
 |
THA/75/012/WP 27 ACUTE TOXICITIES of AMMONIA and NITRITE to
Clarias batrachus and THEIR INTERACTION
to CHLORIDES |
by
Maitree Duangsawasdi and Chouychoosri Sripoomun
Programme for the Development of Pond Management Techniques
and Disease Control (DoF - UNDP/FAO THA/75/012)
Thailand
National Inland Fisheries Institute
Bangkok, Thailand
1981
The "Programme for the Development of Pond Management Techniques and Disease Control (THA/75/012) was implemented in Thailand during 1979–82 as a joint project by the Department of Fisheries (DoF) and UNDP/FAO. The purpose of the project was to improve DoF support services for Clarias farming through strengthening:
the skills of Fisheries staff in aquaculture disciplines such as disease diagnosis and treatment, pond management and extension,
the research on solutions for problematical aspects of Clarias culture,
the system of relaying problems from the farms to DoF and of transferring improved technologies, and
the equipment and facility base of DoF for working on aquaculture problems.
Although the UNDP/FAO participation was structured terminate in August 1981, DoF committed continuation of the project to at least August 1982.
This report is one of several Working Papers prepared on various aspects of the project. A list of titles of reports completed in the series is annexed.
Inquiries concerning the subject matter of any particular report should be directed to the author,
| c/o | The Director National Inland Fisheries Institute Kasetsart University Campus Bangkhen, Bangkok 9 Thailand |
Hyperlinks to non-FAO Internet sites do not imply any official endorsement of or responsibility for the opinions, ideas, data or products presented at these locations, or guarantee the validity of the information provided. The sole purpose of links to non-FAO sites is to indicate further information available on related topics.
This electronic document has been scanned using optical character recognition (OCR) software. FAO declines all responsibility for any discrepancies that may exist between the present document and its original printed version.
2.1 Fish Holding and Feeding Conditions
2.2.2 The test solution delivery system
2.2.3 Stock solution preparation
3.2 Effects of Chloride on Ammonia Toxicity
3.4 Effects of Chlorides on Nitrite Toxicity
4. SUMMARY and RECOMMENDATIONS
List of Figures
1. Diagram of the Test Solution Delivery System
2. Effects of Chlorides on Ammonia Toxicity to Clarias
3. Effects of Chlorides on Nitrite Toxicity to Clarias
List of Tables
1. Response of Clarias Fingerlings Exposed to Ammonia Solution during 48 Hours Period
4. Response of Clarias Fingerlings Exposed to Nitrite Solutions during 48 Hours Period
ACUTE TOXICITIES OF AMMONIA and NITRITE TO Clarias batrachus
and THEIR INTERACTION TO CHLORIDES1
Maitree Duangsawasdi2 and Chouychoosri Sripoomun3
The study on acute toxicity of ammonia and nitrite to Pla Duk Dan (Clarias batrachus Linnaeus) was conducted using the flow-through bioassay method. The test fish of fingerlings size were exposed to five successive concentrations of ammonia and nitrite for 48 hours. The results obtained from this experiment using Probit analysis indicated that the LC50 values of those two toxicants were 15.78 mg/L of total ammonia (or 3.42 mg/L as un-ionized ammonia) and 35.60 mg/L of nitrite respectively.
Interactions between calcium chloride (CaCl2) and sodium chloride (NaCl) on ammonia and nitrite toxicity were also done in this study. The results generally showed that both ammonia and nitrite toxicity to Clarias were lessened when those two chloride compounds were presented in the test solution. The 48 hour LC50 values were increased to 44.34 mg/L of total ammonia and 119.02 mg/L of nitrite in test solution containing calcium chloride and 20.89 mg/L of total ammonia and 151.77 mg/L nitrite in test solution containing sodium chloride respectively.
3 Graduate Student, Environmental Science Department, Kasetsart University, Thailand.
The viability of Clarias culture in Thailand is believed to be restained by the affect of nitrogeneous compounds on fish production. Fish growth is arrested, and disease usually leading to death is abetted, when sub-lethal, stress-inducing concentration occur while lethal levels result in outright mortality. The deleterious concentrations are due to accumulations generated within the culture system as well as those introduced with the source water for infilling culture ponds that carries waste loads from elsewhere.
Ammonia and its oxidization products are assumed to account for nearly all the nitrogen compounds in the Clarias culture system. Ammonia is oxidized in a two step process to nitrite (NO-2) and nitrate (NO-3) where ammonia itself is the sum of un-ionized ammonia (NH3) and ionized ammonia (NH+4). Un-ionized ammonia is believed to be toxic to fish whereas ionized ammonia has little or no toxicity. Nitrite, an intermediate product of nitrification, can also exert its toxic action to the fish especially in the intensive culture system like Clarias. The toxicity of nitrite is believed primarily due to its ability to cause hemoglobin to be oxidized to methemoglobin, a form not capable of transporting oxygen.
Clarias as with all freshwater teleosts are presumed to have an inherent threshold tolerance for ammonia and nitrite. At concentrations below the limit fish can maintain normal physiology and health. At concentrations above the limit physiological impairment, manifesting as stress, and vulnerability to disease increase as the limit is exceeded. Fish can recover from brief exposure to low stressor concentrations but death is inevitable when the exposure to these concentrations is prolonged and when high concentrations occur even for short periods.
