JUNE 14 TO JULY 13, 1980
UNDP/FAO PROJECT THA/75/008
S. K. JOHNSON
TEXAS A&M UNIVERSITY
COLLEGE STATION, TEXAS 77843
U.S.A.
Consultancy Objectives
Participate in “Giant Prawn 1980” presenting a paper on Macrobrachium diseases and taking part in discussion sessions.
Advise project on Macrobrachium pathology, disease prevention and treatment, and water quality.
Advise members of the brackish water division of the Department of Fisheries working with marine shrimp as in II above.
Provide advice to the other FAO Project (Pond Management and Fish Diseases: THA/75/012).
Giant Prawn 1980
Participation in this conference included moderation of a session, panel participation, general information exchange and presentation of a paper on disease of Macrobrachium. The paper is attached to this report.
II. Macrobrachium Project
A. National Freshwater Prawn Research and Training Center, Bangkapong; Chachoengsao, Thailand.
Chachoengsao Station
The Chachoengsao Station was visited three times during my duty in Thailand. One was a brief visit in conjunction with a group tour on June 20. The other two visits were review of facility and work conferences on June 24 and July 8. In these latter visits there was a chance to examine in some detail problems of the Chachoengsao Station.
The Station requires 200 tons of water to operate the hatchery to full capacity. The source of the water is the Bangkapong River. A problem with water quality exists because the river has a seasonal fluctuation of salinity. To overcome unsuitable extremes in salinity the Station stores concentrated salt water and freshwater in holding tanks that were originally designed for grow-out experimentation. This procedure and the salinity extremes of the Bangpakong River are discussed in detail in T. Fujimura's FAO Report of 1978 (THA:75:008/78/WP/2).
The consensus of the Station staff was that most problems with disease occurred during the periods when the river's water was changing from fresh to salty and vice versa. Aside from data on salinity change, very little is known about trends in other water quality parameters that change with river conditions.
A lack of understanding also exists as to the seasonal change in concentration of potential pathogens. The possible influence of pesticide presence and toxic algae blooms deserves consideration. In any case, a certain portion of the prawn larvae continue to die as a result of disease organisms.
The condition of artificial culture is sometimes enough in itself to predispose animals to contagious diseases. The usual trend in aquaculture, however, is that environmental extremes and management imperfections provide for the presence of certain predisposing factors which breakdown resistance to opportunistic pathogens. It is of utmost importance in prawn culture to have an understanding of potential predisposing factors and pathogens so that management adjustments or disease management plans can be developed.
The loss of prawns to disease at the Chachoengsao Station is
readily apparent but the diagnosing of the causes of prawn
loss is almost impossible without a good array of supporting
facts. Such information will require recording of data and
sometimes the procurement of data with specialized testing
and equipment. The Station staff should continue to record
information pertinent to disease predisposal and in some cases,
add new methods for a more comprehensive approach.
Motivation of staff for such tedious efforts as water testing
usually requires the establishment of specific goals with
clearly stated objectives.
In previous years, the Chachoengsao Station was active in developing a production capacity to stimulate prawn farming and to provide prawns for natural waters. The effort in stimulating prawn farming has been successful and now there are many hatcheries in the country offering seed prawns for sale. Perhaps private seed production will now provide for the demand of grow-out farms. The Station has developed a excellent reputation with prawn farmers. With these facts in mind, it is suggested there be an expansion of the extension role of the Station to include various support services to the emerging industry. In spite of the adverse conditions of the Station in regard to water quality, the situation does provide a spectrum of environmental conditions that could simulate most of Thailand's hatchery situations. The development of laboratory support capabilities for prawn farming would help to identify and solve some of the present blocks to increased production in the industry and on the Station.
In light of these considerations, the following suggestions are offered for the development of the Station into a stronger support institution and development of ways to attain competency in attacking certain problems of unknown cause. Other suggestions are intended to present ways of attacking identified problems or at least find out how to attack them.
Water
The water in the Bangkapong River undergoes a seasonal fluctuation in quality depending on whether it is the dry or rainy season. Attempts should be made to further characterize the various physical and chemical nature of the water so that comparisons can be made to known requirements of Macrobrachium rosenbergii. Capability to check the supply water for metals, toxic gases, oxygen, alkalinity, oxygen demand, and ionic constituents is important. The present laboratory has an atomic absorption spectrophotometer but supplies and equipment to initiate a functional service laboratory should be developed. The cost of supplies and equipment should be relatively low for initial set up with additional supply cost varying with particular investigative approaches. Although the constant use of water testing capabilities will help to solve many on-site problems, it would be wise to continue a priority on service to fish farmers in regard to laboratory equipment and staff time.
In addition to assessment of supply water and keeping routine records for culture efforts and special experimentation there should be active efforts toward exploring the benefits of biofiltration of source water and recirculted culture water.
The addition of a well that could produce a supply of good quality water would be helpful.
Toxins
Pesticides - Insecticides can be harmful to all aquaculture species but they are particularly harmful to crustaceans. The establishment of capabilities for pesticide analysis should be considered. Such a function would be primarily service and secondarily research oriented. Commercial firms that test for pesticides are usually not oriented to check the peculiarities of aquaculture samples and because they are often loaded with contractural commitments, specific testing for aquaculture does not have much priority. The research priority of university and government laboratories typically slows analysis and frustrates those who need quick answers to avert crop loss. A pesticide analysis function at Bangkapong would certainly aid the developing industry. Costs for setting up such a function would require a capital outlay of $10,000 -$20,000 with much of the on-going supply costs recovered from analysis fees. The type of equipment varies and should, to a great degree, match the preference of the analyst. A good plan for establishment of the pesticide function would include a short-term (6 months) training of the analyst in a large government laboratory (for example, a U.S. Food and Drug Laboratory) where as a trainee could become acquainted with an array of equipment and its peculiar characteristics. The analyst could then be functional in equipping the laboratory.
An effort should also be made in identifying types and quantity used of the pesticides in the vicinity prawn farming areas. Toxicity levels could be established for prawns at the Station. Biotoxins - Toxic algae in the water supply could present a problem to the larval rearing. More attention should be given to this possibility by monitoring microscopically the phytoplankton. The use of extra-sensitive animals as sentinels could be of advantage in this effort and when monitoring for pesticides. A laboratory functional in pesticide monitoring could easily analyze biotoxins with little extra cost.
Predators and Disease Agents
Hydrozoan Control - One of the major problems indentified by
the Station staff was predation by hydrozoa. These animals
were developing in the water storage units, as well as other
parts of the water supply. Attempts had been made at control
but none were successful.
