106 ind/day/ton of water. However, a comparison between the three systems has shown that the outdoor method gives better results than the other systems in terms of labour manpower, quantity of the yields and the conversion ratio. The aquaculturist believes that the rotifers which feed only on ordinary yeast is considered to be an insufficient food to the fish larvae. It has been found to lack an essential fatty acid which can be synthesized by phytoplankton. In Kuwait, this problem is solved by two ways: first by feeding the rotifers with a small amount of Chlorella in a period of 24 hours before being released to the hatcheries; secondly, by producing qualified yeast that can be fed into the zooplankton culture.
D. Artemia - Artemia salina is used in the culture of shrimp larvae and fish larvae. Artemia cysts are decapsulated in the hatcheries and the nauplii released directly to hatchery tanks. There is a plan to establish three circular plastic tanks with 10 m diameter, to start growing Artemia to adult stage for feeding to shrimp and fish.
In addition, the distribution and isolation of potential food organisms from Kuwait seawater are being studied particularly for strains with high nutritive value which may prove useful for mariculture.
SCS/GFO/81/CR-7
FRESHWATER FARMING IN THE STATES OF PERAK, MALAYSIA
WITH NOTES ON GROWING FOOD ORGANISMS
by
Shahima Bt. Abdul Hamid1
1. INTRODUCTION
1.1 Background information
During the last five years, fish, shrimp and prawn farming especially the farming of the giant Malaysian prawn have expanded considerably. The demand for shrimp and prawn is ever growing and the supply cannot meet the demand. This has resulted in the new fisheries law enforced by the Ministry of Agriculture requiring the change in net mesh size from 1“ to 1–½” and setting the trawling distance for 50-ton weight boats to be 12 miles offshore. Under this new law, many trawlers were affected and many fishermen have turned to freshwater farming.
The giant Malaysian prawn is one of the most economically valuable freshwater crops. Farmers can fetch as much as M$28.00 Malaysian ringgit2 per kg of prawns.
The objective under the 4th Malaysian Plan is to upgrade the living conditions of the farmers and fishermen by encouraging them to have backyard fishponds and also to plant cassava. It has been found that cassava leaves are good for fish in the fishponds. Cassava is also valuable as cassava flour that is made into local cakes as human food. The Malaysian Government also encourages the private sector to set-up fish and prawn hatcheries to catch up with the demand. At present, there is only one marine shrimp hatchery owned by a member of the private sector. In our Fisheries Department, we do not have marine shrimp hatcheries. We have faced lots of problems such as water quality, salinity and the availability of food organisms other than Artemia. So far, we have not come up with anything with regards to growing of food organisms.
1 Head, Prawn and Fish Hatchery Station, Perak, Malaysia
The Fisheries Research Institute in Penang has tried to work on this but no conclusive results have so far been obtained. However, in the freshwater side, we are more or less successful. At present, we have about 4 000 fishponds with areas ranging from ½ acre (0.2 ha) to 1 acre (0.5 ha) scattered all over the district in Perak alone and 3 000 units of cages in lakes and unused mining pools. The main practice is polyculture of fish and the giant Malaysian prawn. The size of the prawn juveniles delivered to the farmers range from 1–½ “ to 2” (3–5 cm).
1.1.1 For my country
My work is mainly on extension and management of a Macrobrachium rosenbergii hatchery which is 217 km from Kuala Lumpur and 120 km from the town. It has been emphasized that for quick development of prawn culture, the setting up of hatcheries is essential for producing large numbers of juveniles for stocking. However, management of prawn hatcheries involves several problems, one of which is the availability of the proper food besides Artemia for the different larval stages. The brine shrimp Artemia is known to be a food of very high nutritive value but is also very costly. However, it is still the major ration. So far, we have not done anything in my hatchery to culture Artemia for their useful use. A proposal has been put up for approval to the Economic Planning Unit and the Ministry of Agriculture.
Feeding of M. rosenbergii is usually at the rate of 15 nauplii/ml. In early stages, the feeding rates are initially high and decreased in the later stages, but it is generally supplemented with a variety of feeds such as fish flesh, egg custard and cockle flesh. Artemia are obtained from cysts hydrated under constant aeration at 28°C and placed in coneshaped fiberglass containers with salinity of 26 0/00 using the standard rearing method. Nauplii are harvested 24 hours after cyst immersion.
For the future programme, we intend to determine the minimum requirements of Artemia nauplii as primary ration for M. rosenbergii juveniles using an inexpensive system and also to improve the hatchery production. Our target is to produce 4 million prawn juveniles to cope up with the great demand.
SCS/GFO/81/CR-8a
WORK ON GROWING FOOD ORGANISMS FOR HATCHERIES AT KITCHARAO
FRESHWATER FISH FARM AND NURSERY, REGION X, PHILIPPINES
by
Carlos G. Bautista1
1. INTRODUCTION
Region X is located in the northern part of Mindanao. It is composed of seven provinces, namely: Misamis Oriental, Misamis Occidental, Bukidnon, Camiguin, Agusan del Norte, Agusal del Sur and Surigao del Norte.
Under the Expanded Fish Production Programme of the Bureau of Fisheries and Aquatic Resources (Region X), the major strategy is to boost fish production by developing commercial bodies of water and fishponds.
The Bureau of Fisheries and Aquatic Resources (Region X) established the Freshwater Fish Farm and Nursery situated in Barangay Anibongan, Kitcharao, Agusan del Norte with an area of 4.3 ha. With a target of producing 4.96 million fingerlings annually, the objectives of BFAR are to produce and disperse fingerlings into communal bodies of waters (lakes, rivers, swamp, etc.) and to train fish farmers on fish culture techniques which includes growing of food organisms.
2. LAB-LAB AND PLANKTON CULTURE
A week or month before the ponds are to be stocked with fry, pond preparation is done. The purpose of the preparation is to create the best condition for lab-lab and plankton growth and to completely eradicate the enemies and competitors of the fry.
The ponds are leveled, if necessary. The topography of the pond bottom should be so made that the catch basin area has the lowest elevation.
Once the pond is properly leveled, it is completely drained and the gates are closed by putting temporary soil diking to keep out water. The pond bottom is dried or exposed to the sunlight until the soil cracks with dryness. This may be accomplished within a week's period.
1 Fish Farm Manager, BFAR Region X, Butuan City, Philippines
After a week of drying, water is allowed into the pond at a depth of 3–10 cm. At this time, the gates are well screened by installing bamboo screens and fine mesh nets to avoid the entrance of fish predators. Application of fertilizer is done with the recommended fertilizer, 16–20–0 at 50 kg/ha; 46–0–0 at 22 kg/ha; and chicken manure at 2 000 kg/ha or 2 500 kg/ha. After 3–7 days, a change of colour appears in the water as a sign of the presence of food organisms, then water is allowed at the desired depth from 60–70 cm. Then stocking of fry is done. The stocking density is from 30–50 fry/m2.
