| BUILDING | qt | price | total | subtotal (total) |
| 1. Hatchery 800 m2 | 1 | 45/m2 | 36 000 | 234 000 |
| 2. Office/lab 400 m2 | 1 | 80/m2 | 32 000 | |
| 3. Staff housing (units) | 8 | 20 000 | 160 000 | |
| 4. Machinery shed | 2 | 3 000 | 6000 | |
| Subtotal | ||||
| ELECTRICAL FACILITIES | ||||
| 5. Electric generator 50 KVA | 2 | 20 000 | 40 000 | 101 000 |
| 6. Diesel motor 80 Hp | 2 | 28 000 | 56 000 | |
| 7. Control cabinet | 1 | 3 000 | 3 000 | |
| 8. Fuel tank | 1 | 600 | 600 | |
| 9. Electrical wiring installation | 1 | 1 000 | 1 000 | |
| 10. Flourescent lamp | 40 | 10 | 400 | |
| Subtotal | ||||
| AIR SUPPLY SYSTEM | ||||
| 11. Roots air blower 800 m3/h, 4 bars | 1 | 4 800 | 4 800 | 12 100 |
| 12. Electric motor 1 500 rpm, 15 KW | 1 | 2 600 | 2 600 | |
| 13. Air-off warning system | 1 | 200 | 200 | |
| 14. Cylindrical air blower with electric motor | 3 | 1 500 | 4 500 | |
| Subtotal | ||||
| WATER SUPPLY | ||||
| 15. Seawater shallow well | 1 | 2 000 | 2 000 | 41 000 |
| 16. Pump with electric motor 50 m3/min | 2 | 12 000 | 12000 | |
| 17. Deep freshwater well | 1 | 12 000 | 12 000 | |
| 18. Pump with 5.5 K W motor 25 m3/min (95 gal/min) | 2 | 6 500 | 13 000 | |
| Subtotal | ||||
| PLUMBING, PIPES AND HOSES | ||||
| 19. PVC 4" | 100m | 8.70/m | 870 | 16 330 |
| 20. PVC 2.5" | 800 m | 5.70/m | 4 560 | |
| 21. PVC .6" | 800m | 2.25/m | 1 800 | |
| 22. PVC .1" | 20m | 1.50/m | 30 | |
| 23. Flexible hose 2.5" | 100m | 11.40/m | 1 140 | |
| 24. Flexible hose 1.5" | 400m | 8.40/m | 3 360 | |
| 25. Flexible hose.6" | 200m | 4.65/m | 930 | |
| 26. Faucets | 160 | 4.50/pc | 720 | |
| 27. Valves, large | 20 | 18 | 360 | |
| 28. Valves, small | 10 | 9 | 90 | |
| 29. Couplings and joints | - | 400 | 400 | |
| 30. Clear hose.1"(air lines) | 100kg | 18 | 1 800 | |
| 31. Epoxy sealing paste | 5kg | 40 | 200 | |
| 32. PVC sealing paste | 10kg | 5 | 50 | |
| 33. Burner (for PVC sealing) | 1 | 20 | 20 | |
| Subtotal | ||||
| FILTER SYSTEMS | ||||
| 34. Mechanical filter | 1 | 400 | 400 | 13 400 |
| 35. Ultraviolet light filter 2401/min (64 gal/min) | - | - | 9 000 | |
| 36. Ultraviolet light filter 60 1/min (16 gal/min) | - | - | 4 000 | |
| Subtotal | ||||
| TANKS AND ITS ACCESSORY | ||||
| 37. Storage tank (100 ton) | 3 | 1 800 | 5 400 | 37 700 |
| 38. Settling or small tank (50 ton) | 4 | 700 | 2 800 | |
| 39. Algae/tank (1 ton) | 20 | 200 | 4 000 | |
| 40. Maturation tank (15 ton) | 4 | 1 000 | 4 000 | |
| 41. Broodstock holding (15 ton) | 1 | 1 000 | 4 000 | |
| 42. Larval rearing tank (2 ton) | 20 | 250 | 5 000 | |
| 43. Spawning tank (1 ton) | 2 | 350 | 700 | |
| 44. Hatching tank (1.5 ton) | 2 | 200 | 400 | |
| 45. Plastic bowls 45 1 (12 gal) (for postlarvae transfer) | 30 | 15 | 450 | |
| 46. Hatching tank | 2 | 200 | 300 | |
| 47. Artemia hatching tank | 10 | 320 | 3 200 | |
| 48. Epoxy paint (clear) | 150 kg | 20 | 3 000 | |
| 49. Lead (for weights) | - | 50 | 50 | |
| 50. Airstones (carborundum) | 200 | 1 | 200 | |
| 51. Net. nylon, 20 mesh | 100 m2 | 5/m2 | 500 | |
| 52. Net. nylon, 150 mesh | 200 m2 | 15/m2 | 3 000 | |
| 53. Net. nylon, 300 mesh | 20 m2 | 30/m2 | 600 | |
| 54. Net. nylon, 1" eye | 50 m2 | 2/m2 | 100 | |
| Subtotal | ||||
| FEED PREPARATION EQUIPMENT | ||||
| 55. Centrifuge | 5 | 4 000 | 20 000 | 31 100 |
| 56. Mincer | 1 | 2 000 | 2 000 | |
| 57. Oven | 1 | 1 200 | 1 200 | |
| 58. Freezer | 2 | 1 100 | 2 200 | |
| 59. Refrigerator | 2 | 1 000 | 2 000 | |
| 60. Blender | 2 | 120 | 240 | |
| 61. Set of sieves | 1 | 400 | 400 | |
| 62. Kitchen grinder | 1 | 60 | 60 | |
| 63. Table and chairs (sets) | 2 | 1 500 | 3 000 | |
| Subtotal | ||||
| UTILITIES | ||||
| 64. Scrubber | 20 | 1 000 | ||
| 65. Cleaning brushes | 20 | |||
| 66. Steel brushes | 10 | |||
| 67. Scraper (metal blades) | 10 | |||
| 68. Buckets 20 1 | 15 | |||
| 69. Tools, set | 1 | |||
| 70. Cooking utensils (burner, dishes etc.) | ||||
| Subtotal | ||||
| LABORATORY EQUIPMENT | ||||
| Instruments | 16 640 | |||
| 71. pH meter | 2 | 700 | 1 400 | |
| 72. DO meter | 2 | 900 | 1 800 | |
| 73. Refractometer | 3 | 1 500 | 4 500 | |
| 74. Photometer | 1 | 250 | 250 | |
| 75. Macroscale | 2 | 50 | 100 | |
| 76. Microscale | 1 | 1 600 | 1 600 | |
| 77. Kitchen scale | 2 | 20 | 40 | |
| 78. Microscope | 2 | 1 200 | 2 400 | |
| 79. Dissecting microscope | 1 | 800 | 800 | |
| 80. Autoclave | 2 | 1 200 | 2 400 | |
| 81. Oven | 1 | 700 | 700 | |
| 82. Burner | 3 | 20 | 60 | |
| 83. Underwater lamp | 3 | 60 | 180 | |
| 84. Thermometer | 10 | 5 | 50 | |
| Subtotal | ||||
| GLASSWARE | ||||
| 85. Slides (microscope), box | 1 | 250 | ||
| 86. Cover slides (microscope) | 3 | |||
| 87. Beaker 50 ml | 10 | |||
| 88. Beaker 100 ml | 20 | |||
| 89. Graduated cylinder 1 000 ml | 4 | |||
| 90. Pipette 1 ml | 15 | |||
| 91. Pipette 5 ml | 15 | |||
| Subtotal | ||||
| 92. Chemicals and chemical kits | 500 | |||
| FURNITURE | ||||
| 93. Wooden racks | 1 500 | |||
| 94. Cabinets | ||||
| 95. Tables and chairs | ||||
| 96. Sink | ||||
| Subtotal | ||||
| VEHICLES | ||||
| 97. Pick-up truck | 2 | 20 000 | ||
| 98. Motor bike | 2 | |||
| Subtotal | ||||
| MISCELLANEOUS AND OTHER UNFORESEEN ITEMS | 10 680 | |||
| TOTAL FIXED ESTIMATED INVESTMENT | 534 950 | |||
| Year | 0 | 1 | 2 | 3 | 4 | |
| salary M$/month | ||||||
| Management | 2 000 | - | 1 | 1 | 1 | 1 |
| Scientific staff | 1 500 | - | 1 | 2 | 2 | 2 |
| Engineering staff | ||||||
| Engineer | 1 500 | - | - | 1 | 1 | 1 |
| Mechanic | 800 | 1 | 1 | 1 | 1 | 1 |
| Office staff | ||||||
| Bookkeeper | 800 | - | 1 | 1 | 1 | 1 |
| Clerk | 300 | 1 | 1 | 1 | 2 | 2 |
| Secretary | 300 | - | 1 | 1 | 1 | 2 |
| General staff | ||||||
| Driver | 400 | 1 | 1 | 1 | 1 | 1 |
| Labourer | 200 | 6 | 6 | 6 | 6 | 6 |
| Year | 0 | 1 | 2 | 3 |
| Management | - | 24 000 | 26 000 | 29 000 |
| Scientific staff | - | 18 000 | 37 800 | 41 500 |
| Engineering staff | 9 600 | 10 500 | 28 500 | 31 400 |
| Office staff | 3 600 | 12 400 | 18 100 | 17 000 |
| General staff | 16 800 | 18 500 | 20 400 | 22 400 |
| Total | 30 000 | 83 400 | 131 200 | 143 300 |
| Year | 0 | 1 | 2 | 3 |
| Electricity | - | 3 000 | 3 000 | 3 000 |
| Vehicles | 1 500 | 1 500 | 1 200 | 1 200 |
| Total | 1 500 | 4 500 | 4 200 | 4 200 |
A. Feed cost for spawners
Assuming a survival rate of 40 percent from nauplii to postlarvae PL 25, 2.5 × 106 nauplii are needed to produce 106 PL 25. If the average spawner hatches 200 000 nauplii, then 13 spawners are needed. To obtain 13 spawners from the maturation tank, the cost to feed them and the non-spawners, e.g. males, is estimated to be M$50.
B. Feed cost for larvae at zoea stage
The cost of chemicals to produce diatoms to feed 2 × 106 larvae, i.e. to obtain 106 postlarvae, and Chlorella for Brachionus is taken to be M$50.
C. Feed cost for larvae from M1 to postlarvae PL 25.
Brine shrimp cysts requirements (See section 4.3)
1) 1240 g cyst/ton water for postlarvae M1 — M3,
PL 1-PL 5, density
= 50 pcs/l
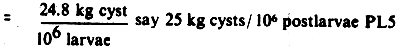
2) 350 g cyst/ton for PL 6-PL 5, density = 50 pcs/l
= 7 kg cyst/106 PL 10
Total requirement per million postlarvae PL 15 is 32
kg, or 32 kg × M$150/kg = M$4800
3) Feed requirement from postlarvae PL 15 to PL 25 The cost to grow one million postlarvae of 25 days old (PL 25) is estimated to be M$200.
