Poul Sørensen
Danish Institute of Agricultural Science, Research Centre Foulum
P.O. Boks 50, 8830 Tjele, Denmark
E-mail: [email protected]
Summary
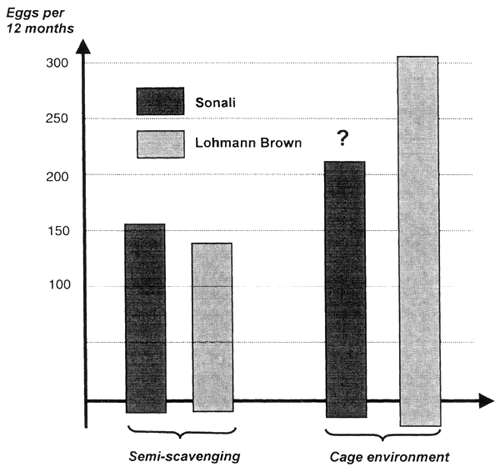
The paper uses Hammond's thesis from 1947 that “the character required is best selected under environmental conditions which favour its fullest expression” as a background for a discussion of two cases in which interaction was shown between breed and production system (environment). The first case compared a floor and a cage system in Denmark, while the second case compared 1200 hens in eight in different breed combinations kept by 280 poor women in Bangladesh. Season as well as location were accounted for. The best breed combination was the Sonali hen which produced 156 eggs in 12 months. In comparison the Lohmann Brown hen that produced 303 eggs in 12 months in the 1995/96 German Random Sample Test produced only 140 eggs in the trial in Bangladesh. The results are considered general for the semi-scavenging model used in Bangladesh. It is concluded (i) that interaction could be expected when two or more environments are very different from the bird's perspective and the breeds compared are different and that (ii) the consequences of a breed environment interaction is that the breeding operations and companies have to perform the breeding and selection in the same environment as the bird has to produce. This statement is against the theory of Hammond (1947) as mentioned in the introduction, and it is also against the interest of the world wide poultry breeding companies as it means that they have to operate with many different breeds and not the one or two they have nowadays.
Key words: poultry, breeding, environment, interaction, semi-scavenging
Introduction
Hammond's (1947) proposal that “the character required is best selected under environmental conditions which favour its fullest expression”, has to a great extend been followed by the breeders ever since. The statement of Hammond means that the test environment of e.g. laying hens, candidates for being chosen as parents for the next generation, should be the one that maximises the egg production of the individual hen. Among the more important environmental elements to be throught of as optimal are:
It is the impression from visits to breeding companies and discussions with their geneticists that the hygienic standard is very high in elite birds, mainly as a requirement from their customer. Also it is clear that the hen has been kept in individual cages for many generations, which implies that their laying pattern has not been disturbed by a social engagement to establish the ranking order.
The consequence in terms of genetic capacity has become a high egg yielding hen when conditions are optimal. That means the hen should be kept in a wellequipped cage system, have free access to a balanced diet, and having a high hygienic standard supplied with a good vaccination program. If these conditions are not met we must admit a risk because the ancestors of the hen have:
Up until now it is known that:
The Hammond thesis presupposes that no genotype X environment interaction exist.
Going through the literature on the subject there are many different findings, and the main conclusions are that the chance for a genotype (G) × environment (E) interaction increases as the distance between the environments grow and the genetic distance between the breeds grow.
In order to illustrate examples of whether or not the G x E interaction exists two cases will be demonstrated.
Test at the Danish Random Sample (Case 1)
Until 1980 it was not allowed to keep hens in cages in Denmark and up to that time some Danish breeding companies used floor systems with trap-nest to record the individual laying of hens as a criterion for selection, one of these was the Skalborg Breed. Already at that time the large international breeding companies had some parent stock in Denmark and they vent into the Random Sample Test for laying stock at Favrholm in 1978 when they were still tested in floor systems. In 1980 the ban against hens in cages was lifted and at a trial at the Random Sample Test at Favrholm, Denmark, in 1982 the same breeds were compared in a newly established cage system.
Table 1. Comparison of the Danish Skalborg breed with international breeds in 1978 in a floor system and in 1982 in a cage system. All breeds of White Leghom type. (Sørensen, 1997b).
| Breed | Test in floor system, 1978 | Test in cage system, 1982 | ||
|---|---|---|---|---|
| Eggs in 365 days per hen placed | Eggs in 365 days Per hen day | Eggs in 365 days per hen placed | Eggs in 365 days Per hen day | |
| Shaver | 265 | 274 | 278 | 298 |
| Babcock | 259 | 264 | ||
| Hisex | 264 | 267 | ||
| Lohmann | 259 | 268 | 276 | 285 |
| DeKalb | 264 | 292 | ||
| Skalborg | 262 | 267 | 240 | 266 |
In comparing Shaver and Lohmann with the Danish Skalborg it is obvious that in the floor system the Skalborg is equal to the international breeds. When tested in cages the international breeds have expressed their full capacity for laying which are 8% larger than on floor whereas the Skalborg produces at the same rate in both environments.
Looking at the relative difference between the two expressions for laying rate it was observed that Skalborg had a 5 time higher mortality in cages compared to the floor, while the international breeds had a mortality rate which was 1.5 time higher in the cages. The conclusion is that although there is no clear breed management interaction it is obvious so that the Skalborg breed is genetically adapted to the floor system whereas the international breeds are genetically adapted to cage systems.