To maintain a continuous supply of lab-lab and other food organisms, the pond should not be overstocked. When ponds are overstocked, lab-lab and other food organisms are exhausted and the fish become stunted, thus increasing mortality.
Supplemental feeds are also needed like ricebran, corn meal, soy bean meal, etc. The feeding rate is 2–5 percent of the total body weight per day.
3. CARP CULTURE AT THE HATCHERY TANKS
After hatching, at about 3–4 days old, carp fry are fed with egg yolk (hard boiled) or powdered milk. Egg yolk or powdered milk is mixed with a little amount of water and fed to carp fry. Most of the fry are still on the sides of the hatchery tank. After 6–7 days, carp fry are transferred to the prepared nursery ponds.
4. FOR TILAPIA CULTURE
Fry are collected from brood ponds and rearing ponds and brought to hatchery tanks, and fed with fine rice bran mixed with corn meal and soy bean meal at feeding rates of 3–6 percent of the total body weight. At about 5–10 days old, tilapia fry are transferred to prepared nursery ponds.
Tilapia are reared in the nursery pond with water depth of 40–50 cm, where algae like filamentous green algae and other species grow. After 7–12 days, 16–20–0 application is recommended at the rate of 15 kg to 20 kg/ha.
These projects are now implemented and the fingerlings produced are being dispersed into communal bodies of water and fishponds throughout Region X.
SCS/GFO/81/CR-8b
A PROGRAMME ON GROWING FOOD ORGANISMS FOR LEYTE FRESHWATER
FISH HATCHERY, BABATNGON, LEYTE, PHILIPPINES
by
Ricardo C. Dasal1
1. INTRODUCTION
The Philippines with its vast fishing grounds and rich aquatic resources can enjoy a continuous supply of fish, should these resources be properly conserved through scientific exploitation. With the present technology, the Government's national programme thrust on the accelerated food production intends to tap these potential resources to provide every Filipino with his fish protein requirement.
The Bureau of Fisheries and Aquatic Resources (BFAR), Region VIII of the Ministry of Natural Resources (MNR) under its expanded Fish Production Programme in consonance with the national programme established the Leyte Freshwater Fish Hatchery at Babatngon, Leyte, Philippines. Occupying 8.4 ha of a suitable site with water source fed by gravity, the project is designed to rear and maintain a sufficient number of freshwater fish breeders particularly carps and tilapia expected to produce 3.28 million fingerlings annually. These fingerlings are dispersed free of charge to cooperators for rice-fish farming, fishponds, backyard ponds, fish cages and fishpen operators and for restocking natural and man-made bodies of water in the region.
Barely satisfying the yearly dispersal demand, the hatchery's pond system which now requires major repair work is undergoing improvement and renovation to include the construction of the headpond with a sand filtration system. The hatchery is being upgraded and prepared for full operation to maximize fingerling production. This activity is not only aimed at reaching the annual target but also to provide a sufficient quantity of fish to be used as feeds in rearing and maintaining about 1.5 million bullfrogs consisting of breeders, growers and froglets for the Region VIII Bullfrog Training and Breeding Centre located in the same site.
1 Fisheries Extension Specialist, BFAR Region VIII, Calbayog City, Philippines
2. CULTURE OF NATURAL FOOD ORGANISMS IN THE HATCHERY
Natural food organisms are being cultured in ponds fertilized with both organic and inorganic fertilizers. Luxuriant growth of plankton organisms is visible a few days after application of the fertilizers as indicated by the “green bloom”. However, the identification of these organisms has not yet been determined. Unknown as they are for the present, these organisms have been noted to improve and/or enhance the growth of fish stock. On food organisms used for feeding the larvae to fry stages of the fish, nothing is actually known or recorded. Hard boiled egg yolk and powdered milk have been used as feeds during these life stages. The main supplementary feed used for both fingerlings and breeders is ricebran.
3. CONCLUSION
The primary problem in the successful management of a hatchery is abundant food that would not only determine the survival of the stock but will likewise determine the viability of the project operation. This training provided a clear idea of what the hatchery should be and how it should be operated and maintained. Furthermore, it showed some ways of how to assess, organize and make use of available resources to the best advantage of the project. Finally, any knowledge and experiences gained from this training will be very instrumental in upgrading the method and techniques in hatchery management. The additional facilities and innovations of existing facilities scheduled to be undertaken could be modernized to conform with the latest trends and technologies.
SCS/GFO/81/CR-8c
THE GROWING OF FOOD ORGANISMS FOR FISH HATCHERIES
IN THE PHILIPPINES
by
Cirilo A. Oandasan, Jr.1
1. INTRODUCTION
In the management and operation of mass seed production of fish nurseries and hatcheries, one of the most significant basic activities is the growing of adequate supplies of appropriate food organisms to assure the favourable growth and survival of the young and reproduction of the breeders as well.
The culture of sufficient quantities and of quality of natural fish food organisms remains a problem in most Philippine fish hatcheries and nurseries. In most cases, the use of artificial feeds as supplement to the natural fish food organisms for larval rearing is practiced.
There is a need for concentrated effort of both the Government and private sectors to work on the culture of food organisms for the growth and mass production of fry and fingerlings.
2. GROWING OF LAB-LAB AND PLANKTON
Lab-Lab is the biological complex of microscopic plants and animals grown in the pond bottom utilized as food of fishes like the Chanos chanos in brackishwater ponds. In the Philippines, the mass production of lab-lab practiced by fish farmers both in brackishwater and freshwater areas depends mainly on natural propagation. This method involves direct fertilization of the pond containing natural populations of phytoplankton and zooplankton. The commonly used organic fertilizers for the production of food organisms in ponds are animal manures especially chicken manure but organic materials of other sources are also used. Compost, ricebran and grass are popular organic fertilizers used in fish nurseries in various parts of the country. With regards to chemical fertilizers, a common single element fertilizer such as superphosphate (0–20–0), is used; an incomplete or two-element fertilizer like monoammonium phosphate (16–20–0) or diammonium phosphate (18–46–0) and complete fertilizer, 14–14–14, are also applied.