TOTAL LARVAE FEED COST PER 106 PL 25 (M$)
| Feed cost of spawners | 50 |
| Nutrients for diatoms | 50 |
| Brine shrimp cysts | 4 800 |
| Postlarvae feed | 200 |
| Total | 5 100 |
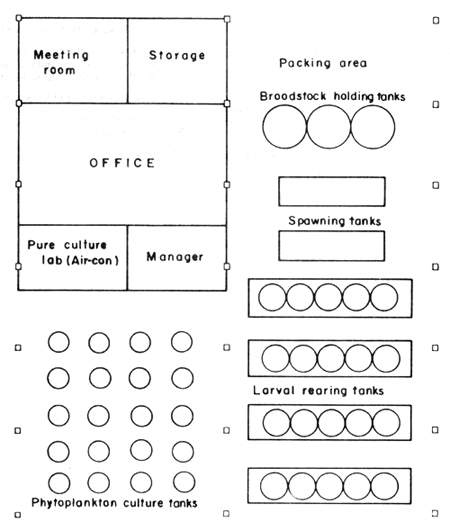
Fig. 1 Layout of the small-scale prawn hatchery
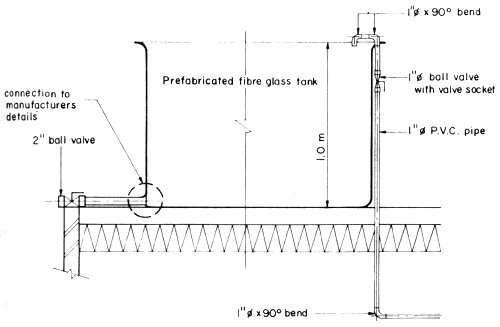
Fig. 2 Broodstock holding tank
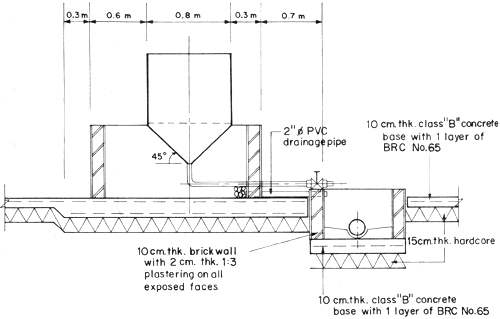
Fig. 3 Spawning tank
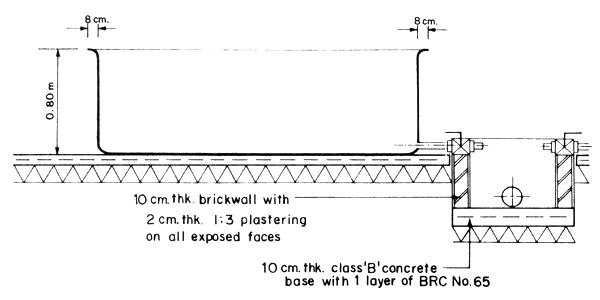
Fig. 4 Hatching tank
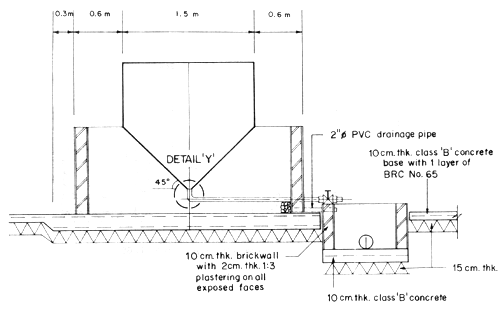
Fig. 5 Larval rearing tank
WP/81/SPH/CP-10
DEVELOPMENT OF BROODSTOCK FOR SMALL-SCALE SHRIMP HATCHERY
(WITH PARTICULAR REFERENCE TO PENAEUS MONODON)
by
J. H. Primavera1
1. INTRODUCTION
The culture of penaeid shrimps (Penaeus monodon, P. indicus, etc.) in brackishwater ponds is increasing, particularly in Southeast Asia. This is because of their importance as a cash crop, earning foreign exchange and a food crop for local markets. The two major inputs of culture are seed supply and feeds.
Shrimp seed or fry (postlarvae and juveniles) are obtained from the wild, or increasingly produced by hatcheries, both government and commercial. Dependent mainly on spawner supply from the wild, these hatcheries face the following problems:
Solutions to the problems associated with wild spawner supply may be the following:
2. BROODSTOCK TECHNOLOGY
2.1 Broodstock systems (Fig. 1)
2.1.1 Tanks
Land-based tanks have a total water volume ranging from 5 to 20 m3. The generally smaller sizes offer two advantages: easy retrieval of broodstock and maintenance of water quality by regular siphoning of debris, etc. Water may be flowthrough, recirculating or with regular (daily, twice weekly, etc.) renewal. Construction materials include cement, ferrocement, fiberglass and others.
The SEAFDEC AQD prototype located in Tigbauan, Iloilo is a 12 m3 ferrocement tank with flow-through seawater (Fig. 2). From 30 ablated females and 20 males/tank, average production is 2 × 106 nauplii/run or 8 x 106 nauplii/year (4 runs of 2 months/run). One broodstock tank unit can supply larvae for 20 m3 of rearing tank capacity (at a density of 50 nauplii/liter). Construction costs total P11 3202/tank and operating costs total P19 6202/tank/year. Tank depreciation is minimal.
A manual (Primavera, 1980a) on penaeid broodstock goes into the details of the reproductive biology (spawner and broodstock sources, sex differentiation, courtship and mating, ovarian maturation stages, spawning and rematuration, and fecundity and egg quality); broodstock technology (transport and acclimation, ablation and stocking, processing of spawners, eggs and nauplii, nutrition); and the land-based tank prototype for P. monodon.
Most existing penaeid broodstock use tanks for species including P. monodon, P. merguiensis, P. indicus, P. japonicus, P. stylirostris and P. vannamei in the Philippines, Indonesia, Thailand, French Polynesia, United States, France and Italy.
2.1.2 Pens
The major requirements for marine offshore pens are protection from wind and wave action and seawater free from industrial and agricultural pollution. Due to location, pen retrieval of broodstock for checking of maturation stages is less frequent than in tanks.
The SEAFDEC AQD pen prototype located in Batan, Aklan province is a 16 x 16 x 4m pen consisting of bamboo posts, braces, mattings and nylon nets (Fig. 3). From 200 ablated females and 100 males, the estimated production capability per year is 10 x 106 nauplii/ run or 30 × 106 nauplii/year (3 runs of 2 months/run). One pen unit can supply larvae for 100 m3 of rearing tank capacity (at 50 nauplii/liter). Construction costs total 11 870 pesos/pen and operating costs total 29 045 pesos/pen/year. With regular maintenance, projected life span of one pen is 3-5 years. Details of this prototype are discussed in previous papers (Primavera, 1980a; Primavera and Gabasa, 1981).
At present, the broodstock pen system is used only in the Philippines, probably due to its site-specific requirements.
2.1.3 Ponds
Ponds have two broodstock-related uses as:
The main drawback of ponds as a broodstock system is the difficulty of retrieval of the females. Seining of P. stylirostris from a clay bottom pond caused maturing females to resorb their ovaries (Conte, et al., 1977). Ablated and control P. monodon held in hapa nets in a brackishwater pond suffered high mortality after 3 months. Very poor maturation (up to stage 3 in ablated females, no development in unablated ones) may be because the animals were pond stock of 4-6 months at harvest (Section 2.2 below).
Alternatively, the pond may serve as a combined broodstock-hatchery-nursery-grow-out system where females mature and spawn, eggs hatch into larvae which grow into postlarvae, juveniles and, eventually market-size animals. In Ragay, Camarines Norte province, Philippines, large suahe (Metapenaeus sp) females are ablated and stocked together with males in a 10 m2 × 2 m deep earthen compartment. Plankton is made to bloom by fertilization and starting two months after ablation, juveniles are regularly scooped out and transferred to nearby rearing ponds. Income from the shrimp crop is 25 000 pesos/year from a 35-ha pond in addition to sales of milkfish, the primary species.
Some 2 500 P. merguiensis females stocked in an earthen pond yielded around 20 000 postlarvae seven weeks after stocking (Lichatowich, et al, 1978). Experimental work on the recently introduced P. japonicus in the extensive vallis of Italy has demonstrated that the whole life cycle, from induced maturation by ablation and spawning (inside a suspended hapa net) through larval and postlarval stages to growth to marketable size can take place in the same pond or valli (F. Lumare, personal communication).
2.2 Broodstock sources
Penaeid broodstock can be procured from either pond or wild sources. The former is preferable, however, because it represents a regular closing of the cycle and elimination of the problems of seasonality and expense attendant to a wild supply. This idea has been achieved for P. indicus — since the first batch of wild females was matured and spawned in captivity with the regular stocking of hatchery-reared fry in the SEAFEC Leganes ponds and retrieval of 2–3 month-old adults for maturation and larval rearing in Tigbauan (Primavera, et al, 1980).
Maturation in ablated wild and pond stock P. monodon can be compared as follows:
egg quality — proportion of good (A1) eggs is 38.87 percent for spawnings from wild stock and 23.50 percent from ablated pond stock of 4–6 months harvest age (Primavera and Posadas, 1981).
Hatching rate — an average of 30.43 percent for spawnings from ablated wild stock and 18.95 percent from ablated pond stock of 4–6 months (Primavera and Posadas, 1981).
The above data suggest that given the same size of at least 90 g, wild stock females are older and therefore more receptive to induced maturation than pond-reared females at normal harvest age of 4–6 months. Using pond stock, our only successful rearing up to the postlarvae have been from spawnings of females ablated at 1–2 years old (Primavera, 1980b).
In addition to the age requirement, other factors in pond broodstock include:
Nutrition — a preliminary study shows higher growth rates of P. monodon pond stock given brood-stock pellets (40 percent protein) compared to those fed tilapia or a combination of pellets and tilapia (Pudadera and Prospero, unpub.).
Stocking density — the same pond study shows P. monodon survival after 3 months of 92–100 percent at 1 000–2 000 juveniles/ha and 51–57 percent at 4 000– 16 000 juveniles/ha (Pudadera and Prospero, unpub.).
Water depth and management — a broodstock pond should be 1–2 m deep and with frequent water change for good water quality. A private shrimp hatchery-pond complex in Chonburi, Thailand sometimes uses approximately one year old female P. monodon collected from deep ponds for ablation purposes.
2.3 Induced maturation
Three basic techniques — eyestalk ablation, nutrition and manipulation of environmental parameters — are used singly or in combination to induce maturation in penaeids (Primavera, 1981). Ablation appears to be the only known method of inducing maturation in some penaeids such as P. monodon. Other species, however, such as P. indicus and P. merguiensis, mature in captivity with or without ablation.
From experience, it is better if ablated P. monodon broodstock are renewed every two months. It is observed that there seems to be a decreasing maturation related to an “exhaustion” of the reserves of the female with successive spawnings and decline in quantity and quality of sperm stored in the thelycum.
Diets of penaeid broodstock include:
Marine annelids based on data suggesting that certain polyunsaturated fatty acids (present in the annelids) are important in maturation. Both ablated and unablated P. setiferus fed a diet supplemented with annelids matured and spawned (Brown, et al, 1979).
In recent study, ablated P. monodon females produced an average of 2 million nauplii/tank (1 tank = 30 females) only 3 weeks after ablation when given a daily feeding of marine polychaetes. This is double the average production of 1 million nauplii/tank after 3 weeks (or 2 million nauplii tank after 6–8 weeks) on the standard feeding of pellets and brown mussel, Modiolus metcalfei (Primavera and Gabasa, 1981).
P. monodon broodstock is fed 60 percent protein pellets and or fresh troca Trochus niloticus described elsewhere (AQUACOP, 1979).