The study herein was developed to determine the lethal concentrations of ammonia and nitrite to Clarias in order to estimate the safe concentrations that Clarias can endure without jeopardy to aquaculture production of the species. The interactions of toxicity with other substances such as chlorides which can be used to decrease the toxicity of ammonia and nitrite were also investigated. The results would thereby can serve as a reference and guideline for management of Clarias culture.
Fish used in this study were Clarias batrachus fingerlings of either sex with a mean weight of 1.10 grams (range 0.52–1.30) and a mean length of 5.7 centimeters (range 4.5–6.5). They were purchased from private catfish farm and were transported to the hatchery at the National Inland Fisheries Institute (NIFI) until required for the experiment.
Fish were transferred from hatchery to the acclimation tank in the wet lab that received a well water of NIFI (hardness as CaCO3 = 149 mg/L, alkalinity = 290 mg/L, pH = 7.6). Water in the tank was continously areated through an air stone and the tank were cleaned every 2 days. The fish were fed once daily on a diet of dry pellet food at about 5 percent of body weight. Fish were maintained in good health on this diet and no significant mortality occurred. Feeding was stopped 48 hours before starting the experiment. The fish were allowed to acclimate to this holding condition for at least 2 weeks prior to use in the study.
Circular polyethylene tanks, 30 cm in diameter and 20 liters in volume, were used in the experiment. Twelve test vessels were placed in a water bath for temperature control during the experiment. Clean plastic bags were placed in the test vessels at the beginning of each experiment, to prevent residual contamination of the test vessel between experiments and, when secured at the top, to prevent fish from jumping out of the test vessels. Plastic bags were discarded after each experiment. The test vessels were rinsed with dilution water for 24 hours before fish were introduced to the test vessels. Dilution water was from the same source of water that supplied the holding tank.
A modified Mount and Brungs (1967) proportioned diluter (Harrison et. al, 1975) Figure 1 was constructed and used to deliver 5 concentrations of test solution and a dilution water control in duplicate to twelve test vessels. The diluter supplied 250 ml of test solution to each test vessel at each cycle. The cycle time for each delivery was 4 minutes which provided 95 percent replacement time in 12 hours (Sprague, 1969).
2.2.3.1 Ammonia stock solution was prepared by using technical grade ammonia solution (27% w/w NH3-N, May and Baker) and diluted with deionized water to get concentration at 10,000 mg NH3/L. This stock solution was kept in dark glass bottle and placed in ice box at low temperature to prevent vapor loss of ammonia gas during experiment. The ammonia stock solution was mixed with dilution water from diluter which provide the highest concentration of ammonia to the test vessel at 56 mg/L and successive concentration at 32, 18, 10 and 5.6 mg/L respectively.
2.2.3.2 Nitrite stock solution was prepared by diluted analytical grade sodium nitrite (NaNO2) with deionized water to provide nitrite solutions to test vessel at the highest concentration of 63 mg/L. By diluting this stock solution with various quantities of dilution water, the diluter delivered nitrite solution to the test vessel in a series of 63, 48, 36, 26 and 20 mg/L respectively.
2.2.3.3 Chlorides solutions were prepared by dissolved analytical grade sodium chloride (NaCl) and calcium chloride (CaCl2) with dilution water to get concentrations at 250 and 500 mg/L for ammonia and nitrite experiment respectively.
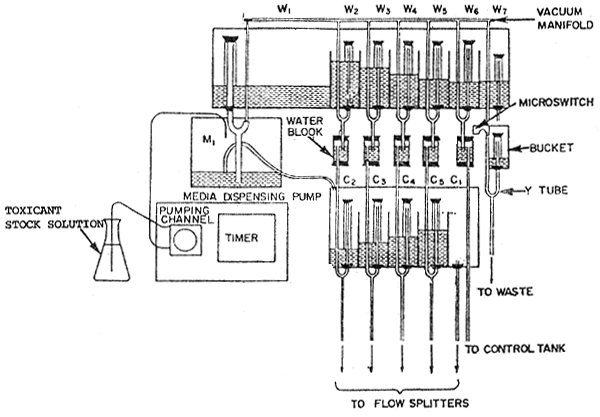
Figure 1. Diagram of the test solution delivery system.
Stock solution of ammonia and nitrite for all experiment were prepared from the same source of analytical grade materials using the same method of preparation. Stock solution for each experiment was prepared in the morning prior to the beginning of the experiment in a glass container. At the beginning of each experiment a proper amount of stock solution was added to each test vessel to make the desired concentration.
Clarias fingerlings of either sex which were acclimated for 2 weeks in the holding tank were equaly distributed in a random manner into 12 test vessels (10 fish per vessel). Fish were held in the test vessels for 48 hours before the beginning of the experiment. All tests were run for a 48 hour period. Feeding were witheld after the fish were put in the test vessels and during the experiment.
Treated fish in each test were checked for mortality at half hour intervals for the first six hours and hourly intervals upto twelve hours and at 14, 16, 18, 24, 30, 36 and 48 hours. Criteria for death were the cessation of respiration and lack of response to any tactile stimuli. Dead fish were removed from test vessels and time of death, weight and length of each fish were recorded.
Water chemistry characteristics of test water in each test vessel were checked for dissolved oxygen, pH, alkalinity, hardness and temperature at 24 hour intervals (APHA, 1973).
Median lethal concentration (LC50, the concentration of test solution that produced 50 percent mortality at a definite time period) and 95 percent confidence intervals were calculated at 48 hour period by using the probit analysis method of Finney (1971).
