The successful farming of any fish species requires the highest standards of husbandry. This in turn depends on a sound knowledge of the principles of fish biology, aquatic ecology, and soil and water chemistry. Such principles are particularly important where intensive culture, with very high stocking densities, is practised, and where availability of good quality water is often at a premium, as is often the case in Thailand.
Environmental Requirements
1. Oxygen. In ideal conditions, water coming into a fish pond or tank should be fully saturated with oxygen. The ability of water to take up oxygen depends on a number of factors, including temperature, pressure, and dissolved salts. The higher the temperature, and/or salinity, the less oxygen can be dissolved in the water (Fig. 1). Although highest levels of water oxygenation may be ideal for clarias, they are not necessarily essential because clariids are capable of obtaining air from the surface of the water, Clarias spend much time on the bottom of the pond but need to swim to the surface to obtain oxygen, it is important therefore that pond depths are not too great. For example pond depths should not be greater than 1½ metres in order to conserve swimming energy. This is particularly important for young fish in hatcheries or brood ponds, where depth should not be greater than 50–75 cm.
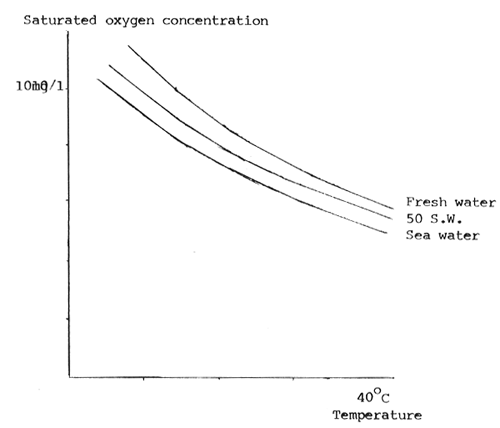
Fig. 1. The relationship between oxygen concentration, temperature and salinity.
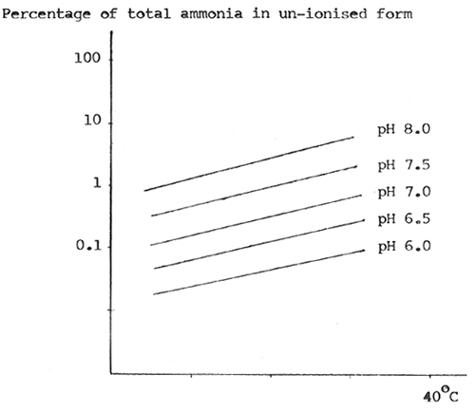
Fig.2. The relationship between unionised ammonia (as percentage of total ammonia), pH and temperature.
2. Temperature. In Thailand the water temperature range is not great (23–35°C). Breeding is usually precipitated by the flush of freshwater. Temperatures higher than optimal for the species are important because of the energy demands they place on the fish, which metabolise at higher rates at high temperature. Consequently any serious disease of the gills or air breathing organ will be much more serious at high temperature than at lower temperatures and it may be necessary to starve such fish at high temperatures, in order to conserve breathing capacity for respiratory purposes and preven oxygen debt mortality. This may occur when the specific dynamic action associated with feeding a high protein diet causes an oxygen demand which cannot be met, due to poor gill function, and low dissolved oxygen levels.
3. Ammonia. Ammoniacal substances are produced as excretory products by fish, and also by breakdown of decaying foodstuffs in the pond. They are found in water in one of two forms: free ammonia (NH3) and ionised ammonia (NH+4). The free ammonia is by far the more toxic of the two, and concentrations as low as 0.017 mg/ litre NH3 are likely to be toxic to the gills and air breathing organ, resulting in reduced growth and poorer resistance to oxygen and other stresses. The relative proportions of free ammonia and ionised ammonia depend on the pH and temperature of the water, the higher the pH the greater the proportion of free ammonia (ie. toxic ammonia)(Fig. 2). In general the water in Thailand is slightly alkaline in pH, so that ammonia can be a major problem in such systems. Other factors, including acidity due to sulphate solids and the effects of photosynthesis may counteract this.