Environmental manipulation includes photo-and thermo-period and light quality experiments on P. japonicus, P. kerathurus and P. monodon.
3. CONCLUSIONS AND RECOMMENDATIONS
To be self-sufficient in spawner/larval supply, a small-scale P. monodon hatchery should have the following:
Alternatively, a number of small hatcheries in close proximity to each other may share of the same brood-stock tank or pen facilities. Either gravid females or larvae may be transported — the latter are more convenient because of the relative sturdiness of nauplii to handling (Primavera, 1981). P. monodon nauplii from Panay island, middle Philippines have been transported overland or by air to other islands with minimum mortality.
Also we must focus some attention on other species such as P. indicus and P. merguiensis that offer distinct advantages over P. monodon in terms of easier maturation and larval rearing, faster turnover rate in ponds, and suitability for local culinary tastes. In the Philippines, they would constitute a “food crop” in comparison to the higher-priced P. monodon.
REFERENCES
AQUACOP, 1979 Penaeid reared broodstock: closing the cycle of P. monodon, P. stylirostris and P. vannamei. Proc. World Mar. Soc., 10:445– 452.
Brown, 1979 A. Jr., et al, Maturation of white shrimp (Penaeus setiferus) in captivity, Proc. World Mar. Soc., 10:435–444.
Conte, 1977 F.S., et al, Maturation of Penaeus stylirostris (Stimpson) and P. setiferus (Linn.) in hypersaline water near Corpus Christi, Texas. Proc. World Mac. Soc., 8:327–334.
Lichatowich, T., 1978 et al, The natural reproduction of Penaeus merguiensis (De Man, 1888) in an earthen pond in Fiji. Aquaculture, 15:377-378.
Primavera, J.H., 1980a Broodstock of sugpo (Penaeus monodon) and other penaeid prawns. Extension Manual No. 7, SEAFDEC Aquaculture Dept., Iloilo, Philippines: 24 pp + 12 figs.
Primavera, J.H., 1980b Studies on broodstock of sugpo Penaeus monodon, Fabricius and other penaeids at the SEAFDEC Aquaculture Department. Intl. Symposium on Coastal Aquaculture, Cochin, India, 12–18 Jan. 1980: 24 pp + 7 tables.
Primavera, J.H., 1981 Seed production of penaeid prawns with particular reference to Penaeus monodon I. Broodstock. Doc. Ref. No. AQU-TRAIN/NACA/020. 51 pp + 5 figs, 2 tables.
Primavera, J.H. and P. Gabasa, Jr., 1981 A comparison of two prawn (Penaeus monodon) broodstock systems — land-based tanks and marine pens. World Conference on Aquaculture, Venice, Italy Sept. 21–25, 1981, 13 pp + 5 figs, 3 tables.
Primavera, 1981 J.H. and R.A. Posadas, Studies on the egg quality of Penaeus monodon Fabricius based on morphology and hatching rates. Aquaculture, 22:269–277.
Primavera, 1980 J.H., T. Young and C. de los Reyes, Survival, maturation, fecundity and hatching rates of unablated and ablated Penaeus indicus H. M. Edwards from brackishwater ponds. Intl. Symposium on Coastal Aquaculture, Cochin, India, Jan 12–18, 1980: 14 pp.
Pudadera, B.Jr., and O. Prospero. The effects of selected feedstuff on growth and survival of Penaeus monodon broodstock. (Unpublished data).
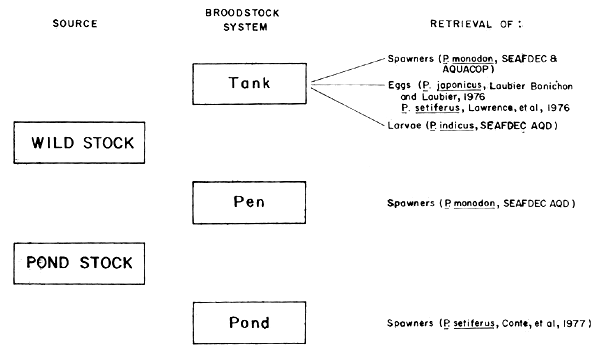
Fig. 1. Stocking and retrieval scheme in different penaeid broodstock systems (Primavera, 1981)
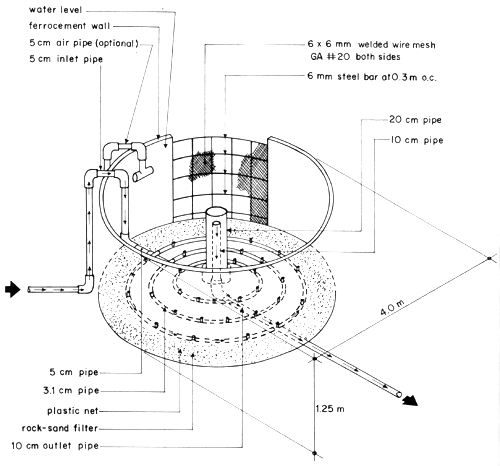
Fig. 2. SEAFDEC AQD prototype of 12 cu. m. ferrocement tank (4 × 1.25 m) for Penaeus monodon broodstock in Tigbauan, Iloilo. Arrows indicate direction of flowthrough water (Primavera, 1980a)
WP/81/SPH/CP-11
RECENT DEVELOPMENTS IN DESIGN AND MANAGEMENT OF SMALL-SCALE HATCHERY FOR PENAEUS MONODON IN THE PHILIPPINES
by
Porfirio G. Gabasa, Jr.1
ABSTRACT
It is a common belief that the zoea of Penaeus monodon are completely filter feeders. Thus, diatoms like Chaetoceros and dinoflagellates are maintained at high feeding densities as much as 80 000 cells/ml in hatchery tanks during the zoeal stage of the P. monodon. This feeding scheme often results in the reddening of the larvae followed by weakening, loss of appetite and eventual mass mortality.
It was found out recently that zoea larvae are not completely filter feeders. It was observed that as early as Zoea 1, the mouthparts of the larvae are already functional and can eat food particles as big as Artemia and Brachionus.
Based on this observation, a new feeding scheme was developed. Bioled egg yolk is fed to the larvae at 15–22 particles (as big as Brachionus) per ml from Zoea 2 to Mysis 3 stages. Tetraselmis is given from Zoea 1 to Mysis 3 stages at a low density level of 5 000 cells/ml. Artemia is also fed at 10–15 individuals/ml from Mysis 1 to Postlarvae 5. If Tetraselmis is not available, bread yeast is given from Zoea 1 to 3 at 0.1–0.3 g/ton as supplementary feed. With this new feeding scheme, the hatchery procedures have been greatly simplified considering that the most difficult and tedious part in larval rearing is the maintenance of algal food especially diatoms.
This feeding scheme was tested in a private hatchery in Batan. Aklan province by the SEAFDEC Aquaculture Department from July to October 1981. All 44 runs were successful, yielding survival rates ranging from 22 to 75 percent and an average rate of 52.9 percent.
The hatchery system was further simplified when experiments at the Batan Substation of the SEAFDEC AQD revealed that as high as 60 percent survival can be attained with minimal aeration. Instead of centralized aeration system using compressors or blowers, portable aquarium-type aerators (5-watt) could be used thus minimizing energy consumption.
Based on these developments, a new model for a small-scale hatchery system is proposed.
1. INTRODUCTION
The jumbo tiger shrimp/prawn (Penaeus monodon Fabricius), known in the Philippines as sugpo, has long been recognized as one of the most economically important shellfish commodities in the Philippines and in most countries in Southeast Asia. The demand for this highly priced shrimp has increased during the past many years but production has never come near to satisfying both domestic and export needs.
Some 10 years ago, P. monodon was not considered important for pond culture. And so, it became available only when a few of this species were caught incidentally from the open seas and sometimes in milkfish ponds. It did not take long, however, for the fishery sector to notice the high economic potential of the P. monodon. Soon many pond owners stocked P. monodon in addition to milkfish, the major fish being cultured in ponds. Later, some enterprising fish producers, having seen the high demand and price of P. monodon, ventured into monoculture of this species.
The growth of the sugpo industry in the Philippines, however, was hampered by a lot of problems, most notably the supply of fry. The quantity caught from the wild has never been enough — and predictably, will never be enough — to satisfy the requirements of a growing sugpo industry. The solution, therefore, was obvious-we have to develop a technology to artificially mass produce fry in controlled enclosures. In other words, a hatchery that can be easily adopted by the private sector. The desire to produce a viable hatchery technology for P. monodon, however, was met with lots of difficulties. For the past 8 years, researchers in the Philippines, particularly in the SEAFDEC Aquaculture Department, have been baffled by the erratic results of P. monodon hatchery. While hatchery techniques have been fairly successful in other penaeids, these same techniques when modified and tried for P. monodon failed to produce satisfactory results.
A technology, as we all know it, takes time to develop. Painstaking research at the SEAFDEC Aquaculture Department for the past 8 years proved this. There had been some failures, but the cummulative result of our efforts is now taking shape; the much needed technology for a small-scale shrimp hatchery has been considerably simplified and improved and is now ready for further verification. This paper describes how we came up with a new model for a small-scale hatchery for P. monodon.
2. EVOLUTION OF CULTURE TECHNIQUES
Two distinctly different methods have been successfully used in the mass production of certain postlarval shrimps: the fertilized or Japanese method and the unfertilized or Galveston method. The former was developed for Penaeus japonicus and was almost perfected over 30 years by Fujinaga and associates in Japan. It utilizes large concrete tanks (50–200 tons) and basically involves culturing the algal foods together with the cultured larvae in one tank. A natural bloom of mixed diatoms such as Melosira, Skeletonema, Nitzschia, and Rhizosolenia is induced and maintained by fertilizing daily the larval culture medium with nutrient salts starting from the nauplius stage up to early postlarval stage. The successful production of shrimp fry is primarily dependent on the skill of the technicians in maintaining algal bloom in the rearing tank through fertilization and water management. This method requires simple technique and equipment. However, productivity per unit volume is low.
The unfertilized or Galveston method was developed at the National Marine Fisheries Services Laboratory in Galveston, Texas, United States for larval rearing of P. setiferus and P. stylirostris in the late sixties. It utilizes smaller tanks (2-ton) with conical bottom. Specific species of algae are cultured in separate tanks and are fed either fresh or frozen in predetermined quantities. Larvae are stocked at high densities and are given the diatom Skeletonema and dinoflagellate Tetraselmis as food for the zoeal stage. Newly hatched brine shrimp nauplii are feed during the mysis and postlarval stages. Although productivity/unit volume is much higher than the Japanese method, it requires sophisticated techniques and equipment especially in the maintenance of uni-algal cultures.
The two culture methods have been tried in the Philippines for rearing P. monodon larvae. The Japanese method using 50-200 tons tanks for the culture of P. monodon larvae was tried at the Mindanao State University Marine Fisheries Research Laboratory in Naawan, Misamis Oriental province in 1973 and later at the SEAFDEC Aquaculture Department in Tigbauan. Iloilo, Philippines. Both attempts failed. Mass mortalities of the larvae especially during the zoeal stage were experienced due to the uncontrolled blooming and collapse of diatoms in the larval culture tanks. There was also difficulty in inducing and maintaining growth of diatoms especially during cloudy and rainy days.