On-site wet laboratory should be developed to explore control
methods for problems such as these. A wet lab could be
established with relatively little expense (less than $500)
and be continually operated for finding practical solutions to
problems in prawn farming. Although it is rightly said that
“what works in the lab may not work in the field”, wet lab
conditions can closely simulate that of prawn hatchery
conditions and provide presumptive answers to many questions
in a short time span. Provision of a wet lab on the site of
such a hatchery is ideal and the setup could serve other
purposes such as toxicity of pesticides and effectiveness of
chemotherapeutics. As the Macrobrachium industry develops in
Thailand, it will be asking particular questions and the
Chachoengsao Station should be equipped to provide quick
answers when necessary.
Bacteria, fouling agents and other disease producing organisms - The Station is equipped with a microscope for routine examinations of prawns but there needs to be an expansion of this capability to include cultivation of microbes. The presence of microbes affecting prawns needs to be demonstrated on occasion as well as the microbial dynamics of culture water. In addition, the expansion of microbial capability will enhance the diagnostic service to local farmers. If there is no one on the staff with skills particular to microbial examination these could be easily obtained by training and counsel with the disease staff at NIFI.
The presence of fouling organisms such as peritrichous ciliates and filamentous bacteria will continue to be present on the prawns during the rearing periods. There are various management possibilities and each should be tried and compared until a management plan for these organisms becomes standard procedure. Written records of all management efforts will provide basis for development of the best working plan on management of the problem.
Extension
The Extension function could be greatly enhanced by the use of audio-video equipment. Such equipment would free staff from many unnecessary conferences. Because of the preparation and forethought required in development of a first class audio-video presentation, clientele often receive more comprehensive information. Staff could also be available for specific questioning and it would take less time. Considerable building space has been allotted at the Station for training. At a relatively low cost, the additional equipment would increase the possibilities of training participation at the Station.
B. Grow-out Farms
The Chachoengsao Station has many opportunities to conduct research which will be beneficial to hatchery managers. The grow-out phase must also continue to receive research support. Cooperative work on area farms is already in progress and will provide many production answers.
Support in disease diagnosis will be needed by farmers, especially in some cases that are beyond the technical ability of the farms. Examples are microbial and pesticide analyses. Efforts should be made to organize diagnostic support procedure and give it appropriate identity.
Disease and problems with water quality affect total production of a grow-out facility. This was clearly seen at the farm near Chiangmai. Demonstration-style testing should show benefits of disease, water-quality management techniques, as well as general production information.
III. Brackishwater Division
Several areas of concern were indicated by staff of the Brackishwater Division in regards to marine prawn and fish culture. These were (1) snail competitors in Penaeus culture, (2) isopods on sea bass in pen culture situations and (3) hatchery loss in Penaeus culture.
Snail control is a problem that has no satisfactory remedy. Chemicals such as Frescon (n-tritylmorpholine - Shell Oil Co., California) and Bayluscide (Bayer 73, Chemagro) have been used with some success in freshwaters but application in food production ponds has many unanswered safety factors. Copper sulfate is a traditional snail control chemical but unfortunately is quite toxic to crustacea. Some success has been obtained by drawdown techniques. Snails seek moist spots and may be killed by dusting with quicklime.
Isopods have been controlled in fish pens in the United States by using several minute baths with formalin in water at a concentration of 500 mg/l. Isopods are not killed but released and fall away for temporary relief.
Discussions with staff on hatchery loss of Penaeus were of limited value in solving problems. Presentations of various problems that I consider important were conducted with staff at Phuket and Songkla. Information was exchanged with disease staff but discussions did not lead to clarification of local problems with prawn health.
There is a need to assign importance to the development of a strong disease staff in the brackishwater area. Support should be given to certain individuals in the brackishwater division who desire to develop career competence in crustacean health. Involvement of the disease staff at NIFI with brackishwater disease problems would be very beneficial in answering questions that relate to mortality of Penaeus spp. and cultured marine fin fish.
IV. Inland Fisheries and the FAO Project: Pond Management and Fish Diseases.
Various fisheries stations were visited between June 25 and 28. On Friday, July 11, a Clarias farm was visited.
Problems with disease and adverse water quality on inland stations were very infrequent according to station chiefs and biologists. Some problems with anchor parasites (Lernaea) and bacteria were seen regularly by sand goby culturists using river basket culture. Antibacterials were being used in the food for control of bacteria but the procedure was of limited benefit. It was apparent that the changing water conditions that occur seasonally were predisposing bacterial infections. In such an open system enhancement of resistance may be the only answer. Perhaps dietary deficiencies are responsible for lowered resistance. If so, a series of experiments could be easily conducted in cooperation with local culturists using vitamin premixes, mineral additions or other diet additives. The Lernaea problem might be attacked with potassium permanganate dips at twenty-five ppm concentration for two hours.
Clarias disease problems were being intensively investigated by the staff at NIFI. Most of the parasite problems were associated with Trichodina. The intensive culture of these fish resulted in heavy loading with toxic waste products. Lime was being applied to raise alkalinity and probably benefited the fish in regard to carbon dioxide and ammonia. Sodium chloride was also being added and was to benefit water quality and aid ionic balance. Chlorides are beneficial in curtailment of methemoglobin formation.
Plans were being formulated for adding sub-sand or sub-gravel units for biofiltration. I suggested that trickle style filtration would be almost as economical and would probably have much more efficiency1. Trickle filters do not form undesirable anaerobic conditions as easily as submerged filters.
A trickle style filter for a production pond would simply consist of a wooden frame set above the pond surface and filled with coarse stones (baseball size). Water is lifted from the pond by pump and allowed to trickle through the biofilter and drop into the pond. Filters set upon levees would require diversion of water back into the pond.
Media and test procedures of special importance in presumptive identification of prawn disease organisms.
Brain heart infusion agar
| dehydrated BHI agar | 26 gm | |
| distilled water | 500 ml |
Heat to boiling to dissolve the medium completely. Sterilize.
Plates: Pour sterilized media into sterile petri dishes (⅓ full). Let stand for 10 minutes with lids off center so as to make an outlet for steam. Cover, let stand until it hardens. Invert, identify media and refrigerate.
Tubes: Fill tubes to desired amount; place caps on loosely and sterilize. Tighten caps after autoclaving. Lay tubes at an angle to solidify leaving a butt at the end of tube.
Method of sterilization: Autoclave for 15 minutes at 15 pounds of pressure (121 C).
Brain heart infusion agar with salt.
Add NaCl to the above medium to make 3% salt medium. Prepare as indicated for BHI
Tryptic soy agar
| dehydrated T S agar | 20 gm | |
| distilled water | 500 ml |
Heat to boiling to dissolve the medium completely. Sterilize. Prepare as for BHI. For prawn work make up 3% salt medium.
Sabouraud dextrose agar
| dehydrated sabouraud dextrose agar | 37 gm | |
| distilled water | 500 ml |
Prepare as above both with and without 3% NaCl.
Nutrient broth
| beef extract | 3 g |
| peptone | 5 g |
| distilled water | 1 liter |
Prepare as above in tubes but do not slant since this is liquid media. Make with and without NaCl.