4. Toxic substances. In addition to ammonia, the decay of fish wastes and uneaten food can produce toxic substances. Hydrogen sulphide (H2S) is produced at certain pH's which is stressful, though not in itself particularly poisonous. Increased levels of CO2, produced during the respiration cycle of phytoplankton, may also cause respiratory stress, and in other species, levels of as little as 10 mg/1 have been shown to induce kidney disease.
Suspended solids levels are always high in catfish ponds because of the stirring-up of mud, but the fish are usually resistant to damage from this source because of the tenacious mucus they produce on their gills. However, if particularly sharp suspended solids such as washings from brickworks or road workings, are washed into ponds these can cause major gill damage. Excessive organic solids, on the other hand, may increase bacterial levels, and deplete oxygen. Pollutants such as chemical sprays and insecticides from rice fields are known to be particularly toxic to fish, and industrial effluents from factories, which may also find their way into pond water supplies, may also be toxic.
Another significant form of environmental stress occurs during sudden changes in water quality, for example during water changes, or in extremely widely varying photosynthesis/respiration cycles.
5. Food and Nutrition. Clariids are omnivorous but require a high level of
animal protein. Currently the majority of farmed clarias are fed on trash fish
supplemented with animal wastes and low grade rice. Clarias, as detritus feeders,
are particulary adapted to deal with decaying protein, but nevertheless, a reasonable
quality of trash fish, containing as little as possible of fatty fish, is required.
Economical production should aim at fast growth rates with minimal waste, so the
method of feeding and correct level of feeding is important. The Food Conversion
Ratio or F.C.R. 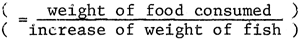 is the key factor in such calculations.
Clarias convert food into fish flesh less efficiently as they grow bigger. Clariids
reared to 200 g on trash fish, with minimal extra food availability from natural
feeding in the pond may have a F.C.R. of 4.5 ie. 4.5 kg of food is required to
produce 1 kg of clarias fish, whereas if calculated for fry of 20 g it might be as
low as 2. It is important for the farmer to be aware of his conversion rates, even
roughly, as otherwise much money may be wasted on food. Excessive feeding also results
in severe fouling of ponds and deoxygenation problems. Too little feeding on the
other hand leads to fighting and starvation. Poor conversion is also a feature of
stressed or diseased fish, particularly where the disease is a slow insidious process,
as in a condition such as tuberculosis or severe gill damage.
is the key factor in such calculations.
Clarias convert food into fish flesh less efficiently as they grow bigger. Clariids
reared to 200 g on trash fish, with minimal extra food availability from natural
feeding in the pond may have a F.C.R. of 4.5 ie. 4.5 kg of food is required to
produce 1 kg of clarias fish, whereas if calculated for fry of 20 g it might be as
low as 2. It is important for the farmer to be aware of his conversion rates, even
roughly, as otherwise much money may be wasted on food. Excessive feeding also results
in severe fouling of ponds and deoxygenation problems. Too little feeding on the
other hand leads to fighting and starvation. Poor conversion is also a feature of
stressed or diseased fish, particularly where the disease is a slow insidious process,
as in a condition such as tuberculosis or severe gill damage.
6. Stress and the general adaptive response. All animals are able to withstand unfavourable conditions to a certain degree. Clarias are particularly resistant to many environmental stresses, due to the harshness of their natural habitat, where drying out, poor water quality and starvation, occur frequently. However, they are not immune to husbandry stresses. While husbandry stresses may not necessarily be fatal in themselves, they may render the fish very much more vulnerable to infections which would normally be easily resisted. Stresses which are particularly dangerous to fishes are grading or transporting in very high density. Severe traumatic damage may be produced by their spines stabbing or scratching each other. Holding in small tanks in bright sunlight, can allow very high temperatures to develop, and squeezing or otherwise damaging fry when transferring them to grow-out facilities causes considerable stress.
Parasitic Disease
Parasites are animal which spend part of their lives living on or at the expense of another animal, in this case the clarias which is then known as the host. Parasites may be found in all tissues of the host, but they are particularly common on the skin and gills because these external surface are easily invaded. The parasites of the clarias may have a common host, but the range of parasite types is very wide. In the wild, although catfishes have many parasites, they are usually found in small numbers and rarely cause any serious injury. In the fish farm, however, they can build up to serious levels very quickly and results can be very serious. Normally a healthy fish and its parasites maintain an even balance, but where the clarias is stressed by mishandling, or water or nutritional problems, then parasite burden can build up very quickly. Although parasites can vary tremendously in shape and size they are usually divided up into two groups, the protozoa or single celled parasites and the metazoa or multi-cellular parasites.