In order to solve the problems encountered in using the Japanese method, some changes in the technique were instituted such as culturing pure species of diatoms in separate tanks, feeding concentrated diatoms, covering outdoor culture tanks with black cloth to control growth of diatom and culturing nauplius up to Mysis 1 stage in indoor tanks and transferring them in outdoor tanks until harvest. With these innovative procedures, the big hatcheries of Mindanao State University Marine Fisheries Research Laboratory in Naawan and SEAFDEC Aquaculture Department in Tigbauan, Iloilo attained some success in producing P. monodon fry. SEAFDEC, in particular, produced 8.215 million postlarvae in 1978. However, out of 52 runs only 30 were successful, obtaining an average survival rate of only 20.6 percent from nauplii to Postlarvae 5. Most of the discarded runs were characterized by reddening of the larvae and attacks of Lagenidium, Sirolpidium and Chitinoclastic bacteria.
By early 1978, the SEAFDEC AQD had established a hatchery technology for the mass production of P.monodon fry using the modified Japanese system in large tanks. Although still far from satisfactory, it nevertheless produced a good amount of fry and paved the way for the development of better hatchery techniques. The SEAFDEC AQD, however, realized that this kind of system requires huge investment and would therefore be difficult for the private sector to adopt. A system must be developed, one that is smaller in scale and easier to operate for the benefit of the private sector who should eventually produce the fry it needs.
So in that same year, a small-scale hatchery adopting basically the Galveston method and using 2-ton tanks with conical bottoms was established by the SEAFDEC AQD. In this system, diatoms were maintained at a high density of as much as 80 000 cells/ml in the tank. Table 1 shows the feeding scheme used in this system. Several trials were conducted at the SEAFDEC AQD and in two private hatcheries in northern Panay since then and until the early part of 1981. Most of the trials were failures, but because the system yielded some successful runs, the trials went on, hoping that a better technique will come out. Reddening of the larvae followed by weakening, loss of appetite and eventual mass mortality is a common occurrence in the hatchery runs. Even with the use of double filtration of seawater and by observing strict sanitary procedures, the results were still unsatisfactory. The continued failures in the hatchery runs prompted the researchers to look for new culture techniques that will yield not only high survival rates but consistent results.
3. NEW TECHNIQUES DEVELOPED AT SEAFDEC AQD
3.1 Feeds and feeding
It is a common belief among hatchery operators that zoea larvae are purely filter feeders. Thus, only microscopic organisms such as diatoms and dinoflagellates are given as food. It has been observed, however, that as early as Zoea 1 the mouth parts are already functional. Zoea 1 and Zoea 2 larvae have been observed grasping big food particles like Artemia and Brachionus, and placing them in their mouths. Microscopic examination of the gut of the larvae revealed the presence of parts of the food they have eaten.
Based on the above observations, a new feeding scheme was tested (Table 2). A major improvement over the old scheme is the use of boiled egg yolk as the main diet for Zoea 2 and Zoea 3 larvae instead of the diatoms Chaetoceros. It should be noted that egg yolk particles are as big as Brachionus. Egg yolk is given to Zoea 2 up to Mysis 3 three to four times daily to maintain the feeding level of about 15–25 particles/ml. Tetraselmis is still used as feed for zoea and mysis larvae but at reduced density level of about 5 000 cells/ml. It is believed that Tetraselmis also plays a vital role in the maintenance of good water quality by utilizing the metabolites present in the water. Artemia is given to mysis and early postlarval stage at higher feeding levels. Bread yeast can be given to zoea as supplementary feed at 0.1–0.3 g/ton in case Tetraselmis is not available.
The use of egg yolk greatly simplifies hatchery procedures considering that the most difficult and tedious part in larval rearing is the maintenance of algal food organisms especially diatoms. Egg yolk is easy to prepare and is readily available in big quantity even in remote areas.
Table 3 shows the results of hatchery runs using the old and new feeding schemes. All these runs were conducted at a private hatchery in close collaboration with the SEAFDEC AQD. Two-ton tanks with conical bottoms and made of fiberglass were used in all the experiments. Wild spawners (Stage III and IV) collected from fish corrals in Batan Bay were used.
The first experiments were conducted from June to September 1979. Of the total 31 runs, only 7 runs or 22.5 percent were successful. Average survival rate of the successful runs from nauplius to P5 was only 2.46 percent. Water temperature ranged from 27°C to 29°C throughout the hatchery run.
From July to October 1981, a total of 44 runs were conducted using the new feeding scheme. All the runs were successful with an average of 52.9 percent survival. Survival rate in the whole run ranged from 20–75 percent. Water temperature throughout the experiment ranged from 26.5°C to 29°C.
The low survival rate using the old feeding scheme could be attributed to the following:
Overfeeding with diatoms during zoeal stage — reddening of the larvae is a common observation when diatoms are fed in high densities. It is possible that diatoms produce metabolites which are toxic to P. monodon larvae. If even diatoms have to be fed, the density level that should be maintained in the tank should not be more than 3 000–5 000 cells/ml.
Underfeeding with Artemia and Brachionus during the mysis and postlarval stages.
3.2 Aeration system
In most hatcheries aeration is provided by a compressor or a Roots blower through PVC air supply line. This aeration system is centralized to aerate all the tanks, hence the same energy is consumed even if only one tank needs aeration. Moreover, when the aeration is contaminated with pathogenic organisms, the whole aeration system has to be cleaned and disinfected, a difficult and time-consuming job.
Based on the observation that survival rate as high as 60 percent can be attained with minimal aeration, it is suggested that electrically operated portable aquarium-type aerator (5-watt) be used. Such an aerator will reduce energy inputs for aeration. It is not only cheap and readily available locally, but can be maintained easily and without much cost. This kind of aerator can effectively aerate a 1-m deep, 2-ton tank.
4. PROPOSED SMALL-SCALE HATCHERY MODEL
Based on the recent findings and experiences at the SEAFDEC AQD, a new small-scale hatchery model for P. monodon has been drawn up. Figure 1 shows the layout of the proposed model. The specifications for each of the components are described below.
4.1 Larval rearing tanks
The size and shape of the larval rearing tanks will not significantly affect the survival of the shrimp larvae. In deciding the tanks' size and shape, due consideration should be given to the ease in construction, and in changing water and harvest of fry. Fig. 2 and 3 show the details of the proposed larval rearing tank. It is 2-ton in volume, bathtub in shape to prevent accumulation of organic detritus at corners. It is shallow, about 0.8 m deep only, thus requiring very minimal aeration. The tanks could be made either of concrete, wooden material or fiberglass. Concrete tanks, however, are more durable, easy and cheaper to construct. Changing of water can easily be done by using a rubber siphon with strainer to prevent drainage of the larvae. A 2" PVC pipe is fitted into the tank to control drainage and harvesting. This pipe eliminates the use of PVC gate valves which are not only expensive but also not locally available. A tank of this size can be stocked with 100 000–200 000 nauplii. The number of tanks needed will depend on the desired production output of the operator.
The larval rearing tank may not necessarily be housed in a massively constructed building. It should, however, have some provision wherein the tank can be easily covered with plastic walling and roofing to prevent salinity change during rains and to be able to maintain high temperature due to easy light penetration.
4.2 Algal culture tanks
Since this hatchery method requires minimal algal feeds, only a few culture tanks are needed. A two to one ratio of larval rearing tanks to algal culture tanks is adequate. The algal culture tank should be about one ton in capacity and can be made of either concrete or marine plywood.
4.3 Seawater supply system
Seawater for larval rearing should be clear and relatively free from silt and from industrial and agricultural pollutants. It should have a salinity range of 30 to 35 ppt.
The seawater supply system consists of a water intake pipeline which draws water from the sea, water pump and water reservoir. A single pipe is all that is required for the seawater intake line. This can draw water directly from the sea or from saltwater sump pit made of concrete circular culvert installed in the sea at 0 + 0 elevation (Fig. 4). In areas where the seashore is sandy and water is calm most of the year, a sump pit is recommended. Pumped seawater is prefiltered and eliminates the need of a sand or bag filter.
For a small-scale hatchery with 10 to 15 tanks, a 2 hp electric pump and a 10 to 15-ton reservoir will be adequate to supply the seawater needs of the hatchery.
4.4 Aeration system
Instead of compressors or blowers which consume a lot of electricity, portable aerators can be used to aerate the larval rearing tanks, algal tanks, and nursery tanks. For a 2-ton tank, two 5-watt aerators are enough.
4.5 Nursery tanks
In this hatchery model, nursery tanks are integrated with the hatchery system. Postlarvae harvested from the hatchery and fry collected from the wild should be reared first in the nursery tanks for about a month before stocking them in grow-out ponds. Stocking the fry in a more controlled environment such as the nursery tank offers several advantages:
The nursery tanks can be made of either concrete, fiberglass or marine plywood. It can be of any size depending on the fry production capability of the hatchery but should preferably be of 1.5 to 12 ton capacity. Moreover, it should be shallow, not more than 1 meter, to allow easy siphoning of sediments in the bottom.
5. PROBLEMS AND RECOMMENDATIONS
5.1 Spawner supply
With the mushrooming of shrimp hatcheries in the Philippines, the supply of spawners has become a major problem. There are at present about 41 hatcheries in the Philippines. However, 13 of these are not operational partly and probably because of the inadequate spawner supply.
Recommendations:
Refinement of prawn gonadal maturation techniques in land-based tanks and marine pens to insure consistent spawner production.
Stocking of selected natural spawning and nursery grounds of shrimps with hatchery-produced fry.
Enactment of laws prohibiting the export and smuggling out of spawners and fry of P. monodon.
5.2 Dependence on Artemia cysts
The present techniques for the consistent production of fry at high survival rates is still very much dependent on imported Artemia cysts as feed for mysis and post-larvae.
Recommendations:
Test suitability of easy-to-produce natural foods such as Brachionus, Moina, Tisbe, etc. as substitutes for Artemia.
Develop artificial feeds to further simplify shrimp production.
REFERENCES
AQUACOP, 1977 Larval rearing of penaeids in a tropical environment. Tarawao, Tahiti.
Cook, Harry L, 1977 Small-scale shrimp hatchery project. Manila, Philippines. FAO/UNDP Brackish-water Aquaculture Development and Training Project.
Mindanao State University, 1975 Annual Report 1975. Naawan, Misamis Oriental, Philippines. Institute of Fisheries Research and Development, MSU.
Mock, 1970 Cornelius R. and M. Alice Murphy, Techniques for raising penaeid shrimp. Proc. First Annual Workshop, World Mariculture Society.
Mock, 1974 Cornelius R. and R. A. Neal, Penaeid shrimp hatchery system. FAO/CARPAS Symposium on Aquaculture in Latin America.
Nurdjana, 1979 M.L. et al, The small-scale backyard penaeid shrimp hatchery.
Platon, Rolando R, 1978 Design, operation and economics of a small-scale hatchery for the larval rearing of sugpo, Penaeus monodon Fabricius. Tigbauan, Iloilo, Philippines. Aquaculture Department of the Southeast Asian Fisheries Development Centre.
SEAFDEC. 1979 and 1980 Annual Reports, Tigbauan, Iloilo, Philippines. Aquaculture Department of the Southeast Asian Fisheries Development Centre.
Tiensongrusmee, Banchong, 1977 Experiences and problems in the design and management of penaeid shrimp hatchery in Thailand. Bankhen, Bangkok, Thailand. Department of Fisheries, Kasetsart University.
Villaluz, Domiciano K. 1969 et al, Production, larval development and cultivation of sugpo (Penaeus monodon Fabricius). Manila, Philippines. The Philip. J. Sci. 98:3–4.