O F basal medium (Hugh Leifson)
Preparation:
| dehydrated O F basal medium | 4.7 gm |
| distilled water | 500 ml |
Heat to boiling to dissolve the medium completely dispense into tubes 4.5 ml each.
| dextrose. | 10 gm | |
| distilled water | 100 ml |
Add 0.5 ml of dextrose solution to each tube.
Parafin oil
Overlay half of tubes with oil for a depth of ½ inch.
Procedure:
Inoculate two tubes, one with oil and one without. Place loop well below surface in medium and swish.
Read at 24 and 48 hours.
Results: positive, acid (A) is yellow color: negative, alkaline (Alk) remains green: gas (G) bubbles under the oil.
NH3 from arginine
Preparation:
Add the following together:
0.1% peptone
0.5% NaCl
0.3% agar
0.03%K2HPO4
0.001% phenol red
1.0% arginine HCl
water to 100%
Titrate with .1 N NaOH until pH is 6.8. The color should be orange. Autoclave. The final pH should be 7.2
Dispense into tubes and cover with ½ inch of oil.
Procedure:
Inoculate as for the O F basal medium. Incubate and read at 24 hours.
Results: positive, intense pink color develops, negative, no change
Cytochrome oxidase test
Procedure:
Smear a large amount of bacteria on Pathotec-CO test paper
(General Diagnostics Division, Warner-Lambert Co., Morris
Plains, N.J. 07950).
Results: positive, a bright blue color develops within 30 seconds;
negative, no change in color.
Catalase
Procedure:
Drop five drops of 3% hydrogen peroxide on a white spot place.
Scoop a moderate amount of bacteria from a colony and place in
the peroxide.
Results: positive, strong bubbling in 3–5 seconds; negative, no bubbling.
Flagella stain (Bacto Flagella Stain, Difco Laboratories, Detroit,
Michigan, U.S.A )
Stain preparation
| dehydrated Bacto Flagella Stain | 1.9 gm | |
| 95% ethanol | 33 ml | |
| distilled water | 67 ml |
Mix water and ethanol then add stain. Shake for 10 minutes.
Procedure:
Grow the bacteria in 5 ml of half-strength nutrient broth for about 18 hours.
Add 1 ml of 5–10% formalin and centrifuge (300 rpm, 10 minutes)
Remove the supernatant fluid by aspiration.
Add 10 ml distilled water and resuspend sediment.
Centrifuge at 3,000 rpm for 10 minutes.
Repeat wash.
Resuspend bacteria in 10 ml distilled water.
Using precleaned slides (corning) pour a drop of suspension onto slide.
Tilt slide and allow to run down to end of slide.
Air dry the film (do not heat).
Place slide on staining rack and add 1 ml of the stain solution. Stain for 10 minutes.
Flood off the stain with water.
Drain and allow to air dry.
Examine slide with microscope searching for bacteria with Flagella. Many may have to be examined before Flagella are found.
Susceptibility testing
From an isolated bacteria colony remove a loop of bacteria and place on BHI plate.
Using a sterile swab (cotton on wooden stick) distribute the bacteria to all parts of the media surface.
If media surface is not particularly moist then addition of several ml of sterile water will facilitate distribution of bacteria.
Add sensitivity disks to the place, spacing so that overlaps of diffusion will not cause confusion when reading.
Susceptible bacteria will not grow on area where antibacterial affects it and a ring of no growth is formed.
Read whether the microorganism is very sensitive, moderately sensitive, slightly sensitive, or resistant.
If the sensitivity discs are not marked it will be necessary to mark name on plate surface just under disc.
Inhibition by vibriostat
Make a 5% solution of 2,4-D amino -6, 7-disopropyl pteridine phosphate (known as 0/129) in distilled water or chloroform. Dip blank antibiotic sensitivity disks in solution and allow preparation to dry. Use as for prepared antibiotic disks by the method described above under susceptibility testing. Inhibition of growth by this compound is characteristic of most vibrios. 0/129 is available from: Gallard Schlesinger Chemical Mfg. Corp. 854 Mineola Ave. Carle Place, L.I. N.Y. 11514 and BDR Chemicals Ltd., Poole, England.
Gram stain
Solutions:
| A. Modified Hucker's Crystal Violet | |
| solution A | |
| ethyl alcohol 95% | 20 ml |
| crystal violet (certified) | 2 g |
| solution B | |
| ammonium oxalate | 0.8 g |
| distilled water | 80.0 ml |
| Mix solutions A and B. | |
| B. Iodine | |
| iodine | 1 g |
| potassium iodide | 2 g |
| distilled water | 300 ml |
| C. Decolorizer | |
| ethyl alcohol | 95 ml |
| acetone | 5 ml |
| D. Counterstain | |
| safranine 0 85% | 6 gm |
| ethyl alcohol | 20 ml |
| distilled water | 200 ml |
Staining procedure:
Air dry smear about 15 minutes.
Flood with methyl alcohol for 2 minutes.
Rinse with distilled water.
Flood smear with crystal violet solution and let stand for 1 minute.
Wash smear briefly with tap water and drain off excess water.
Flood smear with iodine solution and let stand for 1 minute.
Wash with tap water and decolorize until solvent flows colorless from the slide.
Wash briefly with tap water.
Counterstain with safranine for 20 seconds.
Wash briefly with tap water; blot dry and examine.
Result: Gram-positive organisms are blue; gram-negative, red.
Spore stain (Wirtz-Conklin)
Prepare smear on slide as above. Flood entire slide with 5% aqueous
malachite green. Steam for 3–6 minutes and rinse under running
tap water. Counterstain with 0.5% aqueous safranine for 30 seconds.
Result: Spores are seen as green spherules in red stain rods or with red stained debris.
Geimsa Stain
Remove blood and quickly make smear on slide. Air dry. Fix the
film with methyl alcohol for 30 seconds. Apply dilute stain for
10 minutes. Rinse with distilled water and air dry.
Hanging Drop
Apply vaseline or water around the depression of the slide.
Using the inoculating loop, aseptically transfer one drop of bacteria to the center of the coverslip. If bacteria are removed from solid media, mix a loop full in a ml of water and then transfer a drop of mixture to coverslip.
Invert the hanging drop slide and center it well over the drop of bacteria. Press down on the edges of the coverslip so the vaseline or water makes a seal.
Quickly and carefully turn the slide right side up so the hanging drop is suspended in the depression.
Observe the organisms for motility and morphology. Motility: motile, non-motile (don't confuse brownian movement with motility).
Morphology: Long rods, short rods, cocci, tetrads, etc.
Anaerobic jars. Gaspak. (available from BBl Division of Bioquest, Box 243, Cockeysville, MD 21030)
Materials include:
Anaerobic jar
Gaspak packet
catalyst
methylene blue indicator
Procedure:
Add plates or tube to jar.
Place one Gaspak envelope, one Anaerobic indicator and a catalyst in the jar.