Protozoa can only be seen properly with the microscope. Many can survive unfavourable conditions by means of a very resistant spore stage in their life cycle. They are varied in size and shape and are mainly found on the skin, gills and occasionally in the accessory breathing organ of clarias, although a few serious diseases are caused by internal protozoa.
1. Trichodina complex. (Category A). Among the most important protozoan pathogens for clariids are the species of Trichodina and Trichodinella, which make up the Trichodina complex. They are saucer-shaped and have a ring of short hair-like structures, called cilia, with they move over the surface of the fish in their very distinctive spinning fashion. There is an outer cuticular ring which causes damage to the surface as the parasite attaches and an inner circle of toothed denticles which aid in attachment and which make this protozoan easily recognisable (Fig. 3). Although they are found on the skin and gills of all sizes of clarias, they generally represent a severe problem only to the fry and young fish. Specimens of fry have been recorded at NIFI in which massive numbers of trichodinids were found on gills, skin and occasionally in the air-breathing organ. They are frequently associated with mass mortality of fry as a primary pathogen and thus they readily fall into Category A.
Affected fish have a darkened appearance and the skin is often seen to be grey and flaking off. They are inactive and generally do not feed. Affected fish show signs of irritation and may “flash”, that is swim on their side for a second or two and rub against the bottom as if to try to scrape parasites off.
2. Gyrodactylus spp. (Category A.) These monogenean parasites have only been found on the skin but they are often present in significant numbers. They are vivaparous or live-bearing and so when conditions on the skin surface are suitable eg. when there is skin damage, and especially when the fish are stressed, they can very rapidly build up in numbers. Gyrodactylids are up to 2 mm in length and can readily be distinguished from other monogeneans in skin smears under the microscope, by the absence of eye spots and the occurrence of the embryo in the mid-region of the body (Fig. 4). They are often quite active on examination and readily seen under low power magnification. They can be responsible for massive losses when, under as yet unexplained circumstances, a sudden population explosion takes place in apparently healthy fish. Affected fish, of any age, but generally young fast growing stock, have dark patches over the body surface, with sloughing areas of skin. They generally do not feed, and the parasites can be readily seen in skin scrapings, often accompanied by trichodinids.
Fig. 3. Trichodinid from skin of Clarias batrachus fry
a) showing the details of the attachment disc;
b) lateral view; c) details of denticular ring
(Drawn from specimens by N.A.M. Iqbal)
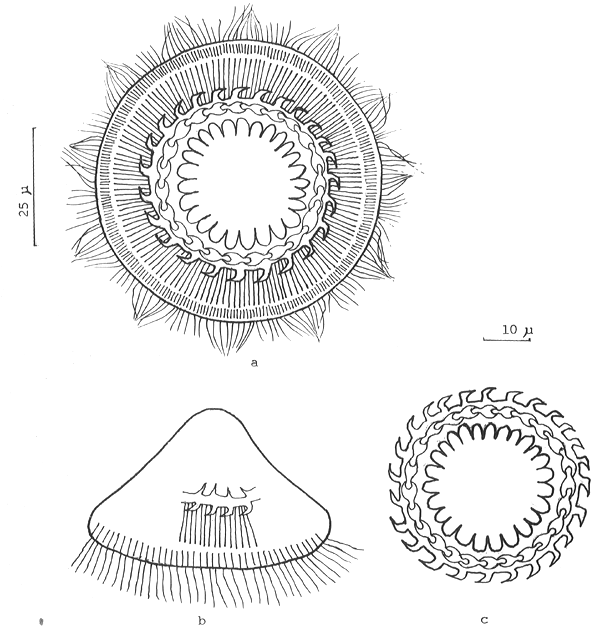
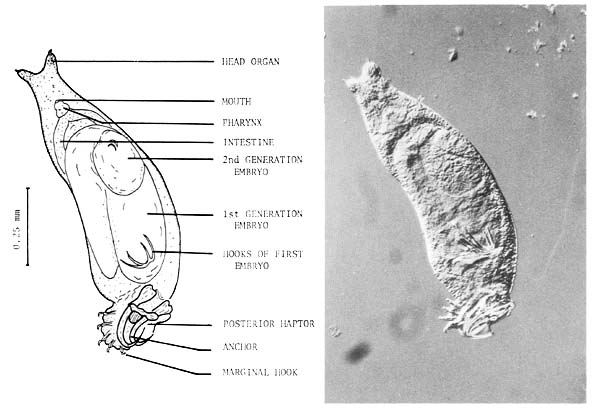
Fig. 4. Gyrodactylus sp. from the skin of Clarias batrachus fry