Table 1. Feeding scheme of P. monodon larvae under the old method
| Larval stage | Nauplius | Zoea | Mysis | Postlarva | ||||
| Z1 | Z2 | Z3 | M1 | M2 | M3 | P1 to P5 | ||
| Chaetoceros cells/ml | 30 000 | 50 000 | 80 000 | 80 000 | 80 000 | 80 000 | ||
| Tetraselmis cells/ml | - | 5 000 | 10 000 | 20 000 | 20 000 | 20 000 | ||
| Rotifer (Brachionus) ind/ml | - | - | 5 | 8 | 10 | |||
| Artemia | - | - | - | - | - | - | 5 | |
Table 2. Feeding scheme for P. monodon larvae under the new method
| Larval stage | Nauplius | Zoea | Mysis | Postlarva | ||||
| Z1 | Z2 | Z3 | M1 | M2 | M3 | PL 1 to PL 5 | ||
| Tetraselmis cells/ml | 5 000 | 5 000 | 5 000 | 5 000 | 5 000 | 5 000 | ||
| Egg yolk particles/ml | - | 15–25 | 15–25 | 15–25 | 15–25 | 15–25 | ||
| Artemia ind/ml | - | - | - | 10–15 | 10–15 | 10–15 | 15–20 | |
| Bread yeast* g/ton | 0.1-0.3 | 0.1-0.3 | 0.1-0.3 | |||||
* If Tetraselmis is not available.
Table 3. Comparative output of P. monodon hatchery runs using old and new feeding schemes
| Method | No. of runs | No. of successful runs | Successful (Percent) | Average survival rate of success- ful runs M - PL 5 (Percent) | Range (Percent) | Period of culture |
| Old | 31 | 7 | 22.5 | 2.5 | 1–9 | June-September, 1979 |
| New | 44 | 44 | 100 | 52.9 | 20–75 | July-October, 1981 |
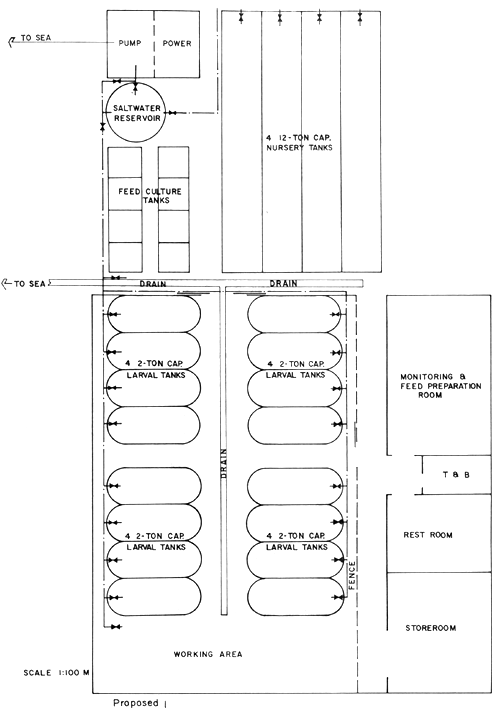
Fig. 1. Proposed plan for a shrimp hatchery
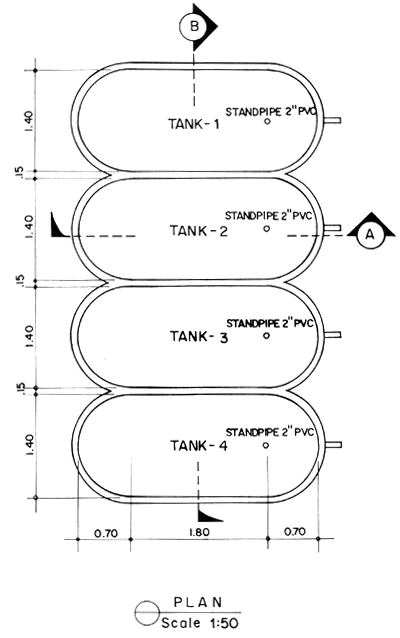
Fig. 2. Plan and section of two-ton larval rearing tank
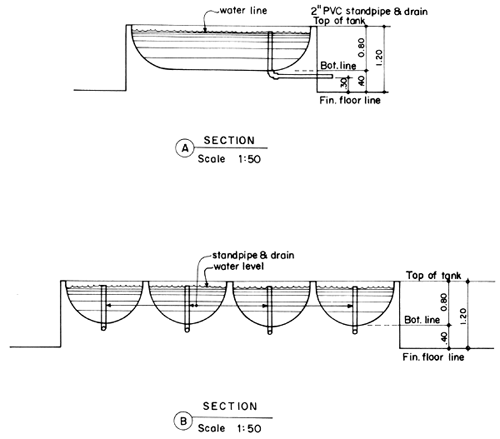
Fig. 3. Cross-section of larval rearing tank
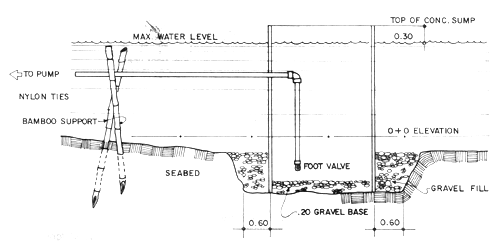
Fig. 4. Detail section of saltwater sump pit
WP/81/SPH/CP-12
HANDLING AND REARING OF HATCHERY-PRODUCED SHRIMP POSTLARVAE FROM SMALL-SCALE HATCHERIES
by
Florentino D.Apud1
1. INTRODUCTION
The supply of appropriate seedlings for pond stocking has been one of the constraints in the development of shrimp industry in the Philippines. Shrimp fry gathered from estuaries were inadequate to meet even the minimum requirement of fishfarmers. In addition, the occurrence of these fry was seasonal, hence, shrimp production in brackishwater ponds remained unpredictable for many years.
The successful production of penaeid fry under controlled condition (Villaluz, et al, 1969) led to mass production of hatchery-bred fry at MSU-IFRD, Naawan, Misamis Oriental province and SEAFDEC Aquaculture Department, Tigbauan, Iloilo, Philippines. This created greater impact to the industry as fishfarmers gained more interest because of better assurance of fry supply. A lot of fishfarmers have ventured into shrimp culture, however, they encountered problems of low survival. Those who initially availed of the use of hatchery-produced fry attained an average survival of approximately 10 percent up to marketable size employing various practices such as: the traditional milkfish nursery system, hapa net system prior to stocking in growout ponds, and direct growout pond stocking of fry. This poor survival could be attributed to improper handling and rearing of hatchery-produced fry prior to final stocking in growout ponds.
The delicate sizes of shrimp fry harvested at stages PL 10 to PL 15 could be an important factor for high mortality. Hatchery-bred fry have been reared and cared under controlled tank conditions. They were provided with good water quality and adequate water supply, adequate food supply, freedom from pests with predators and water salinity, temperature, dissolved oxygen and pH very much under control. Changes in any of these environmental conditions especially in earthen ponds create stress, mortality and losses due to predation. The same thing can happen to wild caught fry. Although it has been exposed to adverse conditions in estuaries, changes in the environmental conditions from the time it is caught, stored, transported and stocked in ponds including stress in handling during the process can greatly affect the survival.
With the establishment of earthen shrimp nursery system at SEAFDEC Leganes Research Station, designed to accommodate the hatchery-bred fry (Apud and Sheik, 1978) or even wild caught fry, and the development of concrete nursery system as an extension of hatchery rearing system in Batan substation (Gabasa, 1980 personal communication) shrimp juveniles production has been improved. The system actually provides an intermediate link where proper handling and rearing of delicate fry to an appropriate size can be effectively achieved prior to final stocking in growout pond environment.
2. HANDLING AND REARING OF POSTLARVAE
The rearing of hatchery-bred shrimp postlarvae to juvenile stage is carried out in nursery system thru concrete tanks, wooden raceways or earthen nursery ponds. The juveniles produced from this system are counterparts of milkfish fingerlings. The size ranges from 0.3 g to 1.5 g. It has been observed that juvenile stages are appropriate for stocking in growout pond conditions and usually result in a better production. The survival rate as observed corresponds directly to the size of fry during stocking. This supports the statement of Shigueno (1970) that the bigger the fry released in ponds, the higher the survival rate regardless of the duration of culture period.
1 Researcher, SEAFDEC Aquaculture Department, Leganes Research Station, Leganes, Iloilo, Philippines
2.1 Design of earthen shrimp nursery
An earthen shrimp nursery system has features that differ from an ordinary milkfish nursery. It is equipped with water pump, water reservoir pond, supply canal and entrance gate separate from drain canal and drain gate facilities for ease in water management. It is provided with filtration system, i.e. harvest/drain filter box of sand pebbles or everted screen bagnet, etc. to prevent entrance of pests and predators. The size of each pond unit in a system varies from 200 to 2 000 m2. There is a diagonal trench connected to harvest/drain box for ease in the retrieval of juveniles. Big ponds aside from being difficult to manage in terms of water supply and control, also make retrieval of small shrimp difficult during harvest. The shape is rectangular or rhomboid with acute corners lying along the prevailing wind direction — northeast and southwest moonsoons. This makes the management of the accumulating and decomposing lablab in the acute corners more convenient.
The pond is provided with substrates such as swamp tree twigs, bamboo twigs, coconut leaves, etc. to provide shelter, clinging material, refuge and added surface area for natural food.
2.2 Nursery pond preparation
The pond is drained and dried for about a week or two to release toxic gases and eradicate some pests and predators. The traditional use of commercial insecticides to eradicate pests and predators is not advisable and should be avoided. Some organic pesticides such as derries roots, tobacco wastes or dusts are utilized. Lime and ammonium sulfate can also be used to eradicate pests and predators. Burned lime at 2 tons/ha in shallow water of about 5 to 10 cm can raise pH of water as high as 8.5. If this lime will be supplied together with ammonium sulfate (100 kg/ha) the NH3 compounds become toxic thus killing pests and predators such as tilapia, gobies, bidbid and other species (Norfolk, 1980, personal communication).
If soil condition is not known, it has to be sampled and analyzed for pH and organic matter (OM) content. A stabilized pond bottom must have at least a pH of not less than 7.0 and organic matter content of 3 percent or higher. Treatment with lime and chicken manure shall depend on soil pH and organic matter levels. Table 1 shows the average soil pH and organic matter content of pilot shrimp nursery ponds of SEAFDEC, AQD at Leganes Research Station taken before and after treatment with lime, Ca(OH)2 (5 tons/ha) and chicken manure (2.8 tons/ha).
Table 1.
Average pH levels and organic matter content of pilot shrimp nursery ponds of SEAFDEC, AQD, Leganes Research Station taken before and after treatment of lime (5 tons/ha) and chicken manure (2.8 tons/ha)
| Before treatment | After treatment | ||||
| September 1977 | January 1978 | June 1978 | |||
| pH | %OM | pH | %OM | pH | %OM |
| 4.38 | 2.26 | 5.81 | 5.12 | 7.61 | 3.41 |
After making the appropriate pond treatments mentioned above, substrates are placed and standard water management for lablab propagation followed. Booster fertilization of inorganic fertilizer at 50 to 100 kg/ha is made if lablab growth is poor.