The 48 hour LC50 value of ammonia for Clarias fingerlings was estimated at 15.78 mg/L of total ammonia or 3.42 mg/L of un-ionized ammonia concentration as determined by the percentage of ionization suggested by Emerson et. al. (1975). All test fish in the test vessels containing 56 and 32 mg/L of total ammonia were killed within 24 hours. The lowest concentration of total ammonia that the test fish are all survived was 5.6 mg/L. The 95 percent confidence intervals for LC50 value was also estimated lying between 13:11 to 19.00 mg/L. Effects of ammonia to Clarias is summarized in Table 1.
The LC50 for ammonia toxicity to Clarias observed from this experiment is higher than the results obtained from other studies. The 48 hour LC50 of un-ionized ammonia to rainbow trout (Salmo gairdneri) was reported at 0.41 mg/L (Ball, 1967). Herbert and Shurben (1965) and Lloyd and Orr (1969) also reported the 24 hr LC50 of un-ionized ammonia to rainbow trout at 0.45 and 0.39 mg/L respectively. Buckley (1978) studied the toxicity of ammonia to coho salmon (Oncorhynchus kisutch) found the 96 hour LC50 at 0.45 mg/L. Thurston et. al. (1978) reported the 96 hr LC50 of un-ionized ammonia to cutthroat trout (Salmo clarki) at 0.5–0.8 mg/L.
Table 1. Responses of Clarias fingerlings exposed to ammonia solutions during 48 hour period.
| Conc. (mg/L) | Log conc. (X) | No. of fish exposed | No. of dead fish | Percent mortality | Expected probit (Y) |
| 56.0 | 1.7482 | 20 | 20 | 100 | - |
| 32.0 | 1.5052 | 20 | 20 | 100 | 6.8651 |
| 18.0 | 1.2553 | 20 | 11 | 55 | 5.3470 |
| 10.0 | 1.0000 | 20 | 3 | 15 | 3.7962 |
| 5.6 | 0.7482 | 20 | 0 | 0 | - |
| 0 | 0 | 20 | 0 | 0 | - |
Notes:
Equation of the probit regression line: Y = -2.2785 + 6.0747X
Slope = 1.53
Estimated 48 hr LC50 = 15.78 mg/L
95 percent confident intervals = 13.11 – 19.00 mg/L
The difference in tolerance of fish to ammonia concentrations may occur which probably due to the speciation of fish as suggested by Buckley (1978). Most salmonid fishes which used in the above studies may probably more sensitive to ammonia than Clarias and other species. Knepp and Arkin (1973) and Colt and Tchobanoglous (1976) reported the 96 hr LC50 of ammonia to channel catfish (Ictalurus punctatus) at 37.5 mg/L of total ammonia and 3.8 mg/l of un-ionized ammonia respectively which are in the same level observed from Clarias experiment. Therefore, the difference in physiological and anatomical of each fish species can influence on the toxicity of ammonia.
Ammonia appears to affect neuronal activity of the fish as indicated by the convulsion observed in this experiment and others. The test fish that exposed to high concentration of ammonia showed erratic movement and swam up to the surface. Later the fish lost its balance, splasm and sank down to the bottom of test tank and died afterward. However, the toxic action of ammonia is still not clear once it has entered the fish. Other investigators reported the symptoms of ammonia toxicity in fish include hyperventilation, violent erratic movement including convulsions and coma (Flis, 1963). Prolonged exposure to sublethal levels of ammonia may cause a reduction in growth rate (Brockway, 1950; Larmoyeux and Piper, 1973), proliferation of gill lamellae, reduced stamina, and increased susceptibility to bacterial gill disease (Burrows, 1964), thickening of the gill lamellae epithelium, reduction in lymphoid tissue in the spleen and hematopoietic tissue in the kidney (Larmoyeux and Piper, 1973), tissue disintegration lesions in blood vessels, and an abundant secretion of mucus (Flis, 1973). Smart (1978) reported that rainbow trout exposed to high concentration of ammonia exhibited high oxygen consumption and swollen of gill lamellae. Smart (1978) also observed that ammonia affect the affinity of hemoglobin to oxygen and the oxygen pressure in dorsal aorta was lowered.
Hillaby and Randall (1979) found that while the un-ionized form of ammonia in water has been shown to be toxic, however, in the blood either the ionized form or the total ammonia load is toxic to fish. The concentration of un-ionized ammonia is dependent not only on total ammonia concentration but also on pH, temperature and ionic strength of the solution. The concentration of un-ionized ammonia increases with pH and temperature but decreases with an increase in ionic strength.
Many external factors, such as temperature (Lloyd, 1961), pH (Smart, 1976), dissolved carbon-dioxide (Alabaster and Herbert, 1954; Lloyd and Herbert, 1960), dissolved oxygen (Downing and Merkens, 1955; Merkens and Downing, 1959; Larmoyeux and Piper, 1973), and bacterial conversion of ammonia to nitrite also contribute to toxic symptoms observed in fish. These external factors may in themselves be toxic to fish or act synergistically with ammonia. Therefore, the toxicity of ammonia to Clarias in natural condition including the mechanism of action still need further investigation.