1 Fish Farm Manager, BFAR Regional Office II, Tuguegarao, Cagayan, Philippines
2.1 Fertilization programme
The following programme has been used successfully for producing lab-lab and plankton:
| Fertilizer (N-P-K) | Amount per application | METHODS AND FREQUENCY OF APPLICATION | |
| Plankton | Lab-lab | ||
| 18–46–0 | 22 kg/ha | Apply all applications in platform | Apply first application by broadcasting and all follow-up application by broadcasting or use of platforms |
| 16–20–0 | 50 kg/ha | Apply every 15–30 days or as needed to keep water visibility between 20–30 cm | Apply every 15–30 days |
| Chicken manure | 2 000–2 500 kg/ha | Apply only on new ponds or in ponds that have little or no soft organic mud. Apply by broadcasting. Use 18–46–0 or 16–20–0 in follow-up application as recommended above | |
2.2 Requirements for growing lab-lab
For the proper and continuous growth of lab-lab, the following factors should be taken into consideration:
Depth of water - for the proper growth of lab-lab, the water in the pond should be kept not more than 12 cm most of the time.
Nature of soil - the most suitable soil observed for lab-lab growth is clay, sandy clay or clay loam.
Turbidity - turbid water has been shown to give poor results.
pH - the pH for normal growth of lab-lab has been observed to be slightly alkaline.
Temperature - low temperature hinders lab-lab growth
3. GROWING OF ZOOPLANKTON (ROTIFERS)
In the Philippines, the rearing of zooplankton such as Moina and Brachionus has been applied economically and carried out without the need of sophisticated equipment. Fish farmers in the Philippines practically produce Moina as feed for fish larvae by enriching the pond with chicken manure at the rate of 200 kg/ha with water depths maintained at 40–50 cm. After a week, the organism is in abundance and fish farmers collect it by using a scoop net with bolting silk cloth for use as feed for the fry and fingerlings or breeders.
Moina has characteristics which make it ideal for aquaculture use. It is easy to handle, it is a non-selective filter feeder of bacteria in manure solution, it reproduces fast, is adaptable to a wide-range of environmental conditions and it has high nutritive value.
4. PROBLEMS
One of the major problems in the growing of fish food organisms for fish hatcheries in the Philippines is the identification of the proper species of food organism for mass production for use in the growth and survival of fish larvae. Most aquaculturists in the government and private sectors lack the necessary equipment and know-how for growing food organisms.
3. CONCLUSION AND RECOMMENDATION
Mass culture of food organisms for the survival and growth of larvae and reproduction of breeders play a vital role in the successful operation of fish hatcheries. As all living animals, fishes and food organisms require adequate nutrition. Determining and growing the proper quality and quantity of food items to be given to larvae for faster growth and reproduction require the concentrated efforts of aquaculturists in both the government and private sectors. There is need for the development of technology packages on growing food organisms to be imparted for application by fish farmers in the country in order to meet the challenge of increasing fish supply for attaining selfsufficiency.
SCS/GFO/81/CR-8d
GROWING FOOD ORGANISMS FOR FISH HATCHERIES AT THE
A. R. APACIBLE SCHOOL OF FISHERIES, NASUGBU,
BATANGAS, PHILIPPINES
by
Merly Palaspas1
1. INTRODUCTION
The A. R. Apacible School of Fisheries, formerly the Batangas School of Fisheries is located in the Southern Tagalog region of the Philippines.
The school has a fishpond and hatchery. The fishpond is about 3.5 ha. This pond has two purposes: as an experimental unit for the students and also for commercial production. It is also a self-liquidating fishpond under the Income Generating Programme. We culture milkfish and Penaeus monodon every year. The food organisms cultivated for the cultured fish are plankton and lab-lab. Production, however, is low because of frequent rain and floods.
Through the initiative of our school superintendent, we managed to build the small-scale hatchery of the school. This small hatchery is also for experimental and commercial purposes. Since our hatchery is on the initial stage of operation, all things are new and little knowledge about the hatcheries is available. This report is based on what we have done in the laboratory. Since I am a school teacher, little time is devoted to the hatcheries so all the work in the laboratory is done by the Japanese technician. However, we have different areas of work to be mastered in culturing food for the fish prawn hatchery. There are six aquaculture teachers working in the hatcheries. The project is located about 150 m away from the sea.
The hatchery has 10 fiberglass tanks rectangular in size and 5 round fiberglass tanks. All these equipment were donated by the Government of Japan to our school. This prawn hatchery project was started last April 1981 and we have a brood stock of 26 pieces of P. monodon, 4 of them are male and the rest are female. Six spawners have already been eyestalk ablated for faster rematuration.
1 Instructor, A. R. Apacible School of Fisheries, Nasugbu, Batangas, Philippines
In order that this project can be progressive, food organisms will have to be cultured in the laboratory, as food of the larvae up to the post-larvae. These organisms are: Chlorella, Artemia salina and Brachionus.
Culture of Chlorella
For phytoplankton, we cultured Chlorella from the National Science Development Board (NSDB). With an inoculum of 200 ml, we started the production of Chlorella. On the first day, we allowed it to stand for 3 days with aeration. After 3 days we filtered the Chlorella and placed it in the five flat-bottom flasks with 1-liter capacity and added filtered seawater to the flask, and the necessary nutrients to the cultured Chlorella such as solution A with KNO3 (nitrogen is basically important to the culture), solution B with NaHPO4 (for phosphorus) and ferric chloride (FeCl3). No vitamins were added to the culture.
With the Chlorella starter, we managed to produce more Chlorella by monitoring them every other day, filtering and removing half of the culture and then placing the separated Chlorella in a rectangular tank to serve as starter for mass production. In these cultures, we add a commercial fertilizer such as 16–20–0 which is available, as the growing of food continues in process. We tried also culturing Chlorella in the round tank. We also used the commercial fertilizer and Tilapia species in the tank to help the water become green in colour. This was set up outside the laboratory with direct sun as light for plant growth. As we proceeded, we noticed that those cultured in flat bottom flask became colourless, so we set up another set of Chlorella culture. Some of the solutions in the bottles became colourless until all the cultures of Chlorella collapsed. I concluded that the culturing of phytoplankton should be properly taken cared of, properly maintained with suitable temperature, the laboratory equipment should be properly organized because it is very difficult to manage the phytoplankton culture as a little mistake means a lot.
Culture of Artemia salina
Another food organism that the school started experimenting on was the brine shrimp, Artemia. It came as a donation from the Japanese Government.
In order to produce 1 million of nauplii, we dehydrated 5 grams of brine shrimp egg (same as in SEAFDEC procedure). As we all know, the Artemia is a tiny animal of the same family as shrimp and prawns. This animal thrives in a very salty water or brine. This animal is commonly used as an excellent live food in hatcheries of shrimps, prawns and other species.