2.3 Handling/transport of fry
Generally the hatchery-bred fry is transported in oxygenated plastic bags as being done with milkfish fry. The temperature of transport water is usually lowered at source with the use of ice to approximately 22–23°C and maintained at this level by adding about 500 g of ice on top of each plastic bag. The ice is wrapped in newspaper or contained in small bag with rice hull. The plastic bag with 10 liter water may contain more or less from 10 000 to 20 000 postlarvae (PL 4, PL 5) depending upon the period of travel. At lower temperature the fry are passive, thus, spend less energy, consume less oxygen and do not get hungry. If fry go hungry, they resort to cannibalism; and if they are fed, the water becomes polluted. At shorter distance and lesser load (6000–8000/bag) reduction of temperature is not necessary. The plastic bag may just be contained in jute sack to prevent increase in temperature during transport. For larger quantities of fry, aerated tanks mounted in a vehicle are usually utilized for transport.
The fry dealers also transport their wild caught shrimp fry in oxygenated plastic bags. The fry are usually at stages, PL 16-PL 30. Storage of these fry had posed a big problem. Similar storage practices for milkfish fry in a basin, wooden box or earthen pot, incurred great mortality. At night time, the fry jumps and they usually stick at the side of the container until they die. Shrimp fry dealers suffered setbacks for many years because of storage and transport problems. Recently, a technique in transporting fry in a plastic bag without oxygen was discovered. This is done by just placing a limited number of fry (approximately 600 to 700) in a plastic bag and abruptly closing the plastic bag thus trapping the air, squeezing the mouth of the bag and tieing it with rubber band. It was observed that fry can survive for many hours and dealers claimed excellent survival. As a result, they use the same technique in storing fry and observed it to be effective within 8–12 hour period.
2.4 Acclimation/counting/stocking
Upon arrival at destination, the shrimp fry regardless of source is first acclimatized to pond water conditions especially in terms of salinity and temperature before it is released into the ponds. There are two common methods in acclimatizing the fry. Those that are transported by plastic bags can be acclimatized by simply allowing the plastic bag to float on pond water for 20–30 minutes until the temperature equilibrate. Then the bags are opened and small amount of water are poured inside the bags to adjust salinity of transport water with that of pond water. Bigger quantity of fry transported in aerated tanks are usually transferred in another tank container provided with aeration where transport water is gradually replaced by pond water.
Counting of limited quantity of shrimp fry (less than 20 000) may employ similar methods used for counting milkfish fry. For a larger quantities, the aliquote method is used, however, this is only effective for postlarvae (PL 4-PL 5) as they can still be made to disperse uniformly inside the container before a representative sample is taken.
The best time or period for transporting and releasing fry is during early morning before 09:00. Other time of the day may do as long as the weather is cool and the period falls within spring tide so that releases can be done while fresh tidal water is entering the pond. The fry when released within the pond either by pail or plastic bags are scattered to prevent them from concentrating at a certain area. Concentration of fry in one area may result in oxygen depletion, food shortage and cannibalism. Stocking rate varies from 20 to 200 fry/m2 depending upon availability of food, water management, stage and quality of fry, cropping season, supplementary feeding, etc.
2.5 Rearing of fry
Rearing period from postlarval stage (PL 4-PL 5) to juvenile stage requires 30-40 days. During this period, supplementary feed may be provided depending upon the stocking density and availability of natural food. At stocking density of approximately 100–200 fry/m2, supplementary feed, such as minced mussel or clam meat, formulated diet, etc. is normally provided. The rate of feed given twice or thrice daily starts with 90–100 percent of biomass/day for the first week, 60 percent in the second week, 30 percent in the third week and 15 percent in succeeding weeks. At this density and feeding rates, water movement few hours a day is advisable although not necessary. At less that 50 fry/m2 natural feed and water replenishment during spring tides may serve the purpose. The water depth during stocking is about 30–35 cm. This is gradually increased every week by 10 cm until it reaches a maximum of 60–70 cm.
2.6 Harvest of juveniles
Harvest is made in the early morning or late in the evening. Normally the juveniles are harvested with the use of screen bagnet. The bagnet is installed at the discharge end of the drain pipe. While the pond is drained, the juveniles are carried by the water current accumulating them inside the bagnet. To avoid stress, the juveniles are removed and transferred to a pail from time to time. They are deposited in aerated tanks or hapa nets installed at the reservoir ponds. Some juveniles left out are accumulated inside the harvest/drain box. They are collected by a circular or triangular scoop net. Those that remain in the trench canal are picked. Otherwise, if they are still too many, water is added from the reservoir pond. Harvest operation is resumed the following morning or evening and juveniles are expected to go with the water current due to slight stress during the previous draining process.
2.7 Counting and transport of juveniles
Counting of juveniles from storage containers (hapa net or aerated tanks) starts as soon as they recover from harvest stress. Large quantities are usually counted by volumetric method using plastic sieve. The sieve is filled with juveniles and the juveniles are counted. Sample counts of 3 to 5 times are made. The average of the sample counts becomes the basis of how many juveniles are contained per sieve. For lesser quantities, say 5 000 to 10 000, head counting may be possible.
Transport of juveniles is similar to that of fry except that the number placed per unit container is greatly reduced. A standard plastic bag, 18" × 36" filled with 10 liters of water may contain only from 300 to 3 000 juveniles depending upon the size of juveniles and distance of the trip. The same container may accommodate 10 000 to 20 000 shrimp postlarval (PL 4-PL 5). The use of aerated containers (canvas or cylindrical plastic tank) is preferrable for the transport of 1 g juveniles with maximum load of 30 000 pieces or 30 kg juveniles for every ton of water. The result shown in Table 2 is encouraging for the transport of smaller (0.26 g) shrimp juveniles even at greater distances from the source.
2.8 Survival and growth of hatchery-produced fry
Based on various studies conducted at Leganes, survival and growth of hatchery-bred fry grown to juveniles stage vary depending upon stocking density, stage and condition of fry upon stocking, water management, feeding, predator control, provision of shelter material and efficiency in the retrieval system. Generally, the lower the stocking density, the higher the survival even without supplemental feeding and with water management dependent on tidal fluctuation. At higher stocking density, say 100 m2, supplementary feeding plus water movement or aeration are needed to achieve high survival. Table 3 shows the survival of P. monodon juveniles at the SEAFDEC pilot shrimp nursery ponds at Leganes stocked at different densities and management techniques.
Table 2.
Survival rates of shrimp juveniles (PL 26) at different packing
densities after 12 hours of transport and handling
(After Yap, et al, 1978)
| Density level (shrimp/bag) | Bag No. | Mortality | Final water Temp. (°C) | Fry Condition | |
| No. | % | ||||
| 1 000 | 1A | 4 | 0.40 | 22.5 | Healthy |
| 1B | 0 | 0.00 | 22.5 | " | |
| 2 000 | 2A | 78 | 3.90 | 22.2 | Healthy |
| 2B | 66 | 3.30 | 22.0 | " | |
| 3 000 | 3A | 351 | 11.70 | 21.70 | Healthy |
| 3B | 100 | 3.33 | 22.1 | " | |
| 4 000 | 4A | 500 | 12.50 | 22.7 | Weak |
| 4B | 1 000 | 25.00 | 22.7 | " | |
| 5 000 | 5A | 1 500 | 30.00 | 33.5* | Very weak |
| 5B | 2 500 | 50.00 | 23.0 | " | |
Table 3.
Survival rates of P. monodon fry (PL 4-PL 5), reared to juveniles stage in
earthen nursery ponds at different stocking densities and management techniques
(After Apud, 1979)
| Density | Water | Management techniques | Feed | Survival |
| No./m2 | movement | water fertilization | % | |
| 22 | Tidal | None | None | 43.6 |
| 50 | Tidal | None | (a) | 29.8 |
| 50 | Tidal | (b) | None | 21.9 |
| 50 | Tidal | (b) | (a) | 19.8 |
| 50 | Tidal | None | None | 25.6 |
| 100 | Tidal | None | (c) | 28.6 |
| 150 | Tidal and pump | None | (d) | 57.4 |
| 150 | Tidal and pump | None | (a) | 25.5 |
(a) Mussel meat at 20 percent body weight given twice daily
(b) Applied with 18-46-0 at 25 kg/ha every 2 weeks
(c) Formulated diet given at 20 percent body weight 3 times a week
(d) Mussel meat given at 100 percent body weight daily for 16 days and 20 percent subsequently
(e) Mussel meat given at 100 percent body weight daily for 26 days and stopped
As indicated in the above table, a survival of 43.6 percent was attained even without feeding and water fertilization and dependent on tidal fluctuation for water management at stocking density of 22 fry/m2. At 50 fry/m2, feeding with minced mussel meat 20 percent biomass and given twice daily got better result (29.8 percent) than with water fertilization only (21.9 percent); both feeding and fertilization (19.8 percent); and without feeding and fertilization (25.6 percent). These results suggest that if water renewal is inadequate feeding and water fertilization may in fact result to low survival which may be possibly due to some effects of excess feed and fertilizer on water quality. At higher density of 150 fry/m2 and with continuous feeding and water movement thru the aide of water pump shrimp survival of 57.4 percent was obtained. Although the same condition was used the survival in other ponds dropped to 25.5 percent possibly because feeding was stopped in the middle of the culture period. The final mean weight of juveniles obtained from the above trials range from 0.4 to 1.5 g.
The results of another study in earthen nursery ponds using different water management schemes as shown in Table 4 support the importance of aeration and supplementary feeding to hatchery-bred fry stocked at higher stocking density (100 fry/m2). As indicated, mean survival as high as 68.6 percent could possibly be attained in ponds with partial flow through and aeration. Analysis of variance showed a significant effect of aeration on the survival rate of fry. Although water movement rate had direct relation with survival, however, it was not statistically significant. Likewise, no interaction effect was observed between aeration and water movements. Average body weight of shrimp juveniles harvested from this experiment was approximately 0.42 g.
A study conducted (N. Tabbu, 1981) using hatchery-bred fry (PL 4-PL 5) at different stocking densities with or without supplementary feeding indicated the need of feeding at higher densities (100–150 fry/m2). Although the differences were not significant the mean survival rates were considerably higher in ponds provided with supplemental diet than those without. The mean survivals at 50 fry/m2 however, were similar in both treatments which suggested that at this density level natural food could be enough to support and achieve survivals of more than 40 percent. Generally, the mean survivals obtained in this study is comparable to the results obtained by Apud (1979) in his above experiment on the use of different water management schemes. The final body weights obtained ranged from 0.5 to 1.5 g as shown in Table 5.
Table 4.
Percent survival of P. monodon fry reared for 28 days, from PL 4/PL 5 to PL 32/PL 33
in earthen nursery ponds using different treatments*
(After Apud, 1979)
| Treatments | Replicates (Blocks) | Mean per treatment | |||
| 1 | 2 | 3 | 4 | ||
| I | 48.6 | 53.1 | 59.9 | 48.3 | 51.1 |
| II | 78.8 | 59.0 | 71.7 | 54.7 | 68.6 |
| III | 54.9 | 78.7 | 64.2 | 49.9 | 61.9 |
| IV | 35.9 | 56.0 | 60.0 | 32.1 | 46.0 |
| Mean per block | 54.5 | 61.4 | 64.0 | 47.6 | 56.9 |
* Treatments
I - flow through 6 hr/day without aeration
II - flow through 6 hr/day + 6 hr/day aeration
III - flow through 6 hr/day (during high tide only) + 6 hr/day aeration
IV - flow through 3 hr/day (during high tide only) without aeration
2.9 Growth and survival of wild caught shrimp fry
Two studies were conducted at Leganes UP-BAC using wild caught shrimp fry. One study was conducted by Cholik (1978) evaluating the effect of different shelter densities (Table 6) in the growth and survival of wild caught fry. The shelter used were coconut leaves cut and bundled into uniform length and size. The fry utilized were at stages PL 14 and above at a constant density of 25 fry/m2. Results as indicated in Table 6 showed that shrimp survival after a month culture period is directly proportional to the density of shelter material. The final body weight was also directly proportional to density of shelter material.