The 48 hour LC50 value of ammonia on Clarias fingerlings, when calcium chloride was added in the test solution at 500 mg/L, was estimated at 44.34 mg/L of total ammonia concentration. The 95 percent confident intervals was lying between 38.99 to 50.53 mg/L of total ammonia. The highest concentration of total ammonia that the test fish were all survived was 20.7 mg/L. Effects of calcium chloride on ammonia toxicity to Clarias is summarized in Table 2.
The estimated 48 hour LC50 of ammonia on Clarias when added sodium chloride in the test solution at 500 mg/L was 20.89 mg/L of total ammonia concentration. The 95 percent confident intervals was 17.38 to 25.09 mg/L. The test fish were all survived in the highest concentration at 5.6 mg/L of total ammonia. The effect of sodium chloride on ammonia toxicity is summarized in Table 3.
In general, the toxicity of ammonia to Clarias was decreased when adding calcium chloride or sodium chloride into the test solution. However, the antagonistic action of sodium chloride to ammonia was lessen when compared with calcium chloride (Figure 2). The difference was probably due to calcium chloride can increase more ionic strength of the solution than sodium chloride. The increasing of ionic strength will increase the percentage of ionization of ammonia in the solution and therefore lowered the un-ionized form which is the most toxic form of ammonia as previously discussed. Herbert and Shurben (1965), Hazel et. al. (1971) and Alabaster et. al. (1979) also reported that increasing of salinity of the test solution can decrease the toxicity of ammonia to fish. However, no data available on the experiment on the effect of calcium chloride on ammonia toxicity in other fish species.
The addition of salt or sodium chloride by the fish farmers into Clarias pond in certain period of time is a common practice for quite some- time. The fish farmers believed that addition of salt can protect disease infection and improve the water quality. However, the amount of salt added are varied from time to time as their experience. Besides, the use of trash fish which mixed with salt for feeding Clarias may contribute certain amount of chloride into the pond. Therefore, the antagonistic effect of chloride, especially sodium chloride, on ammonia toxicity still need further study in the culture conditions inorder to determine the suitable amount of chloride for adding in the Clarias pond for effective prevention of fish mortality from high ammonia concentration during the culture period.
The 48 hour LC50 value of nitrite for Clarias fingerlings was estimated at 35.60 mg/L with 95 percent confident intervals of 30.62 to 41.27 mg/L. The highest concentration of nitrite that the test fish were all survived is 19.8 mg/L. The effect of nitrite on Clarias was summarized in Table 4.
Table 2. Effects of calcium chloride (500 mg/L) on ammonia toxicity to Clarias fingerlings during 48 hour period.
| Conc. (mg/L) | Log conc. (X) | No. of fish exposed | No. of dead fish | Percent mortality | Expected probit (Y) |
| 65.0 | 1.8129 | 15 | 15 | 100 | 12.0377 |
| 48.7 | 1.6890 | 15 | 8 | 53 | 8.5801 |
| 36.5 | 1.5630 | 15 | 3 | 20 | 6.5959 |
| 27.6 | 1.4409 | 15 | 1 | 7 | 4.8253 |
| 20.7 | 1.3160 | 15 | 0 | 0 | - |
| 0 | 0 | 15 | 0 | 0 | - |
Notes:
Equation of the probit regression line: Y = -9.6269 + 8.882X
Slope = 1.29
Estimated 48 hr LC50 = 44.34 mg/L
95 percent confident intervals = 38.99 – 50.53 mg/L
Table 3. Effects of sodium chloride (500 mg/L) on ammonia toxicity to Clarias fingerlings during 48 hour period.
| Conc. (mg/L) | Log conc. (X) | No. of fish exposed | No. of dead fish | Percent mortality | Expected probit (Y) |
| 56.0 | 1.7482 | 20 | 20 | 100 | - |
| 32.0 | 1.5052 | 20 | 17 | 85 | 5.9738 |
| 18.0 | 1.2553 | 20 | 11 | 55 | 4.6595 |
| 10.0 | 1.0000 | 20 | 1 | 5 | 3.3157 |
| 5.6 | 0.7482 | 20 | 0 | 0 | - |
| 0 | 0 | 20 | 0 | 0 | - |
Notes:
Equation of the probit regression line : Y = -1.9480 + 5.2638X
Slope = 1.52
Estimated 48 hr LC50 = 20.89 mg/L
95 percent confident intervals = 17.38 – 25.09 mg/L
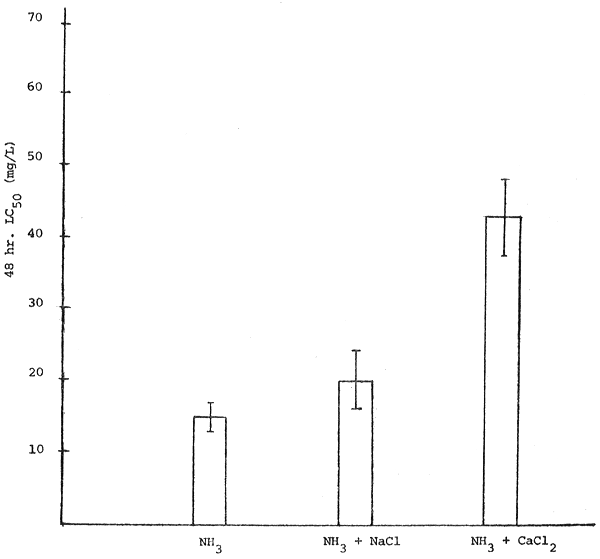
Figure 2. Effects of chlorides on ammonia toxicity to Clarias.