The dried eggs of brine shrimp are placed in vacuum sealed cans. The eggs are placed in seawater with aeration for 24–36 hours, so that the eggs will hatch into very small larvae and it is these larvae that are used as live food in aquaculture. The nauplii will grow out to full-grown adults in 1–3 weeks depending upon feeding condition. Males and females are easily distinguished because females produce offspring after copulation with a male. The offspring can either be in the form of cyst or hatch as free swimming nauplii which in turn can grow into adult. The food given is a mixture of yeast and Chlorella. In culturing this organism, there seems to be no problem.
Culture of Brachionus
Some of the rotifers that were cultured were gathered by means of plankton net from the sea near the school. We found out that there are few species of rotifers present in the sea near our school.
We also cultured Brachionus from Japan using the same procedure used in SEAFDEC. We started with one gram of dry cells of Brachionus. These were hydrated then put in a small aquarium with full aeration, until the cells hatched. The food given was Chlorella and yeast. From the one gram of Brachionus used at the start, there are plenty now in one circular tank and two plastic buckets. When the Chlorella collapses, the food given is yeast only.
SCS/GFO/81/CR-8e
THE ARTEMIA CULTURE PROJECT IN THE PHILIPPINES UNDER THE BUREAU OF
FISHERIES AND AQUATIC RESOURCES - FOOD AND AGRICULTURE ORGANIZATION/
UNITED NATIONS DEVELOPMENT PROGRAMME BRACKISHWATER AQUACULTURE
DEVELOPMENT AND TRAINING PROJECT
by
Erwin Pador1
1. INTRODUCTION
The Bureau of Fisheries and Aquatic Resources (BFAR) is a government agency responsible for the development, improvement, management and conservation of the country's fishery resources. In cooperation with the FAO/UNDP, the Bureau has an on-going Brackishwater Aquaculture Development and Training Project, of which Artemia culture is one of the activities.
The brine shrimp or Artemia salina, classified under Class Crustacea, Order Anostraca, Family Artemidae, is a small animal naturally occurring in salt lakes and brine ponds. Artemia is an excellent live food mainly used in Penaeus monodon hatcheries in the Philippines. It may also be used as supplemental food for Chanos chanos nurseries and other commercially important aquaculture species.
Artemia is presently being imported into the country at great cost such that only research institutions and wealthy farmers are familiar with it, using them in their hatcheries. A cheap local and constant supply of Artemia in the country is expected to strengthen existing aquaculture activities and facilitate introduction of new aquaculture ventures.
Artemia has been introduced in the Philippines, a few years back by Dr. Patrick Sorgeloos, a consultant on this species. No nationwide coordinating effort, however, has been made such that development of Artemia possibilities had been largely dependent on the individual enthusiasm and perseverance of the farmer involved.
It is therefore the aim of the Project to coordinate Artemia efforts, introduce local Artemia cyst production and provide continuous assistance to both the private and public sectors involved.
2. ACTIVITIES
2.1 Survey
Upon the arrival of an FAO Associate Expert on Artemia, a survey was conducted to ascertain the availability of suitable ponds for Artemia. Areas with suitable dry season such as Metro Manila, Cavite, Bulacan, Pangasinan, Negros, Cebu, Bohol and Iloilo were surveyed.
The selection of suitable ponds was made according to several factors, They are as follows:
Duration of dry season in the locality.
Source of high saline water and of fresh seawater.
Deep ponds (approximately 0.5–1 m deep).
Availability of food.
Willingness of farmer to undergo Artemia culture.
Selected ponds were in Pangasinan, Bohol, Negros Oriental, Negros Occidental and Iloilo. All selected ponds were privately-owned since none of the project's facilities have salt beds or evaporation ponds.
Artemia production was originally planned to be integrated with salt production in such a way that brine flows through the Artemia ponds and into the crystalization beds. In this way, no extra land would be needed and Artemia production would provide an additional income with almost no initial investment to the farmer. The farmers were, however, hesitant to engage in this activity for fear of their salt. The only way then was to work in farms offering available space which are independent or semiindependent from the salt-making process.
2.2 Pond preparation
After selecting a suitable pond, preparation procedures were undertaken. Shallow ponds were deepened. Water depth in the pond should be 30–40 cm or more to prevent too high temperature. The pond is drained and crackdried to eliminate predators. Gates are also screened to prevent the entrance of fish and other predators. Dikes are checked for any leakage or seepage. The pond bottom is leveled and compacted. High saline water is then taken in to a depth of 30–40 cm. This is to avoid the growth of lab-lab which could hamper cyst harvest. In the absence of high saline water, salinity of the pond could be increased by gradual evaporation which is time-consuming. The salinity in the pond should be 100–110 ppt and water depth of 30–40 cm before inoculation is maintained. If the water turbidity is 30 cm or more the pond is fertilized to produce a phytoplankton bloom (green water). Inorganic fertilizers, 16–20–0 and 46–0–0, at 50 kg/ha are applied to provide a high nitrogen base.
2.3 Inoculation
Artemia cysts are hatched in transparent jars (10–1 capacity) provided with vigorous aeration. Maximum hatching density is 7–8 g cysts per liter. Hatching is started in such a way that inoculation in ponds could be done during early morning or in the evening to avoid high temperature in the pond. Inoculation density in ponds is 40 nauplii per liter of pond water.
2.4 Management
A salinity of 100–110 ppt must be maintained for about 3 weeks by regular water intake. This would allow the inoculated nauplii to grow into adults and increase through ovoviviparous reproduction. After this, salinity may be increased by evaporation and maintaining sufficient water depth. Cyst production was observed at 122 ppt using the delos Santos strain in the Philippines. More cysts were produced at 130 ppt. Fertilization is done whenever turbidity reaches 30–40 cm. If needed, fertilizers are broadcast during water intake.
2.5 Cyst harvesting and processing
The cysts will usually concentrate in the corner of the pond due to wind action. They can be easily harvested by scooping them with finemeshed nets. The collected cysts are stored in a bucket with brine to separate them from sand and other heavy debris which sink to the bottom. The bucket should be stirred or aerated to improve separation of cyst and heavy debris. The floating cysts are scooped off, washed thoroughly and placed in a bucket of freshwater. The full cysts would sink into the bottom while empty cyst shells, feathers and other light debris will float. The full cysts are then siphoned and dehydrated in brine. This process is called the bi-phase floatation method which separates solutes in solution with different specific gravity. Before drying the cysts, they are washed with freshwater to remove salt. Washing is done as quickly as possible to prevent the initiation of metabolism.
3. CONCLUSIONS
Five of the six inoculated ponds produced Artemia biomass, with two ponds producing cysts. One pond did not produce any with the inoculation because of lack of high saline water to supply the pond thereby affecting temperature and food.