Table 5.
Stocking densities, mean survival and final weight (g) of P. monodon postlarvae (PL 4-PL 5)
reared at 50/m2; 100/m2 and 150/m2 for 45 days in nursery ponds at two feeding regime
(After Tabbu, 1981)
| Treatments | Stocking density | Percent survival (percent) | Final body weight (g) |
| a. Without supplementary feeding | I | 42.5 | 1.5 |
| II | 54.6 | 0.9 | |
| III | 46.9 | 0.5 | |
| b. With supplementary feeding of formulated diet | I | 41.6 | 1.5 |
| II | 67.4 | 1.0 | |
| III | 52.1 | 1.0 |
Table 6.
Mean survival and final weight of wild caught
P. monodon (PL 14-PL 16) and above reared at
25 fry/m2 for 30 days in nursery ponds at four
different shelter densities: 1 shelter/m2 (I)
shelter/2 m2 (II); 1 shelter/3 m2 (III);
and no shelter (IV) (After Cholik, 1978)
| Treatments | Mean survival (Percent) | Mean final body weight (g) |
| I | 90.86 | 0.37 |
| II | 82.00 | 0.30 |
| III | 64.98 | 0.26 |
| IV | 66.25 | 0.26 |
Another study was conducted by Fernandez (1979) using wild caught fry at varying densities of 25 fry/m2; 50 fry/m2 and 75 fry/m2 in earthen ponds provided with shelters. Survival obtained varied slightly but there was no trend nor significant difference among treatments. It appeared that survival at higher densities (50–75 fry/m2) are comparable to that obtained by Cholik (1978). Final body weights obtained were similar ranging from (0.26–0.37 g) for both studies.
Table 7.
Mean survival and final weight of wild caught
P. monodon reared at three different
stocking densities for 30 days in nursery
ponds provided with artificial shelters
(After Fernandez, 1979)
| Treatments | Mean survival (Percent) | Mean final body weight (g) |
| I | 93.64 | 0.20 |
| II | 83.95 | 0.29 |
| III | 85.07 | 0.18 |
3. COMMENTS
The survivals obtained from studies mentioned above indicated a generally high survival for wild caught fry. These results, however, could not be compared to hatchery-bred fry because of varied conditions. The stages of wild caught fry upon stocking were not lower than PL 14 whereas hatchery-bred fry were at PL 4-PL 5. Moreover, the stocking density of wild caught shrimp fry used was lower. On the other hand, regardless of source of shrimp fry results suggest that various factors influence the growth and survival of fry in the nursery ponds. These factors include among others feeding, water management, amount of shelter material, stocking density, pest and predator control, physico-chemical parameters of water, etc. If all these factors are controlled or maintained to the optimum level, production of juveniles from hatchery produced postlarvae (PL 4-PL 5) can be easily achieved at higher survival and growth. Moreover, if older stages (PL 16-PL 21) of postlarvae are harvested in the hatchery and released in nursery ponds survival may be comparable to that of wild caught fry as observed in some of the trials.
REFERENCES
Apud, F.D. and M. Sheik., 1978 Design and construction of a prawn nursery pond system. Readings on Pond Management. SEAFDEC-AIA, Aquaculture Department.
Apud, F.D., 1979 Effects of water movement and aeration system on the survival and growth of hatchery-bred sugpo (Penaeus monodon Fab.) in earthen nursery ponds. UPSEAFDEC Graduate Program (M.S. Thesis)
Apud, F.D., W.C. Yap and K. Gonzales., 1979 Mass production of Penaeus monodon juveniles in earthen nursery pond. Cont. No. 30. SEAFDEC, Aquaculture Department.
Apud, F.D., 1980 Management and operation of prawn pond. Paper presented at the Second Aqua Business Business Project Development and Management Workshop. July 28-Aug. 16, 1980. Metro Manila.
Cholik, F., 1978 A study on the effects of density of sheltering materials on growth and survival of Penaeus monodon fry in earthen ponds. UP-SEAFDEC Graduate Program. (M. S. Thesis)
Gabasa, P., 1980 Personal communication.
Fernandez, P. M., 1979 Effect of stocking densities on the survival and growth of wild and hatchery produced sugpo (Penaeus monodon Fab.) fry in nursery ponds with artificial shelters. UP-SEAFDEC Graduate Program (M.S. Thesis)
Norfolk, J., 1980 Personal communication
Tabbu, N., 1981 Effect of feeding regime and stocking rate on the production of Penaeus monodon juveniles in brackishwater nursery ponds. (M. S. Thesis)
Villaluz, D. K., A. Villaluz, B. Ladrera, M. Sheik, and A. Gonzaga., 1969 Reproduction, larval development and cultivation of sugpo (Penaeus monodon Fab.). Phil. Jour. Sci., 98 (3 and 4): 205–236
Yap, W. G., H. Mochizuki, F. Apud and R. Obregon., 1978 Mass transport of sugpo P. monodon juveniles. (Manuscript)
WP/81/SPH/CP-13
IMPROVEMENT OF THE CLOSED RECIRCULATING WATER SYSTEM FOR GIANT PRAWN (MACROBRACHIUM ROSENBERGII) LARVICULTURE
by
Piamsak Menasveta*
ABSTRACT
The paper described further improvement of the closed recirculating water system with ozonation for larviculture of the giant prawn as reported earlier (Menasveta, 1980). More filter units were added in the larval rearing compartment and the tank wall was thickened for better insulation effect. These modifications significantly improved the survival of giant prawn larvae. The mean production of postlarval prawn was 20 prawns/liter.
1. INTRODUCTION
Larviculture of giant prawn (Macrobrachium rosenbergii) in a closed recirculating water system which proved to give comparable results to an open system has been described earlier (Menasveta and Piyatiratitivokul, 1980). Subsequently, Menasveta (1980) designed a closed recirculating water system with ozonation. This new system could yield better results than the previous ones. This current paper described further improvement of this system by adding three sub-sand filter units into each rearing tank, and the tank wall was thickened for better insulation effect.
2. MATERIALS AND METHODS
The closed recirculating water system used in these experiments was modified from the ‘closed recirculating system with ozonation’ which was previously reported (Menasveta, 1980). Figure 1 illustrates the system. The system has twin tanks, each tank consists of two compartments, i.e. the larval rearing compartment and the water filtering compartment. The larval rearing compartment has dimensions of 8.0 m × 1.0 m × 0.9 m. The water filtering compartment consists of two units located at each end of the tank. The dimensions of each water filtering unit is 1.0 m × 1.0 m × 0.9 m. The filter consists of fine sand layer at the bottom, coarse sand layer in the middle, and oyster shell bed on the top. The total thickness of these three layers is 0.3 m. Underneath the filter layers, there is a space of about 10 cm in height. This space is connected to the larval rearing compartment by three PVC pipes of each of 5 cm diameter. There are three sub-sand filter units one situated at the bottom of each larval rearing compartment. The cross-section of each filter unit is illustrated in Figure 2. It is made of a 40-liter plastic bucket containing four layers of filter, i.e. the nylon screen net on the top, followed by broken coral (calcarious) and coarse sand at the middle, and oyster shell at the bottom. At the center of filter bed, a PVC pipe with a diameter of 1.9 cm (0.75 inch) is plugged in and the air pipe with air stone at the tip is inserted. The tank was of this modified system is thicker than previously reported (Menasveta, 1980); for better insulation effect.
During the larval rearing period, the water level in the rearing tank is maintained at 0.75 m depth. This corresponds with a volume of 6 000 1. The used water is continuously recirculated into the filtering units through the filter pipes by air-lift action. There are three PVC filter pipes at each end of the tank. About half of the total length of each filter pipe which lies on the bottom of the tank is porous and is lined with a screen net with a mesh size of 0.1 mm for preventing larvae from getting into the pipes. The circulation rate in each tank is maintained to 241/min. Water above the filter bed of each filtering unit is continuously ozonated at the rate of approximately 50 mg/hour throughout the period of rearing. It should be noted that water in the larval rearing tank is also ozonated occasionally when the larvae have developed in stage 5. However, the treatment is short, i.e. not more than 2 hours a day.
For each filter unit inside the larval rearing tank, air will lift the water from the bottom of the tank up through the PVC pipe, water on the filter bed will seep down through filter bed to the bottom. This will cause continuous filtration in each filter unit.
At the beginning of the rearing trials, the salinity was set at 15 ppt. The salinity was, however, gradually reduced from 15 ppt to 6 ppt during the course of rearing by replacing the water lost through evaporation and the removal of food residues by siphoning with freshwater.
The M. rosenbergii broodstock used in these trials was the inter-racial hybrid reared in earthern ponds located in Rangsit, Thailand area. For rearing trials, berried females were selected from the broodstock holding tanks and placed in the larval hatching tanks. Upon hatching, larvae were removed and placed in a 20-l plastic bucket for counting. Larvae in each hatch were enumerated by counting larvae in ten 100 ml aliquot samples. Then, either the whole volume or the volume of water containing the desired number of larvae was transferred to the rearing tank.
After the prawn larvae had been transferred from the hatching tank to each of the rearing tanks, they were fed with newly hatched Artemia nauplii during the first three days. From the fourth day to metamorphosis, the prawn larvae were fed with prepared food during the day time. During night time, Artemia nauplii were fed in excess quantities. The prepared feed consisted of a mixture of eggs, ground fish flesh, and ground soft part of shrimp heads. Particle sizes of the prepared feed were varied with each larval stage. Normally, feed particles that could pass through a strainer of 20 meshes per cm were prepared for larvae of 4 to 10 days, 15 meshes per cm were prepared for larvae of 10 to 20 days, and 10 meshes per cm for the larvae of 20 to more than 30 days. The amount of prepared feed used daily was approximately 30 percent of the total body weight of the larvae. The frequency of feeding was once every 2 hours during day time. Uneaten food material and larval wastes at the tank bottom of the modified closed recirculating system were removed daily by siphoning. On some occasions during the rearing trials, certain chemical and physical water quality parameters were measured. The biological aspects of water quality such as species of phytoplankton and zooplankton were also checked.
3. RESULTS AND DISCUSSIONS
Six rearing trials of M. rosenbergii larvae were conducted in two tanks utilizing this recirculating water system. Three sequential experiments were carried out. The results of these rearing trials are summarized in Table 1.
The initial stocking densities of larvae in the six rearing trials ranged from 33 larvae/1 to 42 larvae/1, with a mean of 40 larvae/1. Every trial exhibited good larval survival throughout the whole period of rearing. The first postlarvae was seen after 19 to 20 days of rearing, and 95 percent of the larvae metamorphosed by 28 to 30 days of rearing depending on each trial. Survival ranged from 49 percent to 53 percent with a mean of 51.0 percent. The yield of postlarvae per liter of water ranged from 17 to 22 with a mean of 20. The water quality of the system remained good throughout the whole period of rearing. Ammonia and nitrite nitrogen are always undetectable.