Table 4. Responses of Clarias fingerlings exposed to nitrite solutions during 48 hour period.
| Conc. (mg/L) | Log conc. (X) | No. of fish exposed | No. of dead fish | Percent mortality | Expected probit (Y) |
| 63.7 | 1.8044 | 10 | 10 | 100 | - |
| 47.8 | 1.6794 | 10 | 9 | 90 | 6.0263 |
| 36.0 | 1.5563 | 10 | 4 | 40 | 5.0383 |
| 26.5 | 1.4232 | 10 | 2 | 20 | 3.9701 |
| 19.8 | 1.2983 | 10 | 0 | 0 | - |
| 0 | 0 | 0 | 0 | 0 | - |
Notes:
Equation of the probit regression line: Y = -7.4524 + 8.0259X
Slope = 1.34
Estimated 48 hr LC50 = 35.60 mg/L
96 percent confident intervals = 30.62 – 41.37 mg/L
The LC50 value of nitrite to Clarias from this experiment is higher that values obtained from other studies. Westin (1974) reported the 96 hr LC50 of nitrite to chinook salmon fingerlings (Oncorhynchus tshawytcha) at 0.88 mg/L. Russo et. al. (1974) and Brown and Mcleay (1975) reported the 96 hr LC50 of nitrite to rainbow trout fingerlings at 0.19 and 0.23 mg/L respectively. The 48 hr LC50 of nitrite to mosquitoesfish (Gambusia affinis) was reported at 1.5 mg/L (Wallen et. al., 1957).
The toxicity of nitrite on fish is believed to cause by the formation of methemoglobin in the blood which can not carry oxygen (Smith and Russo, 1975). In this experiment, the rate of respiration of treated fish as determined from the movement of operculum and mouth was higher than the control group. Nitrite, therefore, may exert toxic effect by causing the fish unable to take oxygen from the outside environment even the oxygen level are still higher. The difference in physiological structure in each species may cause the toxicity of nitrite differ. Clarias which has artificial breathing organ or dendrite that can take oxygen directly from the atmosphere, therefore, can tolerate higher level of nitrite and formation of methemoglobin than other species.
However, the acute toxicity of nitrite to fish still unclear. Smith and William (1974) observed that the mortality rate of rainbow trout was not related with level of methemoglobin in the blood. Perrone and Meade (1977) also found that the treated fish that has high level of methemoglobin as 89 percent still survived and showed no sign of abnormality. Crawford and Allen (1977) concluded that the mortality of fish may not caused by the formation of methemoglobin alone but produced by the nitrite toxicity directly. Colt and Tchobanglous (1976) also suggested that the toxicity of nitrite to fish may cause by un-ionized nitrite or nitrous acid (HNO2) which can be pass through the membrane more easily.
The 48 hour LC50 value of nitrite when adding calcium chloride into the test solution at 250 mg/L was estimated at 119.02 mg/L. The test fish can survive in nitrite concentration as high as 76.50 mg/L which at this concentration all test fish were died when there was only nitrite in the test solution. The effects of calcium chloride on nitrite toxicity to Clarias was summarized in Table 5.
The 48 hour LC50 value of nitrite for Clarias fingerlings when adding sodium chloride in the test solution at 250 mg/L was observed at 151.77 mg/L. The highest concentration of nitrite that test fish were all survived was 78.7 mg/L which they were all died at this concentration when exposed to nitrite alone. Table 6 showed the effect of sodium chloride on nitrite toxicity to Clarias.
The results obtained from this experiment indicated that calcium chloride and sodium chloride can decrease the toxicity of nitrite to Clarias as the LC50 values were increased (Figure 3). The antagonistic effects of chlorides on nitrite may cause by the prevention of methemoglobin formation in the fish since chlorides can combine with nitrite in the solution (Tomasso et. al. 1979; Wedemeyer and Yasutake, 1978). Crawford and Allen (1977) also observed that the mortality rate of chinook salmon exposed to nitrite in salt water was lower than in freshwater experiment.
Table 5. Effects of calcium chloride (250 mg/L) on nitrite toxicity to Clarias fingerlings during 48 hour period.
| Conc. (mg/L) | Log conc. (X) | No. of fish exposed | No. of dead fish | Percent mortality | Expected probit (Y) |
| 240.0 | 2.3902 | 10 | 10 | 100 | - |
| 180.0 | 2.2553 | 10 | 9 | 90 | 6.7720 |
| 135.0 | 2.1303 | 10 | 7 | 70 | 5.5391 |
| 101.0 | 2.0043 | 10 | 3 | 30 | 4.2964 |
| 76.5 | 1.8837 | 10 | 0 | 0 | 3.1069 |
| 0 | 0 | 10 | 0 | 0 | - |
Notes:
Equation of the probit regression line: Y = -15.472 + 9.863X
Slope = 1.33
Estimated 48 hr LC50 = 119.02 mg/L
95 percent confident intervals = 105.14 – 134.73 mg/L
Table 6. Effects of sodium chloride (250 mg/L) on nitrite toxicity to Clarias fingerlings during 48 hour period.