Add 10 ml of water to Gaspak envelope and clamp on lid. Hydrogen produced by the packet reacts in the presence of the catalyst to produce an anaerobic atmosphere. CO2 is produced by the packet also.
Incubate for 48 hours.
Bacteriological media available from:
BBL Division of Bioquest, Box 243 Cockeysville, MD. 21030
Difco Laboratories, Detroit, Michigan.
PRAWN DISEASES
Modified from Vanderzant, C. and R. Nickelson. 1969. A microbiological examination of muscle tissue of beef, pork and lamb carcasses. Journal of Milk and Food Technology, 32 (9) : 257–261.
1. Scheme for identification of gram-positive bacteria.
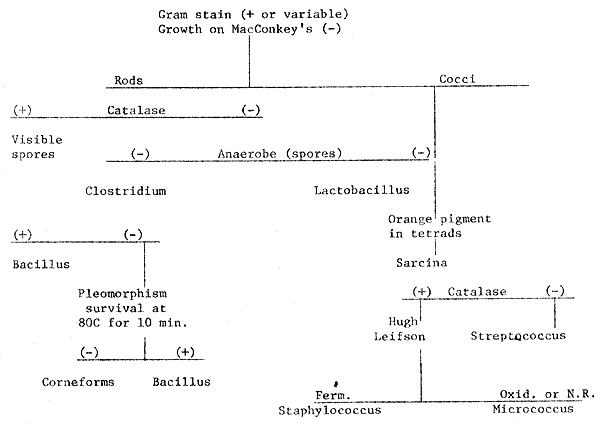
PRAWN DISEASES
2. Scheme for identification of gram-negative oxidase-positive bacteria.
Gram stain (-)
Growth on MacConkey's (+)
Oxidase (+)
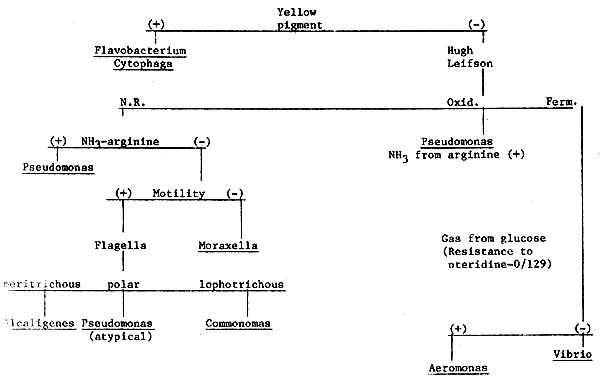
PRAWN DISEASES
3. Scheme for identification of gram-negative oxidase-negative bacteria.
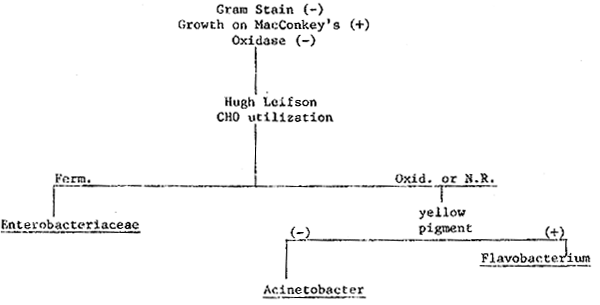
| Material or Equipment | Quantity | Price U.S.$ | |
| BHI agar | 1 | lb. | $44.80 |
| Tryptic soy agar | .25 | lbs. | 7.10 |
| Sabouraud dextrose agar | .25 | lbs. | 7.80 |
| Beef extract | .25 | lbs. | 14.40 |
| Peptone (Bacto) | .25 | lbs. | 7.50 |
| OF Basal medium | .25 | lbs. | 10.30 |
| Dextrose | 100 | g | 3.30 |
| NaCl | 1 | lb. | 4.75 |
| Potassium monophasphate dibasic (K2HPO4) | 500 | g | 20.74 |
| Ager (Bacto) | .25 | lbs. | 12.20 |
| Phenol red | 5 | g | 11.70 |
| L-arginine HCl | 10 | g | 3.40 |
| Sensitivity disks | 300 | disks | |
| 0/129 | 1 | g | 15.00 |
| Mthyl alcohol | 4 × | 8 pts. | 50.31 |
| Crystal violet (certified) | 25 | g | 11.00 |
| Ammonium oxalate | 1 | lb. | 29.24 |
| Iodine | 125 | g | 26.03 |
| Potassium iodide | 500 | g | 31.84 |
| Acetone | 5 | gal. | 36.20 |
| Hydrogen peroxide | 1 | pt. | 3.00 |
| Pathotec - CO test paper | 50 | strips | 4.89 |
| Bacto-Flagella stain | 100 | ml | 15.03 |
| Safranin | 25 | g | 16.20 |
| Malachite green | 25 | g | 12.96 |
| Geimsa stain | 5 | g | 9.72 |
| Anaerobic jar (non-vented) | 122.68 | ||
| Gaspak packet | pkg. | of 10 | 7 48 |
| Catlyst | pkg. | of 10 | 10.83 |
| Methylene blue indicator or | 25 | g | 11.88 |
| Anaerobic indicator gaspak | pkg. | of 100 | 15.30 |
| Perri dishes glass (100 × 15 mm) | pkg. | of 36 | 55.65 |
| Test tubes/screw cap (13 × 100 mm) | case | of 288 | 106.56 |
| Autoclave ($700) or pressure cooker | 1 | 80.00 | |
| Inoculating loops (nickel-chromium, platinum) | 4 | 5.00 | |
| Petri dishes (Disposable 100 × 15 mm) | 500 | 36.30 | |
| Glass slides, depression slides and cover slips | 30.00 | ||
| TOTAL | 891.09 | ||
AUDIO-VIDEO UNIT
| Equipment | Price U.S.$ |
| Wollensak -2570 AV Play/Record(Portable) | $370 |
| Kodak Ektagraphic Slide Projector AF-2 with Ektanar 5" f/2.8 lens | $220 |
| 140 Slide trays (2 extras) | $10 |
| TOTAL | $600 |
Formalin Residue
Determination of formalin residue in water supply using Nash's Reagent to produce the Hantzsch reaction.
Nash (Biochem J., 55 [1953] 416) found that the Hantzsch reaction between acetylacetone, ammonia and formaldehyde fluoresces. Belman (Anal. Chim. ACTA 29 [1963] 120–126) further refined the fluorimetric method. Procedures for tissue residue have been developed by others using trichloroacetic acid. In the process of doing this kind of work, we have used the same reaction in water to confirm concentrations. Instead of using a fluorimeter we used a simple pocket-size u.v. light to illuminate the water samples and visually compared them to standards of known concentration.
Nash Reagent: 2 M ammonium acetate and 0.02 M acetylacetone at pH 6.
Procedure: Add equal volume of Nash Reagent to sample and observe fluorescence.