The result of this investigation showed that the production of metamorphosed prawn per liter of water in these trials examined were much higher than those reported earlier (Menasveta, 1980). The improvement in metamorphosed prawn production might be due to the positive effects of three factors, i.e. the increase of filtering areas, ozonation, and higher and very constant temperature caused by better insulation.
REFERENCES
Menasveta, P. and S. Piyatiratitivokul., 1980 A comparative study on larviculture techniques for the giant freshwater prawn, Macrobrachium rosenbergii (de Man). Aquaculture, 20:239– 249
Menasveta, P., 1980 Effect on ozone treatment on the survival of prawn larvae (Macrobrachium rosenbergii de Man) reared in a closed-recirculating water system. Proc. World Maricult. Soc. 11:73–78
ACKNOWLEDGEMENTS
This investigation was made possible through the funding of International Foundation for Science, Research Grant No. 23. The author thanks Mr. Somnook Sathidsunthorn for his assistance.
Table 1. Larval rearing data of the improved closed recirculating water system
| Larvae | Postlarvae | |||||
| Trial No. | Number in Group | Larvae/1 | Yield | Survival (%) | Postlarvae/1 | Average water temperature (°C) |
| 1 | 250 000 | 42 | 131 000 | 52 | 22 | 30.0 |
| 2 | 240 000 | 40 | 127 000 | 53 | 21 | 30.0 |
| 3 | 210 000 | 35 | 111 000 | 53 | 18 | 29.0 |
| 4 | 200 000 | 33 | 104 000 | 52 | 17 | 29.0 |
| 5 | 270 000 | 45 | 132 000 | 49 | 22 | 30.0 |
| 6 | 250 000 | 42 | 124 000 | 49 | 21 | 30.0 |
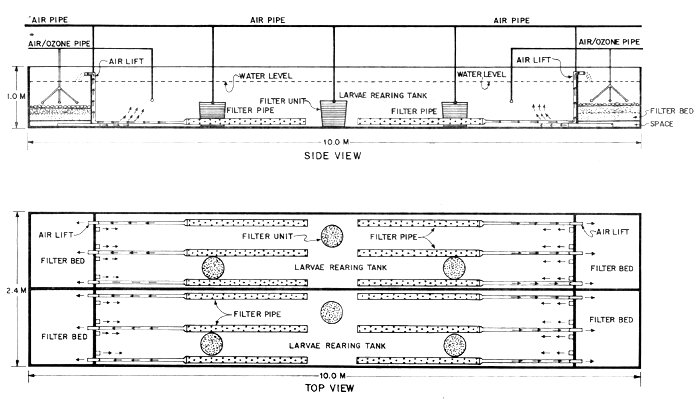
Fig. 1. Schematic illustration of the closed recirculating water system with ozonation
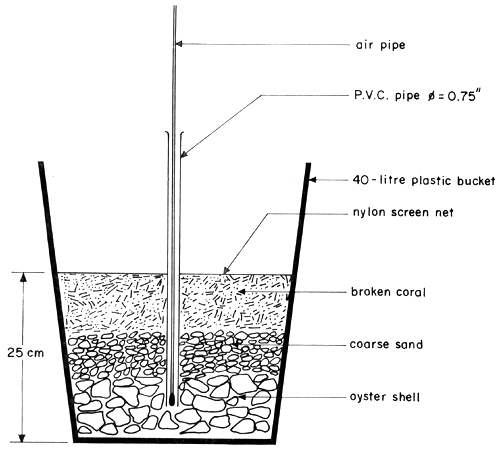
Fig. 2. Cross-section of a filter unit which is situated at the bottom of the larval rearing tank
WP/81/SPH/CP-14
STATUS OF MACROBRACHIUM FARMING IN THE PHILIPPINES
by
Henry Dejarme1, Jurgenne Primavera2 and Fe Estepa2
1. INTRODUCTION
The giant freshwater prawn, Macrobrachium rosenbergii and some smaller species such as M. lanchesterii and M. idella are endemic in rivers and estuaries in the Philippine archipelago.
Although not as popular as Penaeus monodon and other penaeid shrimps, Macrobrachium caught from the wild constitute an important source of animal protein among Filipinos.
Macrobrachium farming is virtually unknown in the Philippines but attempts to develop the aquaculture potential of this prawn are in recent years being initiated at SEAFDEC Aquaculture Department, Tigbauan, Iloilo; MSU-IFRD, Naawan Misamis Oriental province and Central Luzon State University (CLSU), Nueva Ecija province. Some private companies are also reported to have started freshwater prawn farming but information on their progress is not available at the moment.
2. LARVAL REARING
In 1974, SEAFDEC Aquaculture Department researchers at Tigbauan Station began the project on Macrobrachium rosenbergii and carried out some larval rearing trials until 1977 (SEAFDEC, 1974-77). Later on, the project was continued at the Binangonan Research Substation (Padilla, 1980).
At the MSU-IFRD in Naawan, Misamis Oriental province, larval rearing of M. rosenbergii was carried out from 1976 to 1979 (Dejarme, et al, 1980).
Postlarvae were produced from the larval rearing conducted at these two institutions but the production results have not been satisfactory. Many rearing trials were discarded after mass mortalities occured and causes were not clearly understood. Further research is badly needed to modify the larval rearing techniques that have been mostly successful in other tropical countries.
3. POND CULTURE
Postlarvae of Macrobrachium rosenbergii from Hawaii were stocked in cages at Laguna de Bay and in ponds at Bulacan to test their adaptability under local conditions. The study is still on-going (Cruz, 1981).
Earlier pond culture studies have been made on Macrobrachium lanchesterii and Macrobrachium idella at the Central Luzon State University. In these studies, adults from the wild and from previously stocked ponds were further grown in ponds either separately or in combination with other finfishes such as Cyprinus carpio, Tilapia nilotica and Tilapia zillii (Guerrero and Villanueva, 1978; Guerrero and Guerrero, 1976; Guerrero and Gonzales, 1977; Anonymous, 1975; Guerrero and Cagauan, 1978). These studies have generated some information on the culture of Macrobrachium in ponds and in cages but, apparently, follow-up researches are still necessary if we are to develop the freshwater prawn farming into a viable industry in the Philippines.
2 SEAFDEC Aquaculture Department, Tigbauan, Iloilo, Philippines
4. FUTURE PROSPECTS OF MACRO-BRACHIUM FARMING IN THE PHILIPPINES
The country is endowed with vast freshwater resources such as marshes, lakes and estuaries that could easily be developed into prawn farms given the necessary inputs like technology, manpower and ample financial support. Fortunately, the Philippine Government is at present generously supporting the aquaculture development of the country as part of its programme to increase fish production. This may lead to rapid development of aquaculture, including the culture of freshwater prawns.
The Philippine Council for Agriculture and Resources Research, in coordination with fisheries research institutions, has recently prioritized research areas for the development of the aquaculture potential of Macrobrachium in the Philippines (PCARR, 1981). Emphasis are focused on key problem areas which include broodstock development, seed production, pond culture techniques and socio-economics. With the full support from the government, this national programme for Macrobrachium is expected to enhance freshwater prawn farming into a viable industry.
REFERENCES
Anonymous., 1975 Culture of the freshwater shrimps in fertilized ponds with or without Tilapia sp. Inland Fisheries Project Philippines Technical Report. (7):75–78
Cruz, Catalino de la., 1981 PCARR Aquaculture Commodity Fisheries Research Division. Addendum: page 8
Dejarme, 1980 Henry, Jaime B. Dominisac and Sonia Dejarme., Notes on the spawner collection methods and larval rearing of giant prawn (Macrobrachium rosenbergii de Man) at MSU-IFRD, Naawan, Misamis Oriental, Philippines. In: Giant Prawn 1980. IFS Provisionary Report
Guerrero, Luzviminda and A. G. Cagauan., 1978 Comparative study on the pond culture of two freshwater shrimps (Macrobrachium lanchesterii and Macrobrachium idella). Progress Report of Freshwater Aquaculture Centre, Technical Report No. 14
Guerrero, Rafael III and Luzviminda Guerrero., 1976 Culture of Tilapia nilotica and Macrobrachium species separately and in combination in fertilized freshwater fishponds. Philippine Journal of Fisheries, 14(2):232–235
Guerrero, Rafael III and Eden Gonzales., 1977 A study on the polyculture of Tilapia nilotica, Tilapia zillii, gyprinus carpio and M. lanchesterii. Technical Report No. 11 of the Inland Fisheries Project Philippines: 4–8
Guerrero, Rafael III and Eunice Villanueva., 1978 Preliminary report on pond cultivation of Macro-brachium idella. Fisheries Research Journal of the Philippines: 71–73
Padilla, Genoveva G., 1980 Status of macrobrachium research at SEAFDEC Binangonan Research Station, Rizal, Philippines. In: The Giant Prawn 1980, A Provisional Report of IFS
PCARR., 1981 Aquaculture Commodity Fisheries Research Division
SEAFDEC 1974 Annual Report
SEAFDEC 1975 Annual Report
SEAFDEC Performance Report and Evaluation of the Aquaculture Department as of 1976
SEAFDEC Annual Report 1977
WP/81/SPH/CP-15
NOTES ON THE SELECTION OF SITES FOR SMALL-SCALE HATCHERIES FOR CULTIVABLE CRUSTACEANS
by
Banchong Tiensongrusmee1
1. INTRODUCTION
Selection of a suitable site is critically important for a new hatchery. But, despite this, many new ventures appear to have started on unsuitable sites.
This paper is an attempt to provide information on criteria used in selection of site for small-scale hatcheries for cultivable crustaceans. The aim is to assist farmers in suitable site selection, who wish to set up a hatchery for cultivable crustaceans.
2. ECOLOGICAL SUITABILITY
In selection of suitable site for hatcheries for cultivable crustaceans, important parameters needed for assessment are water temperature, salinity, pH, dissolved oxygen, organic loads, sediment loads, nutrients, BOD, COD and meteorological conditions such as rainfall and floods.
3. LAND UTILIZATION CONFLICTS
The site should be examined to establish whether there are possible conflicts with recreation, forestry conservation as well as present and/or future coastal developmental programmes. The value should be evaluated in line with economics and priorities set by government policy.
4. SOCIO-ECONOMIC IMPACTS
Socio-economic impacts in relation to other government programmes should be evaluated. The extent to which the hatchery can benefit the community needs indepth monitoring and careful assessment.
5. ACCESSIBILITY
Good accessibility of site not only facilitates proper management but also determines economics of the venture. Transportation of fry feeds, and harvested products are important factors that need to be examined.
6. MARKETING
Marketing of products contributes to the economic viability of the hatchery. Facility for this activity is needed.
7. BACK-UP FACILITIES AND FINANCIAL SUBSIDY
Consideration must be made of a possibility of back-up facilities in the locality at which the hatchery is built. The possibility of government subsidy, technical assistance from related agencies are useful considerations.
8. AVAILABLE AREA
A certain minimum size of land is needed for the venture, below which it will not be economically viable. The size of hatchery depends on the size of production desired. This has to be assessed before the hatchery is established.
9. AMENITIES
The availability of electricity and readily available freshwater source will add attraction to a particular site for hatchery development. Existing social (schools, medical services, etc.) and economic (markets, roads or waterways, etc.) and other amenities will be helpful.
The above list are by no means complete. Local considerations and assessment on a case to case basis should be practiced before deciding on a site.