Test of an international breed with the Sonali breed in Bangladesh (Case 2)
During the Smallholder Livestock Development Project (SLDP) I project in Bangladesh, an investigation of exotic hens under semi-scavenging conditions was perfromed in which eight different breed combinations were tested (Table 2). In total records from about 280 smallholders with 1200 hens is the background for the table. The data recorded are laying performance, source and amount of supplemented feed and also mortality and sale of bird was recorded. Seasonal variation was accounted for by entering day old chickens hatched during 4 hatches distributed over the year and they were placed in three districts, thereby accounting for locality. On this background the results can be considered general for the semiscavenging model used in Bangladesh.
One of these combinations was the Lohmann Brown that produced 140 eggs in 12 months. The best breed combination was the Sonali hen with 156 eggs in 12 months.
Table 2. Summary of performance of 8 different breed combinations (Rahman et al., 1997)
| Performance | Lohmann Brown | (A×R)×Fa | Fa×AB | R×AB | Sonali (Fa×R) | R×WL | (R×Fa)×AB | WL×AB |
|---|---|---|---|---|---|---|---|---|
| Age at 1st egg,w | 35 | 32 | 32 | 34 | 33 | 32 | 33 | 35 |
| Egg/hen actual | 86b | 104ab | 86b | 105ab | 119a | 97b | 86b | 99ab |
| Eggs/hen 12 months | 140ab | 137b | 125b | 139ab | 156a | 128b | 141ab | 139ab |
| Mortality, % | 22.1ab | 35.0b | 27.6ab | 32.6b | 16.0a | 25.2ab | 21.2ab | 22.9ab |
| Supplemt. Feed, kcal | 146b | 122a | 136ab | 144b | 130a | 134ab | 146b | 135ab |
| Gross Margin, Ta | 135b | 170ab | 113c | 133b | 205a | 145b | 132b | 155b |
Figures with same or no superscript in a row are not significantly different
Abbreviations for breed:
AB = Lohmann Brown
Fa = Fayomi
R = RIR
WL = White Leghorn
A = Female of the Lohmann Brown.
It can be observed that the Sonali hen is the best or as good as any of the other breed combinations compared. Of particular interest is that the Lohmann Brown seems poorer on laying and mortality and needed more supplementary feed than the Sonali hen.
Laying stock sold by the international breeding companies has a capacity to produce above 300 eggs in 12 months, under optimal conditions placed in cages, fed a balanced diet and using optimal vaccination programmes under high hygienic standards. Results from the German Random Sample tests (Sørensen, 1997a) showed that e.g. Lohmann Brown laid 303 eggs in 12 months.
The production capacity of Sonali tested in an optimal environment like the German random sample test, is unknown, but it will be far below 300 eggs per hen and a bit above 200 eggs is the most likely result. The obvious different ranking of Sonali and Lohmann Brown is the two very different environments is termed breed × environment interactions and expresses that the two breed combinations are genetically adapted to a particular environment (figure 1).
Conclusion
There are many such examples of breed × environment interaction in the literature, but even more are seen that has not given any interaction. If one should try to generalise it will be that interaction could be expected when the two or more environments is very different seen from the bird's eye and the breeds compared are also different.
The consequences of a breed × environment interaction is that the breeding operations and companies have to perform the breeding and selection in the same environment as the bird has to produce. This statement is against the theory of Hammond (1947) as mentioned in the introduction, and it is also against the interest of the world wide poultry breeding companies as it means that they have to operate with many different breeds and not the one or two they have nowdays.
References
Hammond, J. (1947). “Animal breeding in relation to nutrition and environmental condition”, Biological Review, 22:195–213.
Rahman, M., Sorensen, P., Jensen, H.A. and Dolberg, F. (1997). “Exotic hens under semi scavenging conditions in Bangladesh”, Livestock Research for Rural Development 9(3). (http://www.hcm.fpt.vn/inet/~lrrd/lrrd9/3/bang931.htm)
Sørensen, P.(1997a). “Results of the German Random Sample Tests 1995/1996”, Dansk Erhversfjerkrœ, 26:239–249.
Sørensen, P. (1997b). “The population of hens looses important genes. A case story”, Animal Genetic Resources, 22:71–78. A FAO publication series.
Figure 1: Breed × Environment interaction for egg production in 12 months
Q.M.E. Huque, S.A. Chowdhury, M.E. Haque and B.K. Sil
Bangladesh Livestock Research Institute
Savar, Dhaka 1341, Bangladesh
E-mail: [email protected]
Summary
Efforts on increasing the productivity of the native chicken of Bangladesh through genetic improvement have been reviewed. The poultry population is increasing at the rate of 6.5%per year and there are three distinct systems of poultry rearing:scavenging, semi-scavenging and intensive. The scavenging system is characterized by poor nutrition (only 14% and 23% of the requirement for protein and energy, respectively), lower productivity (35–45 eggs/year) and high (45–84%) mortality, but yet it contributes over 85% of poultry products in Bangladesh. Semi-scavenging is a model of poultry rearing developed in this country. Intensive poultry farming techniques have not yet been fully adopted. Thus efforts have been made for developing a breed or strain suitable for scavenging and semi-scavenging systems of rearing. Of the six distinct types of pure native chicken studied, native Naked Neck, native Dwarf and Hilly were found to have better production potentialities than others. Among different combinations of native and exotic chickens, RIR × Fayoumi, NN × RIR and NN × Fayoumi had better performances under semi-scavenging conditions. In all cases productivity under scavenging conditions was lower than under intensive systems largely due to poor nutrition. Supplemental feeds, especially protein sources increased the productivity of scavenging and semi-scavenging chicken. The paper concludes with a summary of research requirements.