PROGRAM FOR S. K. JOHNSON, JUNE 14 to JULY 13, 1980
| June 14 | Arrive Bangkok. Prepare for Giant Prawn 1980 Conference |
| June 15 – 21 | Participate in Conference. Chaired session, participated on panel, presented paper. Visited farms, hatcheries and fisheries institutions, as part of conference activities. |
| Sunday 22nd at Bangkok | |
| Monday 23rd | Planning meeting with Michael New FAO and Department of Fisheries officials. Afternoon - visited National Inland Fisheries Institute (NIFI) to discuss aquatic animal health with NIFI staff. |
| Tuesday 24th | Accompany Mr. Somsuk Singholka and Dr. Paul Sandifer to Cachoengsao Fishery Station. Reviewed facility and discussed various aspects of operation. |
| Wednesday 25th | To NIFI. Accompany Dr. Sitdhi Boonyaratplin to Chainat Inland Fisheries Station. Discussed Station activities and program with Station Director Chareon Phanil. Fish larvae were briefly examined for disease. Traveled on to Nakornsawan Station where Station activities and disease problems were discussed with station director Boonthai Thongsamui and staff. Travel to nearby river where pangasius and sand goby culture was taking place. |
| Thursday 26th | Travel to TAK Fisheries Station. Station activities discussed with director Chaiwat Panphrommin and staff. Travel to Chiangmai: Station activities discussed with Director Likhit Nukulrak and Biologist Rewat Ritthaporn. |
| Friday 27th | Chiangmai. Reviewed prawn and fish culture activities of local farms. |
| Saturday 28th | Return from Chaingmai to Bangkok |
| Sunday 29th. | At Bangkok |
| Monday 30th | Travel to Samutsongkram Province |
| Monday 30th | Visited brackishwater fish division then accompanied by Lila Ruangpan and Prapan Thanbupha visited private shrimp farm in Samutsongkram Province. Then to Mitilus unloading site to examine harvest. |
| Tuesday July 1 | Accompanied by Lila Ruangpan and Sophon Ruangpan visited Rayong Fisheries Station. Met with Station Chief Vichit and staff biologists. |
| Wednesday 2nd | Songkhla. Accompanied by Boonsong Sirikul visited station and met station chief Pairoj. Discussions held with Yaowanit Danayadol for the remainder of the day. |
| Thursday 3rd. | Trip to sea bass hatchery and culture area. Accompanied by Yaowanit Danayadol. |
| Friday 4th | Phuket. Accompanied by station chief Anuwat visited station and held discussions with biologist Sanchai Tandavanits and other staff. |
| Saturday 5th | Visit to station again and then to Phuket marine biological center. Travel to Bangkok. |
| Sunday 6th | In Bangkok |
| Monday 7th | Travel to Thai prawn farm: Accompanied by Somsuk Singholka. Examined problem with prawns. |
| Tuesday 8th | Travel to Bangkapong Station to review aquaculture problems with staff. |
| Wednesday 9th | Travel to NIFI to present a workshop on fish disease to staff. |
| Thursday 10th | At NIFI to present workshop on crustacean diseases. |
| Friday 11th | Visit catfish farm north of Bangkok accompanied by Sopa Areerat. Visit with fish disease section staff at NIFI. |
| Saturday 12th | Confer with Somsuk Singholka and Michael New. Prepare for departure. |
| Sunday 13th | Depart Bangkok. |
DISEASES OF Macrobrachium
S. K. Johnson
Department of Wildlife and Fisheries Sciences
Texas A&M University
College Station, TX 77843
U. S. A.
Freshwater prawns die from a variety of disease conditions. The causes of some disease conditions are unknown. Loss is experienced particularly in the hatchery phase where the prawns are cultured most intensively. Fouling by epibionts, predation and oxygen shortage are obstacles to successful pond production.
Various diseases and conditions affecting survival are presented. Their importance is examined and contrasted with similar diseases of other crustaceans. Disease management methods are discussed.
Disease is an important aspect in the culture of any organism and Macrobrachium is no exception. Disease becomes particularly prevalent when animals are reared intensively in hatchery situations. Hatches with low survival rates are in constant need of examination for causes of death. Although Macrobrachium is apparently more hardy than many other kinds of crustacea in the larval stage, the extended period of larval development allows for more problems in total. Juvenile and adult rearing phases experience less disease problems than the hatchery phase, but some of these problems are unique. This paper will review biotic and abiotic causes of Macrobrachium loss and highlight the desirability of certain disease management techniques.
Filamentous bacteria are frequently a problem in larval culture. The bacteria, most often designated Leucothrix sp., attach to gills and external body parts and interfere with normal body movement. This type of bacterium grows extensively in nutrient rich culture water.
Sessile protozoa (Epistylis, Zoothamnium, Cothurnia) can be over-burdening in both hatchery and grow-out phases. Zoothamnium and Cothurnia prefer the gills as sites of attachment whereas Epistylis is apparently nonselective (Hall, 1979). As with filamentous bacteria, sessile protozoa serve as biofilters in hatchery water and therefore tend to flourish in nutrient rich waters. Epibionts readily populate surfaces of crustaceans in pond waters that are nutrient rich. Such ponds typically will be observed as lacking in both rooted and planktonic plant life and as having some degree of turbidity.
Nutritional inadequacy of the prawn is thought to influence epibiont presence by supression of cleaning behavior and lowering resistance of the epicuticle of epibiont attachment. Temperature is influential since it determines the molting rate.
Management schemes for epibionts have met with various degrees of success. In hatchery rearing, chemical control of filamentous bacteria has been partially successful when antibiotics and copper sulfate were used (Solangi, et al., 1979; Lightner and Suplee, 1976). Reduction of the nutrient load by maintaining clean tanks, reducing the stock or tying nutrients up in a competetive species (i.e., Chlorella) (Fujimura and Okamoto, 1970) have been helpful as has restricting biofilter action to a separate part of the hatchery unit (Dugan, et al., 1975).
Ectocommensal protozoa have been successfully treated chemically with formalin, salt (sodium chloride), quinine bisulfate, quinacrine hydrochloride, and copper sulfate (Fisher, 1977; Schnick, et al., 1979; Roegge, et al., 1979; Fujimura, 1966). It should be remembered that these chemicals are selective toxins and the margin of safety for the particular chemical will decrease as the existing condition of the prawn decreases.
Because burdening protozoa interfere with feeding behavior, the addition of abundant high protein food can sometimes aid in increasing growth and molting. Such a practice can increase resistance and allow prawns to outgrow a potential epibiotic problem. Nurdjana, et al., (1977) used this technique in combination with frequent siphoning to successfully control diatom buildup on penaeid prawns. In Taiwan (Liao, et al., 1977), tea seed cake has been used to stimulate molting and thereby aid in relief from protozoa. Other management methodologies for protozoa are similar to those for filamentous bacteria.
Algal epibionts can be supressed to some degree by restricting light (Smith, et al., 1979).