3. Dactylogyrids. (Category B.) Dactylogyrids are very common on the gills of cultured clarias. At least two species are found but no obvious pathology is associated. They can multiply very rapidly and in such cases small numbers may overflow into the buccal cavity. At high levels they are likely to interfere with respiration, and are often found in association with protozoan parasites of the gill. Infected fish may be darker and have a poor appetite but mortalities are not usually associated specifically with Dactylogyrus infections. Unlike Gyrodactylids, the Dactylogyrus monogeneans are oviparous, and the embryonation and hatching of the eggs is dependent on a number of factors especially temperature. Thus, although their numbers are capable of building up rapidly, infections do not explode with the same acuteness as Gyrodactylids, which are, of course, also much more serious pathogens.
4. Metacercariae. (Category B.) These intermediate stages of the digenean parasite life cycle are very numerous in tropical waters. In the Thai clarias culture systems, cercariae released from snails in the ponds or in inflowing rivers, may be present in large numbers in water samples. They penetrate the skin or gills of affected fish and may locate there, or migrate through the body to localise in various other organs such as muscle, heart or peritoneal tissue. In farmed clarias they are common on the gills (Fig. 5). Although in small number they are unlikely to cause serious problems, it is likely that heavy infections in static water are capable of causing mortality in fry and possibly inhibition of organ function in older fish.
5. Myxosporida. (Category B-C). Cysts of Henneguya sp. are found on the skin and gills of catfish in the form of small whitish nodules. When the cyst is squashed large numbers of sopres can be seen under the microscope (Fig. 6). Individual cysts can be seen on fish of all sizes but large numbers generally occur on fry and the results may be serious. Occasionally very heavy infections have been recorded and are thought to cause heavy mortality.
Myxosoma/Myxobolus cysts are seen occasionally in the gonads of older fish but only in small numbers (Fig. 7). Myxidium sp. is found regularly in the bile ducts and gall bladder where it is not thought to be of any significance although it can be readily observed in smears of bile fluid and intestinal scrapings as shiny avoid spores.
The life cycle is thought to be direct and infection is by ingestion of one of the myriadspores produced by rupture of a lesion on an infected fish. The static clarias ponds with a heavy mud sediment are an ideal environment for this parasite which tends to build up over a long period of time. It is essential to remove carcases of dead fish to keep down the incidence of Myxosporida but there is no satisfactoty drug treatment.
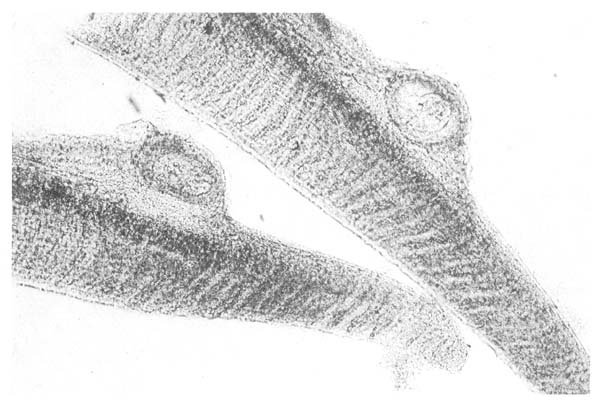
Fig. 5. Metacercaria, the intermediate stage of the digenean life cycle, encysted within the gills of a Thai clarias
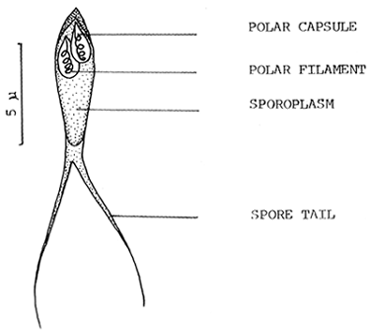
Fig. 6. Henneguya spore squashed from a cyst on thesskin of a Clarias batrachus fry.