Key words: Poultry breeds, systems of poultry rearing, research needs, Bangladesh
Introduction
Land and life are closely entwined in Bangladesh. Over 80% of the Country's 120 million people live in the rural sector and are highly dependent on an agricultural system that is finely attuned to a tropical monsoon climate. Agriculture generated 39% of the GDP and the share of the livestock sub-sector is 2.8% (Brammeret al. 1996).
Table 1. Poultry population in Bangladesh (1989–90 to 1993–94)
| Species | Population in million | Increase 1993–1994 | Annual growth rate (%) | ||||
|---|---|---|---|---|---|---|---|
| 1989–90 | 1990–91 | 1991–94 | 1992–93 | 1993–94 | |||
| Chicken | 89.96 | 95.88 | 102.31 | 109.16 | 116.48 | 26.52 | 5.9 |
| Duck | 13.10 | 13.20 | 13.29 | 13.38 | 13.47 | 0.37 | 0.07 |
Livestock rearing rarely forms a separate land-use enterprise in Bangladesh. Nearly all animals are kept on farms, closely integrated into agricultural production systems. Cattle, buffalo, goat and poultry (chicken and ducks) are the main types of livestock. There are over 116.5 million chickens. However, with increasing population and decreasing land holdings, the number of poultry is increasing at an annual rate of 5.9% (Table 1). At present most of the poultry birds are reared under scavenging conditions. Despite rapid development of the intensive poultry production in the private sector, per capita availability of poultry meat (2.87 kg/annum; BBS 1989) and egg (21 eggs/annum; Huque & Stem, 1993) is still very low. This is largely due to lower productivity of the indigenous birds reared under scavenging conditions. This led researchers to put their efforts on increasing the productivity of native birds through genetic improvement. The theme has been explored through three major questions: What is the present status of poultry production in Bangladesh? What is the performance of pure line exotic or local breeds or their crosses under semi-scavenging or scavenging conditions? How are environmental factors affecting the productivity of the birds under scavenging conditions?
Poultry production status in Bangladesh
As mentioned earlier poultry is an integral part of the farming system. There are about 116.5 million chicken and 13.5 million-ducks in Bangladesh (Table 1).
During the last decade intensive poultry farming has rapidly expanded. According to the Directorate of Livestock Services, there were 47,168 chicken and 26,944 duck farms of 50 to 100,000 bird capacities in 1996 (Rahman, 1997). About 89% of the rural households that rear livestock also rear poultry (BBS, 1996). Huque and Ukil (1994) studied the distribution of poultry birds according to land holdings (Table 2). They found that the number of chicken or ducks per farm was positively correlated with the farm size. They found also the Number and type of chicken reared by the farmers to be influenced by season. The highest number of Birds were present on the farm from January to March (about 42%) and the lowest number from July to September (about 26%). Rearing of poultry birds largely related to the extent of knowledge of poultry production.
Table 2. Distribution of poultry per household according to the farm size (Huque and Ukil, 1994))
| Farm size (area in acre) | Chicken | Duck | Pigeon | Total | ||||||||
| Adult chicken | Grower | Chicks 0–7 weeks | Total | Adult | Grower | Ducklings 0–7 weeks | Total | |||||
| Cock | Hen | Drake | Duck | |||||||||
| Landless (0–0.5) | 0.23 | 1.77 | 1.40 | 2.15 | 5.6 | 0.19 | 0.97 | 0.20 | 0.35 | 1.71 | 0.08 | 9.1 |
| Small (0.51–2.0) | 0.22 | 1.96 | 2.70 | 2.66 | 7.5 | 0.16 | 0.84 | 0.59 | 0.12 | 1.71 | 0.10 | 9.3 |
| Medium (2.01–5.0) | 0.54 | 2.90 | 2.35 | 2.82 | 8.6 | 0.32 | 1.77 | 0.40 | 0.25 | 2.74 | 0.72 | 12.1 |
| Large (>5.0) | 0.27 | 4.24 | 2.84 | 4.07 | 11.4 | 0.84 | 5.93 | 2.64 | 0.57 | 9.98 | 0.82 | 22.2 |
Table 3. Correlation co-efficient of some selected characteristics of farmers with their knowledge on poultry production (Yasmin et al. 1989)
| Dependent variable | Independent variable | Computed value of r (n = 98) |
|---|---|---|
| Knowledge of poultry production | Age | -0.0777NS |
| Education | 0.6360** | |
| Family size | 0.2181* | |
| Occupation | 0.3704** | |
| Farm size | -0.5266** | |
| Income | 0.0798NS | |
| Number of birds | 0.2660** | |
| Extension contact | 0.4976** |
NS: Not significant;
* P < 0.05;
** P < 0.01
Yasmin et al. (1989) studied the knowledge of feeding, breeding, housing, prevention and control of diseases of poultry of 100 poultry Rearers in 10 villages. About 17% had low, 70% medium and 13% high knowledge of poultry rearing. The knowledge was correlated with many social factors, but an interesting feature was that the farm size was negatively correlated (r = -0.5266; P < 0.01; n = 98) with poultry rearing knowledge (Table 3).
The quantity and quality of vaccines available against the major diseases are not up to the desired standard. Taimur et al. (1999) studied the efficacy of six poultry vaccines (Baby Chick Ranikhet Disease Vaccine (BCRDV), Ranikhet Disease Vaccine (RDV), Fowl Pox (FP), Fowl Cholera (FC) and Duck Plague (DP)) produced by the Directorate of Livestock Services. They showed that the efficacy of vaccine deteriorates as it moves from the production laboratory to the District Livestock Officer, further to the Thana Livestock Officer and finally the user (Figure 2). At the production level the vaccines were found to maintain standard (efficacy>90%, except FC). However, the potency declined from the district livestock office to the Thana livestock office and finally falls to between 45–80% potency at the user's level.