| Conc. (mg/L) | Log conc. (X) | No. of fish exposed | No. of dead fish | Percent mortality | Expected probit (Y) |
| 249.0 | 2.3968 | 10 | 10 | 100 | - |
| 187.0 | 2.2718 | 10 | 9 | 90 | 5.9687 |
| 140.0 | 2.1461 | 10 | 2 | 20 | 4.6253 |
| 105.0 | 2.0212 | 10 | 1 | 10 | 3.2906 |
| 78.7 | 1.8963 | 10 | 0 | 0 | - |
| 0 | 0 | 10 | 0 | 0 |
Notes:
Equation of the probit regression line: Y = -18.3096 + 10.6868
Slope = 1.23
Estimated 48 hr LC50 = 151.77 mg/L
95 percent confident intervals = 136.34 – 168.95 mg/L
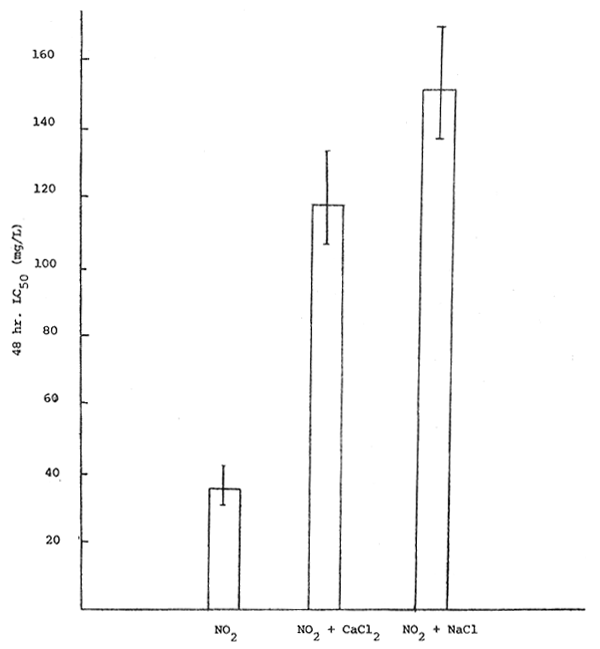
Figure 3. Effects of chlorides on nitrite toxicity to Clarias.
Tomasso et. al. (1979) found that the addition of chloride into nitrite solution at the ratio of 16:1 is enough to prevent the formation of methemoglobin in the test fish. However, the result from this experiment showed that the addition of chlorides at the ratio of 2:1 (Cl-:NO-2) can prevent fish mortality up to 100 percent when compared with the mortality rate of test fish in the same concentration of nitrite alone. The conditions of dilution water such as hardness, pH, alkalinity, temperature and dissolved oxygen concentration as well as species and size of test fish can also contribute some effects on the interactions between chlorides and nitrite (Smith and Williams, 1974; Russo et. al., 1974; Perrone and Meade, 1977). Therefore, the antagonistic effects of chlorides on nitrite toxicity still require further study to determine the suitable ratio of chlorides that can be used to prevent the toxicity of nitrite.
4.1 The 48 hr LC50 of ammonia to Clarias is estimated at 15.78 mg/L as total ammonia or equivalent of 3.42 mg/L as un-ionized ammonia. The approximate safe concentration of ammonia to Clarias is calculated at 0.17 mg/L as un-ionized ammonia concentration (using the application factor of 0.05 multiply by LC50 value as recommended by US, EPA, 1973). The standard level set by EIFAC (1973) for ammonia is 0.02 mg/L as un-ionized ammonia.
4.2 The 48 hr LC50 of nitrite to Clarias is observed at 35.60 mg/L with the 95 percent confident intervals of 30.62 to 41.37 mg/L. The estimated safe concentration of nitrite to Clarias is calculated at 1.78 mg/L (using the application factor of 0.05). The standard level set by US, EPA (1973) for nitrite is 1.0 mg/L.
4.3 Calcium chloride (CaCl2) and sodium chloride (NaCl) have antagonistic effect to ammonia and nitrite toxicity on Clarias. Calcium chloride was more effective than sodium chloride in decreasing the toxicity of ammonia to Clarias as observed from the LC50 values. However, in nitrite experiment, calcium chloride has stronger antagonistic effect than sodium chloride.
4.4. Antagonistic effects of chlorides with ammonia and nitrite still need further investigation in order to determine the suitable level of chlorides added into the fish pond for maximum benefit to Clarias culture.
4.5 In-depth physiological studies may be needed to determine the mechanism of action and toxicity of ammonia and nitrite to Clarias which can be used to prevent high mortality rate in the culture system.
4.6 Long-term bioassay studies on the toxicity of ammonia and nitrite through the life cycle of Clarias will provide useful information for the sound management of Clarias culture.
Alabaster, J.S. and D.W.M. Herbert 1954. Influence of carbondioxide on the toxicity of ammonia. Nature 174:404.
Alabaster, J.S., D.G. Shurben and G. Knowles 1979. The effect of dissolved oxygen and salinity on the toxicity of ammonia to smolts of salmon, Salmo salar L. J. Fish Biol. 15: 705–712.
APHA. 1973. Standard methods for examination of water and Wastewater. American Public Health Association, Inc., New York.
Ball, I,R. 1967. The relative susceptibility of some species of freshwater fish to poisons Ammonia. Water Res. 1: 767–775.
Brockway, D.R. 1950. Metabolic products and their effect. Prog. Fish. Cult. 12: 127–129.
Brown, D.A. and D.J. Mcleay 1975. Effect of nitrite on methemoglobin and total hemoglobin of juvenile rainbow trout. Prog. Fish. Cult. 3: 36–38.