(Drawn from specimens by N.A.M. Iqbal)
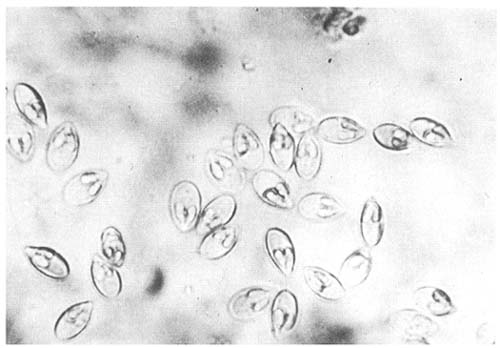
Fig. 7. Myxosoma/Myxobolus spores squeezed from a white cyst occurring on the gonads of mature Clarias batrachus
Fig. 8. Scyphidian from the gills of Clarias spp. This and related forms are very common in clarias ponds
6. Scyphidia complex. (Category C.). This term is used for the groups of diverse ectocommensal protozoans which are associated with high levels of particulate organic matter in the water and on stressed fish. The more simple forms are the Scyphidia (Fig. 8) and Clossatella which have a spiral of cilia at one end and an adhesive disc at the other. Others may be stalked such as Epistylis spp. which is considered to be more pathogenic, especially when found in large numbers on the gills. Colonies of it may extend out of the gills to the edge of the operculum and into the buccal cavity. Vorrticella sp. has a contractile stem and has also been seen occasionally. The parasites of the Scyphidia complex occur commonly on fish from static ponds with water high in organic matter and containing large number of the aquatic bacteria on which they feed. Ulcerative lesions of the skin, rice in bacteria and exudate, are also very suitable environments for these protozoans.
7. Chilodonella sp. (Category C.). This ciliated protozoan swims over the surface of the skin and gills of fish, feeding on bacteria, necrotic skin cells and other organic matter. It has been seen only infrequently on clarias skin and only on fish weakened by bacterial diseases. It is often found in association with other parasites and fungus infection.
8. Cestodes. (Category C.). Tapeworms, either in the form of intermediate stages in the viscera, or as adults, are found occasionally in both Clarias batrachus and C. macrocephalus, but not in significant numbers. Gangesia sp., a proteocephalid of potential significance has however been recorded from C. macrocephalus.
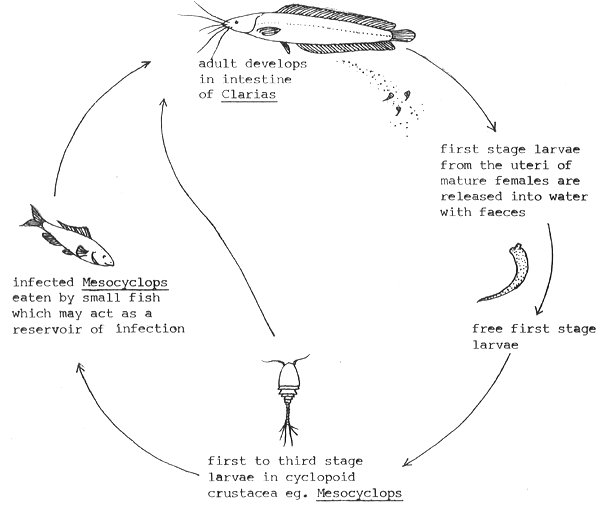
9. Nematodes. (Category C.) Roundworms are only found in small numbers in clarias and are not known to be of any significance. The following species have been recorded: Procamallanus planoratus, Camallanus ophiocephali (third stage larvae), Contracaecum sp. and ascarid larvae from the intestine of Clarias macrocephalus and Camallanus anabantus and Procallanus sp. from the intestine of Clarias batrachus. Fig. 9 shows a typical life-cycle for camallanid nematode. The nematode Thelazo sp. has also been found to occur in the stomach of adult C. macrocephalus.