Three systems of poultry rearing e.g., scavenging, semi-scavenging and intensive exist in Bangladesh. Over 85% of the birds are being reared under scavenging conditions (FAO, 1996). In the scavenging system the birds are sheltered only at night. During the day, the birds are allowed to scavenge on insects, earthworm, residual grain, and green materials around the homestead. Occasionally, the birds are supplemented with rice bran, broken rice, rice gruel and cooked rice in the morning or afternoon.
Rahman et al. (1997) estimated the feed obtained from scavenging to be around 60–70% of the requirements of the birds. They showed that nutrient availability is largely related to cropping pattern. It was evident that the amount of nutrients available is inadequate. Without interventions, the mortality rate of poultry was reported to be 35–85% due to diseases and predators (Saleque and Mustafa, 1996). Despite undernutrition and high mortality, it is remarkable that scavenging birds provide 85% of the poultry meat and 76% of the total egg produced in the country (FAO, 1996).
Semi-scavenging has recently been established in Bangladesh. More than 1 million semi-scavenging smallholder farms have been established and their number is growing at the rate of 100,000 annually (Jensen, 1996). It is an integrated system where different groups of farmers perform different specialized activities as Breeders, Hatchers, Chicken Rearers, Key Rearers, Feed Sellers and Vaccinators (Saleque and Mustafa, 1996; Jensen, 1996). In this system chicken are reared up to 8 weeks in confinement on a standard diet. From nine weeks onward the birds are kept under semi-scavenging conditions and offered 30–70% supplementary feed and scavenge for the rest (Jensen, 1996). In both scavenging and semi-scavenging systems, profitability largely depends on the feed availability and safe access to scavenging conditions.
Intensive poultry farming techniques have not yet been fully adopted. Moreover this industry was severely hit by the 1998 flood. Post-flood rehabilitation was hampered due to the feeding of high moisture grain with consequent severe incidences of aflatixin (produced by the fungi Aspergillus flavus) throughout the country. In one of our trials aflatoxin concentration of different poultry feeds was measured (Figure 3a). Aflatoxin concentrations in maize (250 ppb/kg) and different commercial mixed poultry feed (143–274 ppb/kg) was found to be well above the recommended safety limit (20 ppb/kg). This toxin is hepatotoxic, mutogenic, immunosuppressive and carcinogenic. As a consequence it damages liver, kidney and causes vaccination failure. Because of immune failure, a flock infested with aflatoxin shows incidences of secondary infection like Newcastle diseases, Gumboro, Coccidiosis, Salmonelosis and Colibacillosis (Figure 3b). Mortality due to aflatoxin related diseases was higher with small and medium sized (500–5000 birds) farms than with the large (<5000 birds) farms (Figure 3c).
Performance of different breeds
Of the four wild chicken species available on the Indian Sub-continent (Gallus gallus (Red Jungle Fowl), Gallus sonnerati (Gray Jungle Fowl), Gallus lafayuttii (Cylon Fowl) and Gallus rarius (Green Jungle Fowl), Beede, 1931), indigenous chickens are reported to be derived from the Gallus gallus (Faruque et al. 1987), whereas, Gallus bankiva is believed to be the major contributor to the development of modern commercial breeds (Lush, 1945). Among the native fowls there are some distinct categories namely Hilly, Naked Neck, Aseel, Yasine, Native dwarf and Nondescript deshi. Collection, evaluation and conservation of different genotypes are required due to future changes in the environment, management and food habits (Crawford, 1984). A random mated; unselected indigenous population is a huge treasure of variable genotypes (Yeasmin and Howlader, 1998). Thus, many researchers have made their efforts to develop high yielding breeds/strains using the indigenous chicken.
Indigenous chickens have an inherent scavenging habit; they are more resistant to diseases, less prone to predator attaks and can survive under harsh nutritional and environmental conditions. However, their productivity under scavenging conditions is very low (35–45 eggs per year; Table 4). Yeasmin and Howlider (1998) studied the production potential of indigenous non-descript deshi and native dwarf chickens under intensive rearing conditions. The dwarf chickens were found to have smaller body size, but higher egg production (34 vs. 25% hen-day egg production) and better feed conversion efficiency (5.5 vs. 8.6 g of feed per g egg). However, the dwarf gene of indigenous chicken was found to be autosomal recessive in nature. They concluded that indigenous dwarf chickens are superior to their non-descript deshi counterparts in egg production and should be used for developing egg type birds suitable for rural conditions. In the Bangladesh Livestock Research Institute, a selective breeding programme has been initiated with some native chicken genotypes under intensive production system. Preliminary results on the performance of collected and selected birds are shown in Table 5. Of the five genotypes studied, Naked Neck, Non-descript deshi and Hilly were found to have the highest production potentialities under intensive raring.