Hydrozoans, insects, birds and cannibalism are responsible for a great amount of loss in prawn culture and are interrelated with what is commonly referred to as “diseases”. Hydrozoans have been noted (Sandifer, et al., 1974; Dugan, et al., 1975; Chao and Liao, 1977; Nurdjana, et al., 1977) to establish in larval tanks and serve as predators and competitors. Although Sandifer, et al., (1974) suggested destruction of infested batches and utilization of avoidance as a prevention measure, they and others have noted successful chemical measures. These were 250 mg/liter formalin for one hour (Sandifer, et al., 1974) 100 mg/liter nigrosin and concentrated saltwater (Chao and Liao, 1977).
Cannibalism is of particular importance in maintaining survival rates, especially in the early juvenile stage (Chao and Liao, 1977). Some species have been noted as more aggressive in this respect than others (Goodwin and Hanson, 1975). Molting is apparently an important period of susceptibility (Peebles, 1978). Diseased animals also have weakened mobility and are easily selected as prey. Cover provided for prawns provides for better survival (Smith and Sandifer, 1975). Nutrition must also influence cannibalism possibly as a result of inadequate intake or lack of specific required nutrients. Survival rates have been enhanced by addition of plant material (Maddox and Manzi, 1976).
Insects (Fujimura and Okamoto, 1970) and birds (Smith, et al., 1976a) have been a problem in the grow-out phase. Air breathing insects can be removed by repeated treatments with petroleum products (i.e., diesel fuel and motor oil mixed 20:1) poured over the water surface. Control of larval Odonata is in need of more research since these larvae are not air breathers.
Migratory birds are a constant threat and even those birds that are considered herbivorous will inadvertently consume large numbers of larval and juvenile crustaceans along with plant material. Birds are difficult to repel or control but netting is particularly helpful when hung over small ponds (Singholka and New, 1980).
Fungal diseases have not attained the notoriety in freshwater prawn culture that they have in culture of marine prawns (Ling, 1969; Sick and Beaty, 1974; Goodwin and Hanson, 1975; Smith, et al., 1976; Sindermann, 1977; Burns, et al., 1979). Perhaps freshwater prawns are more resistant than their marine relatives or fungal incidence has not been adequately reported. In cases of fungal presence, salinity changes and chemical control should be helpful.
Toxins, precipitants, and low oxygen are important causes of disease in the general sense or predisposing factors in the more specific sense. Ammonia and nitrite are pollutants of toxic importance. Levels of toxicity for larvae of Macrobrachium rosenbergii have been reviewed by Armstrong (1979) and Llobern (1979). Toxic levels are within the range expected in a hatchery unit with crowded stock or waste-laden culture water. Carbon dioxide is also an important toxic agent and its affects are probably underestimated in rearing units that have low pH and limited aeration.
In waters of where photosynthesis causes a high pH, the shift in carbonate equilibria can cause carbonate to precipitate as calcium carbonate. If this occurs and becomes affixed to larvae it will weight them down and cause deaths. Control of pH with acids has helped in some situations but other chemicals with stabilizing effects such as calcium sulfate (Boyd, 1979) should be explored. Iron is also commonly precipitated. Exudates from certain microbes can cause detrimental effects that are similar to those caused by precipitated materials.
Oxygen shortage and depletion can be problems in hatcheries but grow-out ponds are more seriously affected (Green, et al., 1977; Johnson, 1978). The prevention of oxygen shortage in grow-out ponds by use of the techniques of water replacement and production of current is common in all types of crustacean aquaculture. Certain fouling organisms can compound the effect of low dissolved oxygen levels and remove nutrients which served as a food base for epibionts (Johnson, 1978).
Biotoxins produced by algae and bacteria are known to affect crustacea (Amborski and Amborski, 1979; Sarig, 1971; Korringa, 1976; Johnson, personal observation). Microscopical surveillance of water for dinoflagellates and the use of sentinel animals are possible management techniques.
Injury is usually experienced when hard objects are struck during rapid swimming and jumping. The dorsal portion of the abdomen is commonly injured (Johnson, 1977). Other injuries occur from aggressive behaviour of prawns in transport or other culture conditions where they experience close confinement.
Epicuticle abnormality has been described for crustaceans (Fisher, et al., 1976), and like injury, it is responsible for much of the black spot syndrome which develops as bacteria invade susceptible areas of the exoskeleton. Epicuticle is a main defense against bacterial attack on the exoskeleton and may be found lacking when development is influenced by nutritional inadequacy or chemical action (Fisher, et al., 1976)
Endocuticle abnormalities have been observed by Johnson (1977) and may result from nutritional inadequacy or other stress. Interference with molting is a possible consequence.
Molt arrest is not necessarily an exoskeletal problem but proper shedding of the exoskeleton must occur to permit normal growth. Hatcherymen often see prawns go into molting and see them unable to complete the process. The reasons for this are not understood but it should be remembered that influence of toxins or other potentially stressful conditions such as low dissolved oxygen.
As in other crustaceans the musculature of Macrobrachium may become opaque, particularly in the posterior abdomen, of stressed prawns (Delves-Broughton and Poupard, 1976). Other phenomena such as a milky hypodermal condition of M. ohioni (Johnson, 1977), unusual action of chromatophores and interference with molting are probably related to influence of stressors. There is much to be learned about the effects of stressors on freshwater prawns. Aquacop (1977) associated a necrotic condition in appendages of M. rosenbergii larvae with bacteria and obtained positive results following treatment with antibiotics.
Reddish abdomens were noted in adult prawns reared in Florida (specimens supplied by Ed McSweeny, Weyerhaeuser, Co.). The reddish coloration resulted from much pigment that seemed to be dispersed from chromatophores that had lost their normal integrity. Viral or bacterial agents were not apparent in that situation.
Chromatophore disfunction is often noted in dying larvae. Larvae take on reddish-goldish colorations. The digestive gland will often have peculiar coloration as a result of tissue disintegration somewhere in the body. The basic causes of these symptoms to be discovered.
Areas of the gill cavity are prone to darkening. The gills, the gill bailers, and the carapace covering are variously affected. I consider precipitating chemicals and nitrogenous waste products principal causes. A common darkening of the inner wall of the carapace cover has always been in conjunction with culture water containing high levels of nitrogenous products.
Chemical treatment in Macrobrachium has developed slower than for other cultured crustaceans (Schnick, et al., 1979) but many treatments have universal application. Availability of chemicals is a major concern in aquaculture (Johnson, 1980) because of the extended influence of government regulations. In general, there has been a tendency to rely on chemical treatments for any problem considered a disease. Quite often chemical application merely served as an additional detriment. Perhaps the greatest misunderstandings about chemical treatments are the lack of regard of some chemicals as selective toxins and massive microbial “blooms” that result from the loss of supressive effects of others as treatment chemicals decompose and microbes take advantage of accumulated nutrients.