10. Acanthocephalans. (Category C.). Acanthocephalus sp. has been recorded from Clarias macrocephalus and whilst, as in other species, this is generally considered of no significance, the penetration of the “thorny head” on attachment to the intestinal lining may well be a means of infection by enteric pathogens from the water.
Nematodes, Cestodes and Acanthocephalans have a complex life cycle involving an invertebrate as a first intermediate host. The fish has to ingest the invertebrate, usually a crustacean in order to become infected. The low levels of these worms in cultured clarias probably reflects a lack of zooplankton in the pond water due to poor water conditions.
11. Digenea. (Category C.). Small numbers of adult digenean flukes have been recorded from the intestine of both fry and older fish. They are not thought to be of any clinical significance. Cauhatiana batrachii is the species recorded from the intestine of C. macrocephalus and Orientocreadium batrachoides from the intestine of C. batrachus.
Fig. 9. Life-cycle of a typical Camallanid nematode.
(Drawing by N.A.M. Iqbal)
Bacterial Diseases
1. Bacterial Septicaemia. (Category A.) This is the most important bacterial disease of farmed catfishes and is often found in farms feeding trash fish and in multiple culture systems. Any one of a number of bacteria can be involved but the principal cause is Aeromonas hydrophila which is a normal inhabitant of tropical waters rich in organic matter. Thus it seems likely that since it is ubiquitous in the fishes environment some other factor is also necessary to induce infections. This may be any one of a variety of stresses ranging from bad handling to low oxygen levels. Fish which have been poorly fed, or heavily parasitized, are particularly vulnerable to such infections.
Affected fish generally swim slowly and may gasp at the surface of the water or else lie moribund on the bottom, They are generally very dark and do not feed at all. This latter response to infection is one of the best guides, as clinical signs other than lack of feeding response are often very difficult to determine in the muddy conditions of the typical clarias pond. The fins are often frayed, and greyish with naked fin rays present. They may also be lesions of the skin in the form of large deep red areas of ulceration, often invaded by myxobacteria or protozoa (Fig. 10). Eyes may be swollen, and haemorrhagic and often the abdomen will appear firm and even slightly distended. When moribund fish are opened, there may be red-tinged exudative fluid in the abdominal cavity and the kidney is usually swollen and very soft. The liver is generally yellow in colour and may be swollen and there is often a reddish swollen area in the lower intestine which may expel blood and necrotic tissue via the vent.
Bacterial septicaemia is generally a disease related to specific age groups of fish, being particularly prevalent at 2 weeks post hatching and at 3–3½ months of age. Bacteria other than Aeromonas hydrophila, including Pseudomonas fluorescens and Edwardsiella tarda, also occur in bacterial septicaemias, but not nearly so frequently. Antibiotic treatment (see chapter 6) is required to deal with this disease, but studies at NIFI have shown that often the responsible organisms are unresponsive to many of the most likely antibiotics, with chloramphenicol being the only consistently active drug. Since inappetance is one of the earliest clinical signs, it is difficult to ensure that treatment mixed with food is in fact being taken in by the fish. Thus it is important to remove all seriously affected fish and kill them, and to try to improve water and food quality for the remainder, to remove contributing factors.
2. Bacterial Ulcerative Disease. (Category A.) This is a common condition, but it is suspected that it has a number of different aetiologies. These probably all relate to traumatic damage from fighting or over-crowding around feeding stations. The lesions are invariably deep dermal ulcers with severe bacterial necrosis of skin and muscle (Fig. 11). Usually there is a mat of myxobacteria or saprophytic organism over the surface, but a variety of gramnegative bacteria can be isolated from the depths of the lesion. Generally there is no evidence of generalised tissue damage, unlike the case with bacterial septicaemia, but fish can nevertheless become moribund and die, possibly from osmotic imbalance.