Table 4. Production performance of indigenous chickens of Bangladesh (From Huque; 1992)
| Parameters | Mean/Range | References |
|---|---|---|
| Live weight(g) | 1141 | Huque and Ukil, 1994 |
| Carcass weight (% of live weight) | 55 | Huque and Ukil, 1994 |
| Egg production/hen/year | 35–45 | Bulbul, 1983; Ahmed and Islam, 1985; Sazzad, 1986; Huque et al. 1990 |
| Egg weight (g) | 35–39 | Ahmed and Islam, 1985; Ahmed and Huque, 1990 |
| Age at point of lay (d) | 190–200 | Huque and Haque, 1990;
Sazzad, 1986; Huque et al. 1990 |
| Length of laying period/clutch | 2–3 weeks | Huque et al. 1990 |
| No. of eggs/clutch | 3–4 | Sazzad, 1986; Huque et al. 1990 |
| No. of clutch/year | 10–15 | Huque et al. 1992 |
| Hatchability (%) | 84–87 | Sazzad, 1986; Huque et al. 1992 |
Table 5. Preliminary results of the performance of some collected and selected native chicken genotypes under intensive rearing conditions
| Parameter | Naked Neck | Hilly | Yasine | Aseel | Non-descript deshi | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Collected | Selected | Collected | Selected | Collected | Selected | Collected | Selected | Collected | Selected | |
| Adult weight (kg) | 1.2–2.5 | 1.2–2.5 | 1.5–3.5 | 1.5–3.5 | 1.5–3.5 | 1.5–3.5 | 1.7–4.5 | - | 1.0–2.0 | 1.2–2.0 |
| Age at 1st laying (d) | 180–210 | 130–180 | 180–210 | 140–180 | 210–240 | 170–220 | 240–300 | - | 180–210 | 130–180 |
| Annual egg production | 98 | 141 | 91 | 101 | 58 | 98 | 33 | - | 44 | 121 |
The Directorate of Livestock Services has imported different exotic breeds and Amber (1994, personal communication) recorded the production performances of 22 different genetic combinations of both native and exotic birds under intensive rearing conditions (Table 6). He found the productivity of the pure native to be very low and among the crossbred, the RIR×Fayoumi combination, called Sonali was the best, which, he suggested for rearing under semi-intensive conditions. In the Bangladesh Livestock Research Institute, the performance of pure Naked Neck, Hilly and their crosses with different exotic breeds: RIR, White Leghorn and
Table 6. Production performances and livability of birds of different genetic combinations under intensive rearing in Bangladesh (Abdul Jalil Amber, Personal Communication)
| Genotype | Livability (%) | Egg production (%) |
|---|---|---|
| Indigenous non descript (D) | 75.0 | - |
| Australorp (AUST) | 74.4 | - |
| Barred Plymouth Rock (BPR) | 50.0 | - |
| White Leg Horn (WLH) | 81.5 | - |
| Rhode Island Red (RIR) | 66.5 | - |
| Fayoumi (FY) | 78.5 | - |
| RIR×FY | 91.7 | 53.72 |
| RIR×WLH | 82.5 | 55.85 |
| RIR×BPR | 83.8 | 56.79 |
| BPR×FY | 85.4 | 58.13 |
| BPR×WLH | 82.5 | 52.77 |
| BPR×RIR | 75.0 | - |
| WLH×FY | 89.5 | 61.63 |
| WLH×RIR | 85.0 | 58.71 |
| WLH×BPR | 78.0 | - |
| WLH×AUST | 88.8 | 53.12 |
| FY×WLH | 91.5 | 60.66 |
| FY×BPR | 84.7 | 55.09 |
| RIR×D | 86.9 | - |
| WLH×D | 72.3 | - |
| FY×D | 80.0 | - |
| D×FY | 85.0 | - |
Fayoumi, were studied under intensive and scavenging rearing conditions (Table 7).
Table 7. Preliminary results on the performance of Naked Neck and its crosses with different high yielding exotic breeds: RIR, White Leghom, Fayoumi reared both under intensive and scavenging conditions
| Parameters | Condition | Breed combinations (mean values) | ||||||
|---|---|---|---|---|---|---|---|---|
| NN×NN | NN×RIR | NN×WLH | NN×FY | RIR×RIR | WLH×WLH | FY×FY | ||
| Age at 1st egg | Caged | 163 | 157 | 154 | 157 | 169 | 162 | 177 |
| Scavenging | 234 | 184 | 218 | 208 | 210 | 207 | 217 | |
| Wt at 1st egg | Caged | 1433 | 1408 | 1370 | 1300 | 1736 | 1380 | 1243 |
| Scavenging | 1171 | 1268 | 1229 | 1119 | 1771 | 1370 | 1250 | |
| Egg yield: 45 weeks | Caged | 88 | 83 | 105 | 87 | 94 | 107 | 80 |
| Scavenging | 13 | 37 | 18 | 35 | 22 | 28 | 24 | |
Although under intensive rearing, birds of exotic background (WLH × WLH, RIR × RIR) had better performance than the Naked Neck or its combinations, the combinations of NN × Fayoumi and NN × RIR had better performance under scavenging conditions. Egg production performance of scavenging birds (24.0%) was approximately 40% of those under intensive rearing (60.5%). This difference was attributed to the poor nutritional status under scavenging conditions. In a similar trial, performance of eight exotic breed combinations under semi-scavenging conditions in three different locations (Jessor, Manikganj and Rajshahi) was studied (Rahman et al., 1997). The results are shown in Table 8. Here, location effect was more pronounced than the breed or season effect. However, the combination of RIR × Fayoumi was found to be superior to the other breeds or breed combinations - in line with the results reported by Amber - and this combination was followed by RIR × WLH. The estimated protein and ME scavenged were approximately 50% and 40% of their requirements. They concluded that dietary supplementation and reduction of mortality are important factors to be addressed for improving the smallholder poultry production system.