Salinity alterations have been noted as helpful against fungus (Dugan, et al., 1975; Goodwin and Hanson, 1975) and hydrozoa (Chao and Liao, 1977) and should be effective in some cases against protozoa. Temperature manipulation has been of little value except in raising it to optimal levels.
Stock and water pretreatment with antibacterials and other chemicals have been used as preventatives in the hatchery but the effectiveness has not been evaluated in the production level. Disinfectants for equipment follow patterns for other phases of aquaculture and efforts have been directed toward hydrozoans (Sandifer, et al., 1974) and epibionts (Sindermann, 1977).
Cleaning and water exchange are important hatchery practices. Epibionts have been controlled when siphoning of exuvia and dead artemia was practiced as part of the management methodology (Nudrjana, et al., 1977). Water exchange can be used to enhance water quality and discourage epibiont and bottom debris development (Dugan, et al., 1975; Johnson, 1978; Fujimura and Okamoto, 1970). In pond culture epibionts can be controlled by water exchange. The use of water exchange during periods of vegetation decomposition will also assure adequate oxygen levels not only for survival but also for optimal prawn growth.
Although benefit has been obtained from the use of algae in hatchery water, too much algae can become a burden to culture. Chemical treatments to retard algae have been unsuitable in most cases. The restriction of light by covers can be helpful in curtailing algal development. Too much light, however, can cause distress in prawns (Fujimura and Okamoto, 1970) and along with diet may be responsible for many of the chromatophore abnormalities experienced in larval culture and post-transport.
The numerical reduction of stock is an important management practice for hatcheries. It can reduce the spread of epibionts and reduce the effects of stressors that accompany poor water quality.
The use of nutritional enhancement as a disease management tool awaits a more complete understanding. Larvae burdened by epibionts have reduced feeding activity and its secondary effects. The addition of an abundance of food sometimes correct these shortcomings and normalize disease resistance (Nurdjana, et al., 1977). Application of a more nutritionally complete diet has influenced survival in Macrobrachium (Murai and Andrews, 1978), and in penaeids, the lack of ascorbic acid has been related to dietary disease and poor tissue healing (Lightner, et al., 1979).
Mechanical filtration is used to clean water in recirculating culture systems and also to prevent introduction of unwanted biotic and abiotic substances into the system. Filtration has helped to avoid introduction of hydrozoans, fish, and microbes. Ion-exchange filtration for toxic waste removal may prove to be an economical practice in hatchery work for either recirculated or new (incoming) water. In the case of bacterial filtration, the technique may serve to cause more problems than correct. Because bacterial processes are important in nutrient decomposition and water quality maintenance, filtration often just adds another unnatural obstacle to the stability of the rearing unit. Unwanted side effects and unneccessary costs are avoided when mechanical filtration is designed for specific objectives derived from real needs.
Biofiltration is an important aspect of hatchery rearing. In cases where filamentous bacteria and other epibionts flourish, the prawns themselves serve as substrates for biofiltration within the rearing water. Biofiltration units are usually established apart from the actual rearing space so as to localize the biofiltration away from the animals. Sometimes biofiltration takes place in the water of rearing space in the form of companion species such as plants (Cohen, et al., 1976; Fujimura, 1966), mollusks, or fish.
Ultraviolet lighting is very similar in effects to microfiltration. Microbes in the incoming water are killed but their remains pass on serving as substrates for growth of other bacteria. The most desirable feature is in preventing the introduction of agents known to cause problems. Ozonation can be considered a method of selective toxicity for potential biological pathogens or a method of chemical treatment for dissolved metabolic products. It has been used in a variety of crustacean culture situations and has been reported to have the additional advantage of enhancing water quality (Blogoslawski and Brown, 1979; Yamaguchi, 1978). Ultraviolet lighting and ozonation have not become established methods in crustacean aquaculture. Costs and technical difficulties in both of these methods have prevented them from being widely accepted. The influence of equipment manufacturers is partly responsible for sustaining interest and use of these methods.
Amborski, R.L. and Grace F. Amborski. 1979. Opportunistic pathogens and a bacterial disease of the Louisianna crawfish Procambarus clarkii. pp. 92–107. Proceedings of the Second Biennial Crustacean Health Workshop, Galveston, Texas, April 20–22, 1977. Texas A&M University Sea Grant Publication 79–114. 400 pp.
Aquacop. 1977. Observations on diseases of crustacean cultures in Polynesia. Proceedings of the 8th Annual Meeting World Mariculture Society, 8: 685–703.
Armstrong, D.A. 1979. Nitrogen toxicity to crustacea and aspects of its dynamics in culture systems. pp. 329–360. Proceedings of the Second Biennial Crustacean Health Workshop, Galveston, Texas, April 20–22, 1977. Texas A&M University Sea Grant Publication 79–114. 400 pp.
Blogoslawski, W. J. and C. Brown. 1979. Use of ozone for crustacean disease prevention: a brief review. pp. 220–230. Proceedings of the Second Biennial Crustacean Health Workshop, Galveston, Texas, April 20–22, 1977. Texas A&M University Sea Grant Publication 79–114. 400 pp.
Boyd, C. E. 1979. Water quality in warmwater fish ponds. Auburn University, Agricultural Experiment Station, Auburn, Alabama. 359 pp.
Burns, C. D., M. E. Berrigan and G. E. Henderson. 1979. Fusarium sp. infections in the freshwater prawn Macrobrachium rosenbergii (de Man). Aquaculture 16: 193–198.
Chao, N. H. and I. C. Liao. 1977. Status of the propagation of the giant freshwater prawn, Macrobrachium rosenbergii, in Taiwan. Journal of the Fisheries Society of Taiwan 5: 30–40.
Cohen, D., A. Finkel and M. Sussman. 1976. On the rule of algae in larviculture of Macrobrachium rosenbergii. Aquaculture 8: 199-207.
Delves-Broughton, J. and C. W. Poupard. 1976. Disease problems of prawns in recirculation systems in the United Kingdom. Aquaculture 7: 201–217.
Dugan, C. C., R. W. Hagood and T. A. Frakes. 1975. Development of spawning and mass larval rearing techniques for brackish-freshwater shrimps of the genus Macrobrachium (Decapoda, Palaemonidae). Florida Marine Research Publication No. 12. Florida Department of Natural Resources, St. Petersburg, Florida. 28 pp.
Fisher, W. S. 1977. Epibiotic microbial infestations of cultured crustaceans. Proceedings of the 8th Annual Meeting World Mariculture Society. 8: 673–674.
Fisher, W. S., T. R. Rosemark and E. H. Nilson. 1976. The susceptibility of cultured American lobsters to a chitinolytic bacterium. Proceedings of the 7th Annual Meeting World Mariculture Society. 7: 511–520.
Fujimura, T. 1966. Notes on the development of a practical mass culture technique of the giant prawn, Macrobrachium rosenbergii. FAO Indo-Pacific Fisheries Council. 12th Session, Honolulu, Hawaii. 4 pp.