Inoculations in ponds were done very late in the dry season because of unexpected rainfall. Most salt farms were still in the process of evaporating their first batch of brine only by the middle of March.
It is hoped that by the next dry season, we could start early and obtain better results.
SCS/GFO/81/CR-8f
WORK ON THE GROWING OF FOOD ORGANISMS
FOR THE TACURONG FRESHWATER DEMONSTRATION FISH FARM AND NURSERY
IN REGION XII, PHILIPPINES
by
Leo B. Pamposa1
1. INTRODUCTION
Region XII, located in the central part of Mindanao, is composed of five provinces, namely: Sultan Kudarat, North Cotabato, Maguindanao, Lanao del Sur and Lanao del Norte. The inland waters (lakes, marshes, etc.) of the region abound with marine and freshwater species which when fully tapped will help enhance the food production programme of the region in particular and the country in general.
The Bureau of Fisheries and Aquatic Resources (BFAR), Region XII, is mandated to properly develop these bountiful natural resources to its maximum production with the end in view of promoting self-sufficiency in fish. The expanded fish production programme of the Bureau has launched and implemented different projects for this purpose.
The Bureau has two freshwater demonstration fish farms and one brackishwater aquaculture centre aimed at increasing fingerling production of tilapia and carp for dispersal and to properly train fish farm managers, extensionists and fish farmers on fish culture methodologies especially on the culture of growing food organisms for outdoor fish hatcheries.
Natural growing of food organisms such as plankton has been done under pond conditions.
Concerted efforts are needed to develop techniques for mass production of food organisms so as to ensure sufficient supply for fish larvae and fry in fish hatcheries.
2. GROWING OF FOOD ORGANISMS AT THE TACURONG FRESHWATER FISH FARM
The fish farm at present is undertaking three projects, namely: (a) fingerling production and dispersal, (b) rice paddy fish culture project, and (c) integrated fish farming (started July 1981). These projects, however, are dependent on food organisms in the pond.
2.1 Lab-lab and plankton culture
Pond preparation and maintenance of the nursery are necessary to grow natural food under outdoor conditions. First, the pond is drained and dried to eradicate extraneous species and hasten organic matter decomposition. Snails, mudfish and other extraneous species are eliminated by applying chemicals such as Thiodan (Organo-phosphate chemical) at the rate of one liter per ha.
The pond bottom is cultivated and leveled to make available nutrients from sub-surface soil and to attain complete drainage of the pond at harvest. After this process, the water is let into the pond to attain a depth of 3–5 cm. Inorganic fertilizers such as 45–0–0 and 16–20–0 are applied at the rate of 20 kg/ha and 15 kg/ha, respectively. The water is gradually increased to a depth of 12–15 cm so as to allow the growth of lab-lab and plankton in the nursery pond. Tilapia fish larvae and fry taken and collected from the breeding hapas and ponds are then stocked.
3. FEED OF CARP FRY
For carp culture, fry are fed with egg yolk mixed with powdered milk in the hatching tank one week after hatching. The mixed foods are placed on the feeding tray to avoid decomposition and pollution of the water. After three days the fry are transferred to the lab-lab nursery pond.
4. HAPA CULTURE (FRY TO POST FRY) OF TILAPIA NILOTICA AND T. MOSSAMBICA IN RELATION TO FOOD ORGANISMS IN THE POND
Hapa culture is the culture of Tilapia in a fine mesh enclosure or inverted mosquito net with a ratio of 2:6 (male to female) Tilapia breeders/ 1.5 cu. m. The net is mounted in the pond with a depth of 0.7–1 m and intensive growth of plankton. Before stocking, the pond is fertilized with 8 kg of chicken dung, 5 kg of 45–0–0 and 4 kg of 16–20–0/1000 m2. Food organisms growing in the pond pass through the mesh of the hapa and the fry utilize them as food.
Tilapia and carp fingerlings are reared in the pond with a depth of 1 m where growth of macro-algae like filamentous green algae, Hydrilla and Chara species (digman) thrive.
In some instances, organic fertilizers like rice stalks and ipil-ipil leaves are utilized as compost. These are stocked at the centre of the pond surrounded with fence so as not to scatter at a certain depth of 10–12 cm. After 7–10 days in the water, it decomposes and 16–20–0 fertilizer at the rate of 2 kg/.01 ha (1000 m2) is applied. Two days after application, a prominent yellow-greenish colour appears in the water as a sign of the presence of food organisms (lab-lab and phytoplankton). The water is increased to a depth of 15–20 cm and the fry and fingerlings are stocked.
Supplemental feeding with fine ricebran (darak) and pounded dried ipil-ipil leaves is also done at the rate of 3–5 percent of the fish total body weight. Application is maintained at the rate of 50–60 kg/ha.
5. GROWING FOOD ORGANISMS FOR THE RICE PADDY FISH CULTURE PROJECT
In the rice paddy fish culture project, tilapia and carp fingerlings are stocked following the same process in the growing of food organisms in pond. Fish is stocked after 7–10 days at the rate of one fingerlings per 2 m2. The water is maintained at a depth of 50–70 cm.
Integrated farming like fish-cum-chicken and fish-cum-duck culture is being done in the fish farm to enhance the availability of manure as organic fertilizer.
These projects are now implemented and fingerlings produced in the fish farm are dispersed to communal waters and fishponds throughout the region to enhance the food production programme of the country.
SCS/GFO/81/CR-9
THE GROWING OF FOOD ORGANISMS FOR FISH HATCHERIES IN SINGAPORE
by
Tan Kay Heok1
1. INTRODUCTION
Mass culture of food organisms for fish larvae has been a continuing work in the Primary Production Department (Singapore) from the onset of fish culture research studies. Freshwater Moina and Daphnia are cultured for feeding to carp larvae and aquarium fish. Artemia nauplii are hatched from imported cysts and fed to the giant freshwater prawn and marine shrimp. Diatoms are also cultured for marine shrimp larvae. Chlorella or Tetraselmis is used for feeding the rotifer, Brachionus plicatilis, which is then fed to the larvae of the grouper, Epinephelus tauvina.
With increasing importance of marine fish farming in the Republic, there is a need to develop a technique for the mass production of fish fingerlings to meet the demand by farmers for fish seeds. Hence, there is also a concerted effort by the department to develop techniques for the mass production of organisms used as food by fish larvae so as to ensure sufficient quantity and types for the larvae at different developmental stages. This work is expected to be a continuing one.