Buckley, J.A. 1978. Acute toxicity of un-ionized ammonia to fingerling coho salmon. Prog. Fish. Cult. 40: 30–32.
Burrows, R.E. 1964. Effects of accumulated excretory products on hatchery reared salmonids. U.S. Fish. Wildl. Serv. Bur. Sport Fish. Wildl. Res. Rep. 66: 1–12.
Colt, J. and G. Tchobanoglous 1976. Chronic enposure of channel catfish, Ictalurus punctatus, to ammonia: Effects on growth and sargival. Aquaculture. 15: 353–372.
Crawford, R.E. and G.H. Allen 1977. Seawater in hibition of nitrite toxicity to chinook salmon. Trans. Am. Fish. Soc. 106: 105–109.
Downing, K.M. and J.C. Merkens 1955. The influence of dissolved-oxygen concentration on the toxicity of un-ionized ammonia to rainbow trout (Salmo gairdneri Richardson) Ann. Appl. Biol. 42: 243–246.
EIFAC, 1973. Water quality criteria for European freshwater fish. Report on ammonia and inland fisheries. Water Res. 7: 1010–1022.
Emerson, K., R.C. Russo, R.E. Lund and R.V. Thurston 1975. Aqueous ammonia equilibrium calculations: effect of pH and temperature. J. Fish. Res. Board Con. 32: 2379–2383.
Finney, D.L. 1971. Probit analysis 3rd ed. Cambridge: University Press.
Flis, J. 1963. Anatomicohistopathological changes induced in carp (Cyprinus carpio L.) by ammonia water. I. Effects of toxic concentrations. Acta Hydrobiol. 10: 205–224.
Harrison, S.E., W.R. Lillie, E. Pessah, J. Loch, J.C. Macleod, and J.F. Klaverkamp 1975. A modular system for aquatic toxicity studies. Fisheries and marine service. Technical report No. 592 Winnipeg, Manitoba. 15 p.
Hazel, C.R., W. Thomsen and S.J. Meith 1971. Sensitivity of striped bass and stickleback to ammonia in relation to temperature and salinity. Calif. Fish. Game. 57: 138–153.
Herbert, D.W.M. and D.S. Shurben 1965. The susceptibility of salmonid fish to poisons under estaurine conditions - II. Ammonium chloride. Int. J. Air. Water Pollut. 9: 89–91.
Hillaby, B.A. and D.J. Randall, 1979. Acute toxicity and ammonia excretion in rainbow trout (Salmo gairdneri). J. Fish. Res. Board Can. 36: 621–629.
Knepp, G.L. and G.F. Arkin 1973. Ammonia toxicity levels and nitrate tolerance of channel catfish. Prog. Fish. Cult. 35: 221–225.
Larmoyeux, J.D. and R.G. Piper 1973. Effects of water reuse on rainbow trout in hatcheries. Prog. Fish. Cult. 35: 2–8.
Lloyd, R. and D.W.M. Herbert 1960. The influence of carbon dioxide on the toxicity of un-ionized ammonia to rainbow trout (Salmo gairdneri Richardson). Ann. appl. Biol. 48: 399–404.
Lloyd, R. 1961. The toxicity of ammonia to rainbow trout (Salmo gairdneri Richardson). Water Waste treat. J. 8: 278–279.
Lloyd, R. and L.D. Orr 1969. The diuretic response by rainbow trout to sub-lethal concentrations of ammonia. Water Res. 3: 335–344.
Merkens, J.C. and K.M. Downing 1959. The effect of tension of dissolved oxygen on the toxicity of un-ionized ammonia to several species of fish Ann. appl. Biol. 45: 521–527.
Mount, D.I. and W.A. Brungs 1967. A simplified dosing apparatus for fish toxicity studies. Water Res. 1: 21–29.
Perrone, S.J. and T.L. Meade 1977. Protective effect of chloride on nitrite toxicity to coho salmon (Oncorhynchus kisutch) J. Fish. Res. Board Can. 34: 486–492.
Russo, R.C., C.E. Smith and R.V. Thurston 1974. Acute toxicity of nitrite to rainbow trout (Salmo gairdneri). J. Fish. Res. Board Can. 31: 1653–1655.
Smart, G. 1976. The effect of ammonia on gill structure of the rainbow trout (Salmo gairdneri). J. Fish Biol. 8: 471–475.
Smart, G.R. 1978. Investigations of the toxic mechanisms of ammonia to fish-gas exchange in rainbow trout (Salmo gairdneri) exposed to acutely lethal concentrations. J. Fish. Biol. 12: 93–104.
Smith, C.E. and R.C. Russo 1975. Nitrite-induced methemoglobinemia in rainbow trout. Prog. Fish. Cult. 37: 150–151.
Smith, C.E. and W.G. Williams 1974. Experimental nitrite toxicity in rainbow trout and chinook salmon. Trans. Amer. Fish. Soc. 103: 389–390.
Sprague, J.B. 1969. Measurement of pollutant toxicity to fish I. Bioassay methods for acute toxicity. Water Res. 3: 793–821.
Thurston, R.V., R.C. Russo and C.E. Smith 1978. Acute toxicity of ammonia and nitrite to cuthroat trout fry. Trans. Am. Fish. Soc. 107: 361–368.
Tomasso, J.R., B.A. Sinco and K.B. Davis 1979. Chloride inhibition of nitrite-induced methemoglobinemia in channel catfish (Ictalurus punctatus). J. Fish. Res. Board Can. 36: 1141–1144.