Fujimura, T., and H. Okamoto. 1970. Notes on progress made in developing a mass culturing technique for Macrobrachium rosenbergii in Hawaii. FAO Indo-Pacific Fisheries Council, 14th Session, Bangkok, Thailand. 187 pp.
Goodwin, H. S., and J. A. Hanson. 1975. The aquaculture of freshwater prawns/Macrobrachium species. Summary of Proceedings Workshop on Culture of Freshwater Prawns, St. Petersburg, Florida. The Oceanic Institute. Waimanalo, Hawaii. 95 pp.
Green, J. P., T. L. Richards and T. Singh. 1977. A massive kill of pond-reared Macrobrachium rosenbergii. Aquaculture 11: 263– 272.
Hall, J. T. 1979. Ectocommensals of the freshwater shrimp, Macrobrachium rosenbergii in culture facilities at Homestead, Florida. pp. 214–219. Proceedings of the Second Biennial Crustacean Health Workshop, Galveston, Texas. April 20–22, 1977. Texas A&M University Sea Gran Publication 79–114. 400 pp.
Johnson, S. K. 1977. Crawfish and Freshwater shrimp diseases. Texas A&M University Sea Grant Publication 77–605. 19 pp.
Johnson, S. K. 1978. Some disease problems in crawfish and freshwater shrimp culture. Texas A&M University Extension Fish Disease Diagnostic Laboratory Publication FDDL-Sll. 5 pp.
Johnson, S. K. 1979. Aquaculture and aquatic animal medicine. Florida Sea Grant-International Association of Aquatic Animal Medicine State of the Art Symposium on Aquatic Animal Medicine. Marineland, Florida, April 25, 1979. Florida Sea Grant Publication. In Press.
Korringa, P. 1976. Farming marine fishes and shrimps. Developments of aquaculture and fisheries science, 4. Elsevier Scientific Publishing Company. Amsterdam, New York. 208 pp.
Liao, I-Chiu, F. R. Yang and S. W. Lou. 1977. Preliminary report on some diseases of cultured prawn and their control methods. Reports on Fish Diseases Research (1), JCRR Fisheries Service, 29: 28–33. (in Chinese with English abstract)
Lightner, D. V. and V. C. Supplee. 1976. A possible chemical control method for filamentous gill disease. Proceedings of the 7th Annual Meeting World Mariculture Society, 7: 473–481.
Lightner, D. V., B. Hunter, P. C. Magarelli, and L. B. Colvin. 1979. Ascorbic acid: nutritional requirement and role in wound repair in penaeid shrimp. Proceedings of the 10th Annual Meeting World Mariculture Society, 10: 513–528.
Ling, S. W. 1969. Methods of rearing and culturing Macrobrachium rosenbergii (de Man). FAO Fisheries Report 57, Volume 3. 1969.
Llobera, J. A. 1979. Toxicity of ammonia to larvae of Macrobrachium rosenbergii. M. S. Thesis. Texas A&M University.
Maddox, M. B. and J. J. Manzi. 1976. The effects of algal supplements on static system culture of Macrobrachium rosenbergii (de Man) larvae. Proceedings of the 7th Annual Meeting World Mariculture Society. 7: 677–698.
Marai, T. and J. W. Andrews. 1978. Comparison of feed for larval stages of the giant prawn (Macrobrachium rosenbergii). Proceedings of the 9th Annual Meeting World Mariculture Society, 9: 189–193.
Nurdjana, M. L., B. Martosudarmo and B. Tiensongrusmee. 1977. Observations on disease affecting cultured shrimp in Jepara, Indonesia. Bullentin Brackishwater Aquaculture Development Center, 3: 204–212.
Peebles, B. 1978. Molting and mortality in Macrobrachium rosenbergii. Proceedings of the 9th Annual Meeting World Mariculture Society. 9: 29–46.
Roegge, M. A., W. P. Rutledge and W. C. Guest. 1979. Chemical control of Zoothamnium sp. on larval Macrobrachium acanthurus. pp. 295– 299. Proceedings of the Second Biennial Crustacean Health Workshop, Galveston, Texas. April 20–22, 1977. Texas A&M University Sea Grant Publication 79–114. 400 pp.
Sandifer, P. A., T. I. J. Smith and D. R. Calder. 1974. Hydrozoans as pests in closed-system culture of larval decapod crustaceans. Aquaculture. 4: 55–59.
Sarig, S. 1971. Diseases of fishes - book 3: the prevention and treatment of diseases of warmwater fishes under subtropical conditions, with special emphasis on intensive fish farming. S. F. Snieszko and G. R. Axelrod (eds.). TFH Publications. Jersey City, New Jersey. 127 pp.
Schnick, R. A., F. P. Meyer, L. L. Marking and T. D. Bills. 1979. Candidate chemicals for crustacean culture. pp 245–294. Proceedings of the Second Biennial Crustacean Health Workshop, Galveston, Texas, April 20–22, 1977. Texas A&M University Sea Grant Publication 79–114. 400 pp.
Sick, L. V. and H. Beaty. 1974. Culture techniques and nutrition studies for larval stages of the giant prawn, Macrobrachium rosenbergii. Technical Report Series No. 74–5. Georgia Marine Science Center, University of Georgia. Skidaway Island, Georgia. 30 pp.
Sindermann, C. J. 1977. Diseases of freshwater shrimp. In C. J. Sindermann (ed.). Disease diagnosis and control in North American marine aquaculture, developments in aquaculture and fisheries science 6. Elsevier Scientific Publication Co. Amsterdam, New York. 329 pp.
Singholka, S. and M. B. New, 1980. The status of Macrobrachium farming in Thailand. Proceedings of the 11th Annual Meeting World Mariculture Society. In Press.
Smith, T. I. J. and P. A. Sandifer. 1975. Increased production of tank reared Macrobrachium rosenbergii through use of artificial substrates. Proceedings of the 6th Annual Meeting World Mariculture Society. 6: 55–68.
Smith, T. I. J., P. A. Sandifer and W. C. Trimble. 1976a. Pond culture of the Malaysian prawn, Macrobrachium rosenbergii (de Man) in South Carolina 1974–1975. Proceedings of the 7th annual Meeting World Mariculture Society. 7: 625–645.
Smith, T. I. J., P. A. Sandifer, and W. C. Trimble. 1976b. Progress in developing a recirculating synthetic seawater hatchery for rearing larvae of Macrobrachium rosenbergii. Proceedings of the 4th Annual Food-Drugs from the Sea Conference. Mayaquez, Puerto Rico, 1974 (Marine Technological Society): 167–181.
Solangi, M. A., R. M. Overstreet, and A. L. Gannam. 1979. A filamentous bacterium on the brine shrimp and its control. Gulf Research Reports. 6: 275–281.
Yamaguchi, R. 1978. Ozone treatment in a closed culture system for Macrobrachium rosenbergii. M. S. Thesis. Texas A&M University.