Table 8. Performance of different breed combinations under semi-scavenging conditions (Rahman et al. 1997)
| Parameter | Breed combinations (least square mean values) | |||||||
|---|---|---|---|---|---|---|---|---|
| AB = Lohman Brown | AB×RIR×Fayoumi | Fayoumi×AB | RIR×AB | RIR×Fayoumi | RIR×WLH | RIR×Fayoumi×AB | WLH×AB | |
| Age at 1st egg (week) | 34.5 | 32 | 32 | 34 | 33 | 32 | 32.5 | 34 |
| Hen days egg product | 86 | 104 | 86 | 105 | 119 | 97 | 86 | 99 |
| Mortality (%) | 23.5 | 35.9 | 29.0 | 35.4 | 18.1 | 25.2 | 26.5 | 25.5 |
| Protein intake (g/head/d) | 7.3 | 6.0 | 6.9 | 7.2 | 6.4 | 6.7 | 7.1 | 6.6 |
| ME intake (Kcal/h/d) | 146 | 122 | 136 | 144 | 130 | 134 | 146 | 135 |
| Gross margin Taka/hen | 135 | 170 | 113 | 133 | 205 | 146 | 132 | 155 |
Ducks
About 95% of the ducks reared in this country are of the indigenous type (Huque, 1991).
Table 9. Performances of Khaki Campbell, Indigenous ducks and their crosses under scavenging conditions (Huque and Hussain, 1994)
| Parameters | Khaki Campbell (KC) | Indigenous duck (D) | KC × D |
|---|---|---|---|
| Annual per duck egg production | 58 | 39 | 56 |
| Egg production per duck-day (%) | 16.6 | 10.8 | 20.1 |
| Onset of lay (days) | 154 | 181 | 173 |
| Weight at onset of lay (kg) | 1418 | 1365 | 1401 |
| Mortality (%) | 58 | 72 | 72 |
Huque and Hussain (1994) studied the performance of Kahki Campbell (KC), indigenous (D) and their crosses (KC × D; Table 9). It was found that the number of eggs produced per year was much higher in KC and KC × D than the D. The study showed that egg production was not related to the genetic merit, age or season, but rather to the nutritional condition of the bird.
Effect of nutritional supplementation
It is evident from above studies that unless nutrient requirements of the birds are being met no benefit of genetic improvement can be harnessed. Rahman et al. (1987) studied the production performance of RIR, Fayoumi and RIR × Fayoumi chicken under semi-scavenging condition supplemented either with 25, 75 or 130 g of concentrate. The results are shown in Figure 4. Production parameters were linearly correlated with the level of supplementation. Similarly, in another trial, production performances of scavenging ducks without or with 65 g concentrate supplementation was measured (Huque et al. 1993) and supplementation was found to improve egg production by almost four times (Table 10).
Table 10. The production performance of scavenging ducks with or without 65 g of concentrate supplement (Huque et al. 1993)
| Parameter | Age at 1st laying (d) | Age at peak production (d) | Duck-day egg production (%) | Total egg production | Survival (%) |
|---|---|---|---|---|---|
| Without supplement | 210 | 272 | 15.72 | 42 | 82.2 |
| With supplement | 163 | 177 | 66.50 | 137 | 87.5 |
Clearly supplementation of scavenging and semi-scavenging poultry can significantly increase the productivity. However, there is no clear indication about the type and amount of feed needed for supplementation. A recent report (Chowdhury and ørskov, 1997) showed that with adequate protein supply, a ruminant animal could maintain its productivity even with sub-maintenance energy level as long as it has fat in its stores for energy, which means - if the concept can be applied to poultry - that for periodically undernourished scavenging poultry, supplementation of protein has high priority.
However, most of the protein sources are costly and available in insufficient quantities at the farmer's level. Therefore the possibility of using unconventional protein sources like duckweed, earthworm, silkworm pupae, shrimp waste, snails, rumen ingest, different slaughterhouse byproducts should be explored. The Bangladesh Livestock Research Institute has developed a year round duckweed production technique using farm manure (either fermented or unfermented). In two separate trials the possibility of feeding dry duckweed to broilers (Table 11) and fresh duckweed to laying ducks (Table 12) were studied.
Table 11. Effect of replacing of diet with dried duckweed in broiler (Salahuddin, personal communication)
| Parameter | Level of dried duckweed in diet | |||
|---|---|---|---|---|
| 0 | 15 | 25 | 35 | |
| Feed intake (g/bird) | 4600 | 4350 | 4410 | 4390 |
| Live wt (g/bird) | 1800 | 1500 | 1350 | 1100 |
| Feed conversion ratio (kg feed/kg gain) | 2.55 | 2.96 | 3.26 | 3.99 |
Table 12. Effect of different levels of fresh duckweed on the performance of laying ducks
| Parameters | Levels of fresh duckweed (g/d) | ||||
|---|---|---|---|---|---|
| 0 | 640 | 960 | 1280 | 1600 | |
| Duck-day egg production (%) | 24.05 | 46.85 | 38.92 | 36.20 | 36.84 |
| Improvement over the ‘0’ (%) | - | 94.8 | 61.8 | 50.5 | 53.2 |
It is apparent from Table 11 and 12 and similar works in Cambodia (Samnang, 1999) and Vietnam (Rodriguez and Preston, 1999) that duckweed can profitably be incorporated into the poultry diet. However, the form and extent to which duckweed can be used for semi-scavenging chicken and ducks need to be studied.
Conclusion
Five points are apparent from the above discussion:
A suitable breed needs to be developed for semi-scavenging or scavenging production systems.