U.S. Environmental Protecting Agency. 1973. Water Quality Criteria 1972. U.S. Government Printed Office. Washington, D.C. p. 86–88.
Wallen, I.E., W.C. Greer and R. Lasater 1957. Toxicity to Gambusia affinis of certain pure chemicals in turbid water. Sewage and Industrial Wastes. 29: 695–711.
Wedemeyer, G.A. and W.T. Yasutake 1978. Prevention and treatment of nitrite toxicity in juvenile steelhead trout (Salmo gairdneri). J. Fish. Res. Board Can. 35: 822–827.
Westin, D.T. 1974. Nitrate and nitrite toxicity to salmonid fishes. Prog. Fish. Cult. 36: 86–89.
The Programme for the Development of Pond Management
Techniques and Disease Control
(DoF-UNDP/FAO THA/75/012)
Reports
| THA/75/012/WP 1 | Report on Aquaculture Training Undertaken at the International Center for Aquaculture, Auburn University, U.S.A. Chanchai Sansrimahachai |
| THA/75/012/WP 2 | Third Semi-Annual Report (Sept. 1/80-Feb. 28/81) of Progress on the “Programme for the Development of Pond Management Techniques and Disease Control (DoF-UNDP/FAO THA/75/012)”. Alex N. Fedoruk |
| THA/75/012/WP 3 | Management in Clarias Culture, Thailand. James Muir |
| THA/75/012/WP 4 | Collecting Clarias Fry from Natural Waters. Montree Muangboon |
| THA/75/012/WP 5 | Preliminary List of Diseases of Cultured Clarias in Thailand. National Inland Fisheries Institute, Thailand and Institute of Aquaculture, Striling, Scotland. |
| THA/75/012/WP 6 | Electrophoretic Analysis of Tilapia from the Dusit Palace Stock, Thailand. Brendan McAndrew |
| THA/75/012/WP 7 | Water Quality Conditions as Disease Related Stressors in Clarias Pond Culture. Vijai Srisuwantach, Rangsarn Soungchomphan and Pathipath Sae-Eng. |
| THA/75/012/WP 8 | Analysis of NIFI Clarias Diet No. 12. Albert J. Tacon and M. Beveridge |
| THA/75/012/WP 9 | Summary of the Report “Raising Clarias Fry on an Artificial Diet” Prasert Sitasit and Alex Fedoruk |
| THA/75/012/WP 10 | A Management Perspective on Stress and Infectious Diseases in Clarias Farming. Alex N. Fedoruk |
| THA/75/012/WP 11 | Feeds for Catfish (Clarias batrachus Linn.) Fry. Samran Dhamrongrat and Prasit Kasesuchi |
| THA/75/012/WP 12 | Assessment of a Vitamin Mineral Premix in an Artificial Feed for Pla Duk Oui (Clarias macrocephalus) Fry. Krittiya Taechajanta and Prasert Sitasit |
| THA/75/012/WP 13 | Management and Research Approaches in Clarias Culture, Thailand. B. Hepher |
| THA/75/012/WP 14 | Synopsis of the Report "Production Costs and Incomes in Clarias Pond Culture, Bang Pla Ma District, Suphanburi Province. Vanich Varikul and Alex Fedoruk |
| THA/75/012/WP 15 | Comparison of the Effects of Trash Fish and Pelleted Diets in Clarias Grow-Out Operations. Vijai Srisuwantach, Rangsarn Soungchomphan and Prasert Sitasit |
| THA/75/012/WP 16 | Water conditions as Limiting Factors in Clarias Culture. John A. Colman and S. Chinabut |
| THA/75/012/WP 17 | A Bibliography of Clarias (Family Clariidae) in Thailand. Alex Fedoruk and Kamonporn Pawaputanon |
| THA/75/012/WP 18 | Snakehead (Channa striatus) Farming in Thailand. Kok Leong Wee |
| THA/75/012/WP 19 | Assessment of the Program for the UNDP/FAO Supported Catfish Project in Thailand. Ronald J. Roberts |
| THA/75/012/WP 20 | Selected Clarias Bibliography from Countries other than Thailand. Wantanee Kate-Pasook and Alex Fedoruk |
| THA/75/012/WP 21 | Length-weight Relationships of Cultured and Wild Clarias batrachus in Thailand. Vijai Sriwantach and Dhana Yingcharoan |
| THA/75/012/WP 22 | A Review of Factors Influencing the Growth of Clarias batrachus in Thailand. Vijai Srisuwantach and Atchara Wongsangjun |
| THA/75/012/WP 23 | Optimal Rations in the Grow-out Phase of Clarias batrachus Culture. Vijai Srisuwantach, Rangsarn Soungchomphan, Patiphath Sae-Eng and Chutipong Wongsongsarn |
| THA/75/012/WP 24 | Report on the Clarias Seed Fish Farmers Seminar, NIFI, February 17 and 18, 1981. Kamonporn Pawaputanon |
| THA/75/012/WP 25 | Report on the Clarias Workshop for Fisheries Extension Officers, NIFI, July 7–9, 1981. Kamonporn Pawaputanon |
| THA/75/012/WP 26 | Fourth Semi-Annual Report (March 1/81-Aug.30/81) of Progress on the “Programme for the Development of Pond Management Techniques and Disease Control”. Alex Fedoruk |