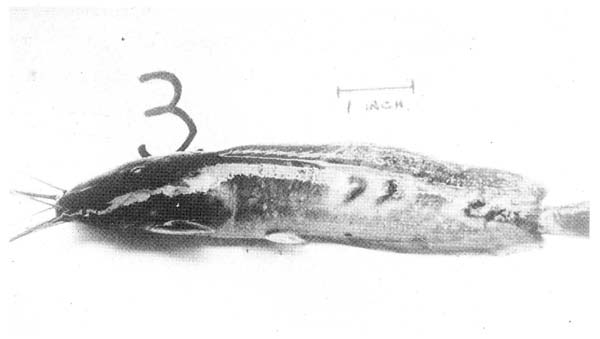
Fig. 10. A clarias moribund with Bacterial Septicaemia. The skin lesions and erosion of fins are typical of the late stages of this generalised infection.
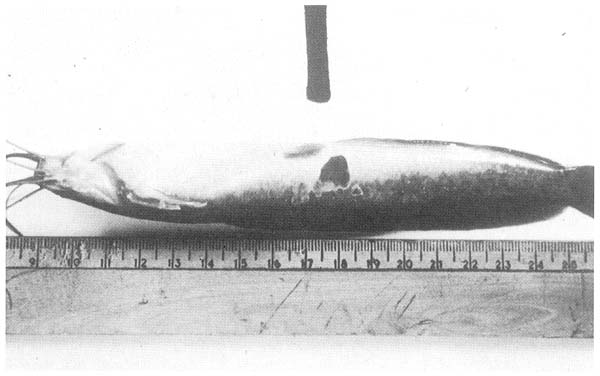
Fig. 11. Bacterial ulcers on the surface of an otherwise healthy fish. The lesions are localised and in this case there is not yet a covering mat of fungus or bacteria.
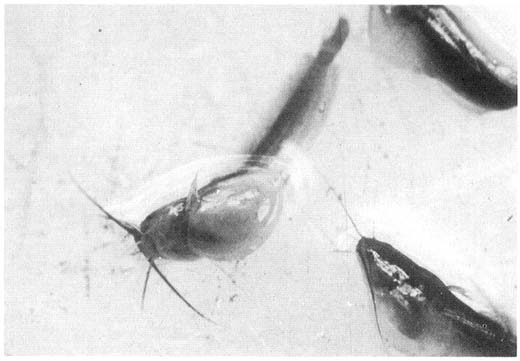
Fig. Fry with acute severe abdominal dropsy. The cause of this condition is not known but may well be a virus.
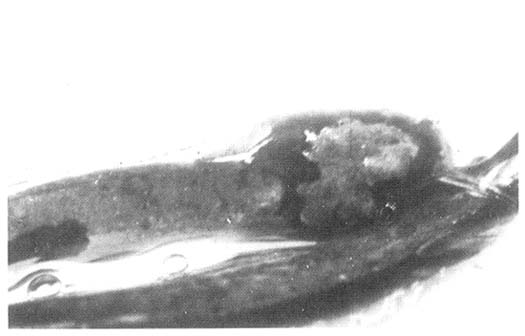
Fig. A mat of saprolegnia fungus over the surface of a skin lesion of a young clarias.
3. Focal Hepatic Necrosis. (Category B-C.) The exact status of this disease is not well understood. In surveys of farms with heavy mortalities, fish are often found which are normal apart from having fatty livers with large foci of necrosis associated with bacteria. The bacteria vary from case to case, and may be secondary but can be seen in large numbers in microscopical sections.
Viral Diseases
No viral diseases of clarias have been reported. It is unlikely however that they do not occur, and any sudden increase of mortality in fry, especially when parasitic or bacterial causes have been eliminated, must be considered suspicious of viral infection. Until further studies are carried out it is not possible to speculate further at this time, but particular fry problems associated with severe abdominal dropsy and high mortality are worthy of further investigation in this respect (Fig. 12).
Fungus Infections
1. Saprolegnia. (Category B.). The only fungal infection currently found in Thai calrias is Saprolegnia infection. Saprolegnia is recognised in all freshwater fish as a ready and potentially very serious contaminant of ulcerative lesions. However, in clarias in culture conditions such ulcers very rapidly become infected with scyphidian and myxobacterial secondary invaders so that opportunities for Saprolegnia per se are limited. However, it is seen on occasion, particularly on dorsal lesions, as a whorling, greyish plaque covering ulcers and extending from them (Fig. 13).