Under semi-scavenging condition crossbred birds (NN × RIR, NN × Fayouni, RIR × Fayoumi)perform better than their respective pure breed parents.
Chicken and ducks under semi-scavenging conditions are severely deficient both in energy and protein, thus any improvement in genetic potential will have little benefit unless these are being corrected.
Supplementation of even small amounts of well-balanced concentrates to semi-scavenging poultry can increase their productivity many fold.
Unconventional protein sources like duckweed, earthworm, and snails can be used for feeding laying ducks and chicken under semi-scavenging conditions. However, the form and the extent to which duckweed can be used for semi-scavenging chicken and ducks need to be studied.
Research need for production management:
Studies that need to be undertaken include:
Comparative production performances of selected breed or breed combinations under different management conditions.
Comparative production performances of NN × Fayoumi, NN × RIR and RIR × Fayoumi under semi-scavenging conditions with supplementation of concentrate.
Effect of supplementing different levels of energy and protein concentrate to scavenging or semi-scavenging birds.
Small scale duckweed production and its utilization at different levels either in i) dry, ii) sun baked or in iii) fresh conditions to semi-scavenging birds.
Research need for health management:
Role of parasitism on the affectivity of vaccination programs.
Studies on maternal immunity and early vaccination program.
Susceptibility of local chicken to exotic diseases e.g., Gumboro, Avain Leucosis and studies on the development of Marek's diseases. There is a need for poultry disease surveillance and a sera-immunity system in Bangladesh.
References
Amber, A.J. (1994). Research on establishing appropriate breed for rural poultry production, Directorate of Livestock Services (Personal Communication).
Barua, A., and Howlider, M.A.R. (1990). “Prospects of native chicken in Bangladesh”, Poultry Advisor, 23(7), 57–61.
Bangladesh Bureau of Statistics (1996). Statistical Year Book of Bangladesh, Seventeenth Edition.
Beebe, W. (1931). Pheasant, Their Lives and Homes, Vol. I, New York: Doubleday Doran.
Brammer, H., Asaduzzaman, M., and Sultan, P. (1996). “Effects of climate and sea level changes on the natural resources of Bangladesh”, in R.A. Warrick and Q.K. Ahmed (eds.), The Implications and Climate and Sea-Level Change for Bangladesh, Kluwer Academic Publishers, 143–203.
Chowdhury, S.A. and ørskov, E.R. (1997). “Protein energy relationships with particular reference to energy undernutrition: A review”, Small Ruminant Research. 26, 1–7.
Crawford, R.D. (1984). “Assessment and conservation of animal genetic resources in Canada”, Canadian Journal of Animal Science, 17, 93–97.
Faruque, M.O., Hasnat, M.A., Mostafa, K.G., Takashi, A., Kurosawa, Y., Ota, K. and Nsmikawa, T. (1987). “Conservation of livestock genetic resources in Bangladesh: Past, present and future”, in Genetic studies on breed differentiation of the native domestic animals - Bangladesh, Part II, Hiroshima University, 129–137.
Huque, Q.M.E. (1991). “Duck production system in Bangladesh”, Asian Livestock, 16 (2), 18–23.
Huque, Q.M.E. and Stem, C. (1993). Current status of poultry production and marketing system of Bangladesh, Bangladesh Agricultural Research Council/USAID/Checci & Company Consulting Int, Dhaka, Bangladesh.
Huque, Q.M.E. and Hussain, M.J. (1994). “Comparative performance of three genotypes of ducks under rural conditions”, Bangladesh Journal of Scientific Research, 12 (20157– 160).
Huque, Q.M.E. and Ukil, M.A. (1990). Feeding pattern of birds (chicken and ducks) under scavenging conditions, Poultry Production Research Division, Bangladesh Livestock Research Institute, Savar, Dhaka, Bangladesh.
Huque, Q.M.E. and Ukil, M.A. (1994). “Existing poultry production and utilization system in the traditional villages in Bangladesh”, Bangladesh Journal of Training and Development, 7 (1), 35–43.
Huque, Q.M.E., Ukil, M.A. and Hossain M.J. (1993). “Supplementary feeding of laying ducks under scavenging conditions”, Bangladesh Journal of Livestock Research, 1 (1), 57–62.
Jensen, H.A. (1996). “Semi-scavenging poultry flock”, in Frands Dolberg and Poul Henning Petersen (eds.), Integrated Farming in Human Development, Proceeding of the Workshop March 25–29, 1996, Tune Landboskole, Denmark, 56–63.
Lush, J.L. (1945). Animal Breeding Plans, Iowa State University Press, Third Edition, p. 16.
Netke, S.P. (1991). “The Aflatoxin content of the poultry feed shall not exceed 200 ppb”, Indian Poultry Review, Oct. 1991.
Rahman, M. (1997). Development of feed and livestock industries in Bangladesh, Department of Livestock Services, Khamar Bari, Dhaka, 1215, Bangladesh.
Rahman, M., Islam, M.N., Sarker, N.R., and Islam M.M. (1998). “Effect of supplementary feeding on production performance of RIR, Fayoumi and their crossbred chicken in rural Bangladesh”, Bangladesh Journal of Livestock Research 1 (1–5), 184–193.