2. CULTURE OF PHYTOPLANKTON
2.1 Maintenance of axenic stocks
Axenic stocks of all the phytoplankton species are maintained in 100-ml flasks, each containing 60–70 ml of Sato's medium. To avoid contamination of the stock, only sterilized seawater is used in preparing the culture medium. The axenic stock is maintained with minimum handling. No aeration is supplied to the flask in order to keep the growth rate of the stock at a minimum.
1 Assistant Primary Production Officer, Republic of Singapore
2.2 Batch culture
To initiate batch culture, a portion of the axenic stock is first inoculated into 500-ml sterilized glass bottles, each containing about 350 ml culture medium. The bottles are arranged in inverted positions and strong aeration is provided from the mouth of each bottle. Continuous illumination is provided by a fluorescent lamp above these bottles. The phytoplankton in these bottles are allowed to grow for 6–10 days after which sub-culture is carried out to 2.5-liter glass bottles followed by 25-liter carboys. The stock in the carboy is used as starter stock for mass culture.
2.3 Mass culture
2.3.1 Tetraselmis sp. and marine Chlorella
The fertilizer used in the mass culture of Tetraselmis and marine Chlorella are as follows:
| Ammonium | - | 100 g/m3 |
| Urea | - | 10 g/m3 |
| Superphosphate | - | 30 g/m3 |
For mass culture, the starter stock from a carboy is first allowed to thrive in 0.5-m3 or 1-m3 fiberglass tanks containing seawater enriched with the above fertilizers. The phytoplankton in these tanks will thrive within 1–2 weeks and by then, the whole content is introduced into a 8 m3 or 10 m3 tank for mass culture. The phytoplankton in these mass culture tanks are ready for use in rotifer cultures and fish larval rearing when the density reaches 10–15 × 106 cells/ml which can normally be attained within 1–2 weeks after inoculation.
2.3.2 Diatoms
The diatoms being cultured are Chaetoceros sp. and Skeletonema costatum. The fertilizers used and their proportions are given below:
| KNO3 | - | 300 g/m3 |
| NaHPO4 | - | 30 g/m3 |
| Na2CiO3 | - | 15 g/m3 |
| Clewat 32 | - | 30 g/m3 |
The culture procedures for diatoms are similar to those for Tetraselmis sp. and Chlorella. The diatoms can be fed to marine shrimp larvae one week after inoculation and two-thirds of the water is replaced daily with fresh seawater and re-enriched with the above fertilizers.
3. CULTURE OF ZOOPLANKTON
3.1 Rotifer (Brachionus plicatilis)
Rotifers are cultured in 5 m3 concrete tanks. The tanks are first filled with 2 m3 of green water of either Tetraselmis sp. or marine Chlorella and then rotifers are inoculated at densities of 5–10/ml on the same day. One m3 of green water together with Baker's yeast are added to the tank daily. The rotifers can be harvested on the 4th day and densities of 50–80 rotifers/ml are obtained.
3.2 Copepods and Diaphanosoma sp.
Copepods (mix species) and Diaphanosoma (cladoceran) are cultured in 1 m3 fiberglas tanks. At the beginning, Chlorella sp. is allowed to thrive in seawater enriched with chicken manure at 400 g/m3 in the tank. Stocks of copepods and Diaphanosoma are introduced on day 3. Baker's yeast is given at 1 g/m3 daily as food. The organisms are harvested from day 8 onwards with a 500–800 μm plankton netting and half the volume of culture water is replaced and re-enriched after each harvesting.
3.3 Artemia
The rearing of Artemia nauplii to adults is done in either 1-m3 fiberglass or 5-m3 concrete tanks. Newly-hatched Artemia nauplii are used and fed with phytoplankton such as Tetraselmis or Chlorella. About half the volume of water in the culture tank is replaced daily with seawater containing the phytoplankton. Baker's yeast is added incidentally as supplement food for the Artemia. Harvesting starts from day 5 onwards during which the Artemia reaches the size of about 8–10 mm in total body length.
4. MOINA AND DAPHNIA CULTURE
In Singapore, there are several commercial Moina ponds, ranging in size from 0.2–0.4 ha. During the culture period, the ponds are continuously enriched with farm effluent. Not more than 25 percent of the total volume of the pond water is being replaced by new effluent daily.
For small-scale culture, animal manure is added directly into the culture tank at 2.5 kg for every m3 of water. The enrichment process takes about 3 days after which Moina is inoculated into the tank. Harvesting commences 8–10 days later and lasts for a week. A similar method is used for culture of Daphnia but only half the amount of animal manure is used for water enrichment. With proper management of the culture tank, the harvesting period of Daphnia can be extended to one month.
SCS/GFO/81/CR-10
STATUS OF PROGRAMME ON GROWING FOOD ORGANISMS
FOR FISH HATCHERIES IN SRI LANKA
by
P.M.A. Jayasuriya1
1. INTRODUCTION
The Ministry of Fisheries of Sri Lanka has decided to establish a National Aquatic Resources Agency (NARA) which will have eight major research divisions under it. The NARA will be the principal national institution charged with the responsibility of carrying out and coordinating research development and management activities on the subject of aquatic resources. The agency would be a body corporate with autonomous status.
The principal objects and functions of the agency would be firstly, to ensure the full application and utilization of scientific and technological expertise for the implementation of the national development programme on the subject of aquatic resources. Secondly, to promote and conduct research activities directed towards the identification, assessment, management and development of aquatic resources. NARA will be primarily engaged in activities relating to the following fields:
Very little work has been done on the growing of live food organisms in Sri Lanka. Preliminary work was initiated recently and the suitability of certain diatoms as feed was examined.
1 Research Officer, Ministry of Fisheries, Colombo, Sri Lanka
2. FUTURE PLANS
One of the main projects envisaged by NARA is an establishment of a hatchery for penaeid shrimp and Macrobrachium rosenbergii. A thorough knowledge of the feeding techniques for various stages of larval forms as well as adults is required for the establishment and operation of a hatchery. Feeding techniques would be done using phytoplankton such as Chaetoceros and Skeletonema. Culturing diatoms should be started under laboratory condition. So there should be basic laboratory facilities such as chemicals for the media, growing tanks, air pumps and filters. Light and temperature-controlled rooms and some equipment such as autoclaves, balances, refrigerators and microscopes will be needed for the culturing work.
At present, technical expertise in this field is not available in Sri Lanka and I am confident that this programme of training will be very useful for me to contribute actively to the development of hatcheries in Sri Lanka.
SCS/GFO/81/CR-11
BRACKISHWATER AQUACULTURE AND GROWING FOOD ORGANISMS FOR
FISH AND SHRIMP HATCHERIES IN THAILAND
by
Niphond Haemaprasit1
1. INTRODUCTION
Fish constitute an important and cheap source of animal protein in the diet of the people of the world including Thailand. Presently, many countries have developed their economic zone to a 200-mile limit. It is estimated that approximately 250 000–600 000 less metric tons or a decrease of 15–39 percent in marine fish products will be harvested annually as a result of the establishment of such limit. As a result, the Department of Fisheries, Brackishwater Fisheries Division has already paid more attention to increase the activity of coastal aquaculture. In this context, the growing of food organisms for feeding the fish and shrimp larvae is of importance to Thailand.
2. PRESENT STATUS OF BRACKISHWATER AQUACULTURE IN THAILAND
The development of aquaculture and fisheries along the coastal zone, in the mangrove areas and on mudflats is being emphasized. This is in order to compensate for the decrease in marine products, resulting from over-exploitation of demersal species and from the changes in fishing operation following the declaration of the extended economic zone by neighbouring maritime nations. The Brackishwater Fisheries Division of the Department of Fisheries of Thailand is fully aware of this situation and is preparing to solve any technical problems including cultural techniques by carrying out the following programmes described below.
1 Senior Fisheries Biologist, Department of Fisheries, Thailand
2.1 Shrimp culture
Thailand started its shrimp culture in 1970, with the purpose of developing efficient techniques for commercial-scale. Since then, the rearing techniques have improved greatly and there are eight brackishwater fisheries stations of which four have succeeded with experiments on seed production in the large commercial-scale since 1974 and four have carried out experiments on upgrading the production and pond management of fish and shrimp. Lately, success with the seed production of Penaeus monodon, P. merguiensis, P. semisulcatus and P. indicus has been achieved.
The production of shrimp juvenile stages is done under intensive culture conditions and growth in the pond is enhanced by feeding with trash fish and compounded diets. The yield with such management yields 940–1 880 kg/ha/crop, compared to 315 kg/ha/year by the extensive method.
2.2 Finfish culture
There are many suitable marine or brackishwater fishes such as seabass (Lates calcarifer), groupers (Epinephelus sp.), sea catfish (Plotosus caneus), mullet (Mugil sp.), milkfish (Chanos chanos), etc. At present, Thailand has succeeded with the artificial breeding of seabass since 1976 and many millions of the juvenile not only support fish farms in Thailand but are also exported to Taiwan, Hong Kong, Malaysia and Singapore. The production of seabass in earthen ponds is 2 500 kg/ha/6 months and 1 875–3 125 kg/100 m2/year for cages. The other fish culture system in Thailand being carried out utilizing grouper just started in 1980 and we hope it will succeed like the seabass in the next few years.
2.3 Shellfish culture
The shellfish culture in Thailand covers the areas mostly on the mudflat and intertidal areas of the Gulf of Thailand and Andaman Sea. The common shell fisheries include the production of sea mussel (Mytilus smaragdinus), oyster (Crassostrea lugubris, C. commercialis), ark shell (Anadara granosa) and short-neck clam (Paphia sp.). Shellfish artificial culture in Thailand is also carried out by the Aquaculture Department of the Chulalongkorn University and Kasetsart University.
3. THE DEVELOPMENT OF GROWING FOOD ORGANISM FOR BRACKISHWATER AQUACULTURE IN THAILAND
The growing of food organisms for fish and shrimp culture started in 1971. The first culture was with Chaetoceros calcitrans, C. ceratopora, Skeletonema costatum and Cyclotella sp. for shrimp larvae. The deep interest in the growing of food organisms was started by the success in seabass culture which used a lot of Chlorella sp. and rotifers for the hatching of seabass larvae. Many kinds of phytoplankton and zooplankton were also used for shrimp and fish culture (Table 1).
Table 1. Food organisms for fish and shrimp hatcheries
| Organism | Organism fed | Stage of larvae being fed |
| PHYTOPLANKTON | ||
| Chaetoceros sp. | Shrimp | Nauplius, Zoea |
| Chlorella sp. | Rotifer | - |
| Tetraselmis sp. | Shrimp rotifer | - |
| ZOOPLANKTON | ||
| Rotifer | Shrimp | Mysis to postlarvae |
| Fish | After yolk sac is absorbed | |
| Artemia | Shrimp | Postlarvae |
| Fish | Fry |
At present, the Department of Fisheries has five stations involved in work on growing food organisms for fish hatcheries. These include: (i) Marine Laboratory in Bangkok; (ii) Songkhla Fisheries Station (National Institute of Coastal Aquaculture); (iii) Satul Fisheries Station; (iv) Phuket Fisheries Station; and (v) Rayong Fisheries Station. All these stations are on the isolation of local species of food organisms and on improving the techniques of mass production of plankton. Some 13 pure culture or stocks of diatoms (Table 2) have been isolated at the Marine Laboratory in Bangkok.
Table 2. Stock of the unicellular algae and diatoms under culture in Marine Laboratory at Bangkok
| Name of alga | Size (u) | Colour | Movement |
| Chlorella sp.1 | 3 | Green | - |
| Chlorella sp.2 | 8–10 | Green | - |
| Chlorella sp.3 | 1–2 | Green | - |
| Chaetoceros calcitrans | 3–2 | Brown | - |
| Skeletonema costatum | 3 + 5 | Brown | - |
| Dunaliella tertiolecta | a + 6 | Green | + |
| Tetraselmis sp. | 5 + 7 + 13 | Green | + |
| Platemonas sp. | 20 + 25 | Green | + |
| Chlamydomonas sp.1 | 15 + 25 | Green | + |
| Chlamydomonas sp.2 | 20 + 25 | Green | + |
| Isochrysis sp. | 5 – 7 | Brown | + |
| Thalassiosira sp. | 20 + 10 | Brown | - |
| Cyclotella sp. | 15 + (20 – 30) | Brown | - |
SCS/GFO/81/CR-12a
SOME RESULTS ON THE CULTURE OF CHLORELLA IN VIETNAM
by
Le Vien Chi1
ABSTRACT
Experiments on the culture of Chlorella, identified as C. pyrenoidosa, in controlled conditions under various environments were undertaken. Various standard algal culture media, i.e., Detmer, Molish, Leningrad, and Tamiya were tried. Organic fertilization experiments using different dosages of chicken manure and mixed inorganic and organic fertilization were also tried. The effect of different salinity levels on the culture was also tested. Preliminary results were obtained and preliminary conclusions and recommendations on the possibility of these results were given in the full paper.
1 Fishery Biologist, Institute of Agriculture Research, Haiphong City, Vietnam
NOTE: Due to editorial difficulties, it is regretted that the full paper cannot be printed here.
SCS/GFO/81/CR-12b
SOME RESULTS OF SCENEDESMUS CULTURE IN THE LABORATORY
AT THE NHATRANG FISHERIES UNIVERSITY
NHATRANG PHUKHANH PROVINCE, VIETNAM
by
Vu Thi Tam1
1. INTRODUCTION
Phytoplankton are a necessary part of the diet of many aquatic organisms. Many algal species have been used as food organisms in the culture of fish and shrimps.
In Vietnam, green algae have been researched on particularly Chlorella and Spirulina which are cultured in universities and stations for the rearing of fish and shrimp. Experiments on Scenedesmus culture have been carried out in the laboratory at the Nhatrang Fisheries University. Two problems have been solved, namely:
Isolating Scenedesmus from mixed samples.
Selecting a suitable culture medium.
2. ISOLATING SCENEDESMUS
Scenedesmus was collected from a pond of freshwater where Scenedesmus was present. Two methods were used for isolating the alga. These are described below.
2.1 Capillary pipette method
A Petri dish was used as an isolation dish. Some 20–30 drops of pond water were placed on the dish. A sterile capillary pipette was used for transferring Scenedesmus from the Petri dish into another sterile one, containing a suitable liquid medium. Scenedesmus was then transferred to a sterile bottle containing the liquid cultural medium.
1 Aquaculturist, Nha Trang Fisheries University, Socialist Republic of Vietnam
2.2 Isolation on agar
The agar Petri dish was prepared as solidified medium dish (2% agar). A drop of collected pond water was placed in the centre of the agar's surface. A flame-sterilized hook was streaked in a zigzag manner from the drop. The agar dish was then incubated for 3–6 days under suitable conditions for growth. After the incubation period, the Scenedesmus colonies were transferred to a sterile bottle containing a liquid medium.
2.3 Results
From the above isolation methods, we observed that the first method was better, because it was easier to work with and isolation time was shorter.
3. SELECTING A SUITABLE CULTURE MEDIUM
3.1 Different media used
Scenedesmus was cultured in the following media:
Inorganic medium: Detmer
Natural water + pig fertilizer medium (with 10 g/l; 15 g/l and 20 g/l).
Urine medium (with concentrations: 1.0 percent, 1.5 percent, 2.0 percent, 2.5 percent, 3.0 percent and 3.5 percent).
The same culture conditions were given for each culture medium:
Initial density: 104 cells/ml
Temperature: 25–30°C
pH: 7–8
Light source: the bottles were placed near the window
3.2 Result
After 10–15 days of growing Scenedesmus in the three media, we observed that the urine medium with concentration of 1.5 percent, 2.0 percent and 2.5 percent, and natural water, pig fertilizer medium (15 g/l; 20 g/l) had better development of Scenedesmus than in the inorganic medium.
In short, a urine medium and the medium with natural water and pig fertilizer may be used for Scenedesmus culture. Both media are not only cheap but also readily available.
SCS/GFO/81/CR-12c
NOTES ON GROWING DIATOMS FOR FEEDING SHRIMP LARVAE AT THE
AQUACULTURE RESEARCH INSTITUTE IN VIETNAM
by
Vu Van Dung1
1. INTRODUCTION
Diatoms play an important part in the feeding of larvae of shrimp. There are many media for the culture of these algae. The remaining problem is that of culturing enough algae to feed large numbers of shrimp larvae. We have applied two methods for diatom culture which are described below.
2. METHODS OF DIATOM CULTURE
2.1 Monoculture method
Skeletonema costatum is taken from Doson beach by phytoplankton net and isolated by dilution method. Some chains of S. costatum are isolated from other species by sterilized micropipette. They are placed in sterilized balloons and placed under 40 w day light lamps. When strings of S. costatum are produced, we carry out the isolation again.
We have used the following media for culturing the algae: Fel-Schreiber, Erd-Schreiber, Alen-Miquel, simple medium.
Seawater
Seawater is taken from Doson bay and passed through the membrane filter then it is enriched with nutrient salts per medium named. The media are sterilized in autoclave at 115°C for about 30 minutes and then they are divided into the balloons of about 100 ml for each.
After inoculating the algal balloons, they are placed under the three 40 w daylight lamps or on a table by the window.
1 Aquaculturist, Institute of Aquacultural Research, Haiphong, Vietnam
2.2 Results
After three experiments, we obtained the following results as shown in Table 1.
Table 1. The growth of Skeletonema costatum in the different media
| Culture period (days) | Results (cell/ml × 100) | |||
| Fel Schreiber | Erd Schreiber | Alen Miquel | Control | |
| 1 | 0.65 | 4.9 | 4.6 | 4.0 |
| 2 | 1.0 | 5.0 | 4.2 | 3.2 |
| 3 | 1.1 | 4.7 | 4.0 | 3.0 |
The Erd Schreiber is the best one for development of S. costatum. The dense growth of S. costatum usually appears within 3–4 days with inoculative number that is about 50 000 or 80 000 cells per ml.
The composition of the Erd Schreiber medium is as follows:
| KNO3 | 200 mg |
| K2HPO4.H2O | 10 mg |
| K2SiO3 | 1 mg |
| FeCl3 | 0.005 mg |
| Seawater | 1 000 ml |
2.3 Mix culture
Seawater is strained through a zooplankton net to separate the zooplankton. Then it is enriched with nutrient salt. The additives that are used to enrich seawater are KNO3, Na2HPO4, Na2SiO3 with proportion of 10:1:1 and concentration of 1–2 ppm.
The combination of algae is taken from Doson bay and passed through a zooplankton net to separate zooplankton and then added into the tank.
The tanks used consist of two 4.0 m3 tanks and one 200 m3 tank. The solution of algae is aerated by means of an airstone and compressor. The salinity is about 25–30 ppt and the temperature fluctuates from 20–26°C.
2.4 Result
The bloom of algae usually appears within 3–4 days. The average density of algae in the tank is about 3–5 × 104 cells per ml. The species of algae that usually become dominant is Skeletonema costatum and the second is Chaetoceros. The culture changes in colour from white yellow to dark yellow and we do not stock diatom for a long time. Because composition of seawater varies during the years and the season, it is difficult to determine which additives support the best growth of algae. Also, the species of alga that becomes dominant is not always suitable as food for the larval shrimp.
We carried out successfully in the following way the culturing of algae to feed larval shrimp:
Use Tank No. 1 for stock culture.
We alternate cultures, starting with a new one every 4–5 days to supply algae for Tank Nos. 2 or 3.
We use Tank No. 2 to culture diatom actively and it will supply algae for Tank No. 3 when it has not enough algae to feed the larvae shrimp.
Tank No. 3 serves as nursery tank.
It is used to rear the larval shrimp and it is enriched by nutrient salt.
All the tanks are in the open air.