Rahman, M., Sorensen, P., Jensen, H.A. and Dolberg, F. (1997). “Exotic hens under semiscavenging conditions in Bangladesh”, Livestock Research for Rural Development, 9 (3). http://www.cipav.org.co/lrrd/lrrd9/3/bang931.htm
Rodriguez, L and Preston, T.R. (1999). “Observation on local (indigenous) and Tam Hoang (exotic) chickens given free access (when confined at night) to duckweed (Lamnaceae) offered alone or mixed with rice bran”, Livestock Research for Rural Development, 11 (1). http://www.cipav.org.co/lrrd11/1/ly1111.htm
Saleque M.A. and Mustafa, S. (1996). “Landless women and poultry: The BRAC Model in Bangladesh”, in Frands Dolberg and Poul Henning Petersen (eds.), Integrated Farming in Human Development, Proceeding of the Workshop March 25–29, 1996, Tune Landboskole, Denmark, 38–55.
Samnang, H. (1999). “Duckweed versus soya bean meal as protein source for scavenging chickens in the integrated farming system”, Livestock Research for Rural Development, 11 (1). http://www.hcm.fpt.vn/inet/~utaf/the98/Samnang/Samnang2/Samnang2.htm
Taimur, M.J.F.A., Sill, B.K., Johan, S. and Pradhan, A.M. (1999). “Utilization of poultry vaccine and monitoring their potency at various levels in Bangladesh”, Animal Health Research Division, BLRI, Savar, Dhaka, Bangladesh.
Yasmin, L., Hussain, M.A. and Miah, M. (1989). “Characteristics of backyard poultry farmers affecting their knowledge on poultry production”, Bangladesh Journal of Training and Development, 2 (1), 22–30.
Yeasmin, T. Howlider, M.A.R. (1998). “Comparative physical features, egg production and egg quality characteristics of normal and dwarf indigenous (Deshi) hens of Bangladesh”, Journal of Applied Animal Research, 13, 191–196.
Professor P.B. Spradbrow
Division of Veterinary Pathology and Anatomy
The University of Queensland
Brisbane, 4072 Australia
E-Mail: [email protected]
Summary
Newcastle disease is a serious and commonly fatal disease of chickens caused by a paramyxovirus. Other avian species are also infected, but usually with less severe consequences. In most developing countries Newcastle disease is the most important infectious disease affecting village chickens. The usual source of infection for village chickens is usually other chickens. The role of other birds as carriers to initiate outbreaks in villages is not well documented. Both epidemic and endemic forms of Newcastle disease occur in village conditions. Thermostable Newcastle disease vaccines are now available that can give substantial protection of village flocks against Newcastle disease. The benefit of these vaccines in increasing flock size or flock output can be measured. New techniques are required to allow accurate cost-benefit analysis.
Key words: Newcastle disease. Village chickens. Epidemiology. Thermostable vaccines.
Introduction
This paper is restricted to a consideration of Newcastle disease as it affects the birds that I refer to as village chickens, and that others regard as rural chickens or family chickens. I have been for many years project leader to programs funded by the Australian Centre for International Agricultural Research (ACIAR) and concerned with the development of Newcastle disease vaccines for use in village chickens. Of recent years there has been increasing interaction between the disparate organisations that have an interest in village chickens and the potential contribution of chickens to the alleviatin of poverty amongst the rural poor. This group now includes FAO, IAEA, INFPD, World Bank, Danida and numerous NGOs. I welcome this opportunity to visit Denmark and meet with the Network for Poultry Production and Health in Developing Countries.
The view of ACIAR has been that little progress could be made with village poultry until Newcastle disease was controlled. Now that suitable vaccines are available it is possible to adopt a more holistic approach to the whole problem of rural chickens, and indeed of all rural poultry.
The virus
The virus of Newcastle disease is classified within the genus Paramyxovirus of the family Paramyxoviridae. This immediately tells us certain immutable characteristics of the virus. The virus will have a genome of single stranded RNA. The inexact replication of the RNA will frequently produce variants with differences, often subtle differences, in phenotype from the parent particle. Unless there is suitable selection pressure, these variants will not prosper. We must be aware that the populations of Newcastle disease virus that spread in the field, or the populations that make up a vaccine stock, are not clonal. Selection pressure can alter the average behaviour of the population. Of particular interest to this discussion are the variations that can evolve in pathogenicity and in thermostability.
The infectious virus particle (the virion) will have a lipoprotein envelope that will be essential for infectivity. The proteins on the envelope will be specified by the viral genome. These will be antigenically important and they will contribute to the host specificity and the spectrum of pathogenicity of the virus.
We will also be able to suggest other properties of Newcastle disease virus, by biological analogy with other paramyxoviruses. In particular, we will expect that Newcastle disease virus will be antigenically stable across its geographical range, and with time. Although variants will be detectable with monoclonal antibodies, or by sequence analysis, polyvalent antiserum will not easily distinguish between strains.
Newcastle disease viruses are usually cultivated in the cells lining the allantoic cavity of embryonated hen eggs. Some strains kill the embryos; others do not. The virus will also grow in cell cultures of avian-origin, and in some mammalian cells. The replication of some strains of the virus is indicated by the destruction of the host cells, a process termed cytopathogenicity. Not all strains of Newcastle disease virus are cytopathic and detection of these strains in cultured cells can be difficult.
All strains of Newcastle disease virus will agglutinate chicken red blood cells in vitro (and sometimes red blood cells from other species). The process is known as haemagglutination and is the basis of the common serological test, the haemagglutination-inhibition test, used to detect antibodies to this virus. Other serological tests are available.
The hosts
It is probable that most avian hosts, domestic and feral, can be infected with strains of Newcastle disease virus. Kaleta and Baldauf (1988) listed more than 250 species of free-living and caged birds that have been infected with Newcastle disease virus. This is in addition to the common species of domestic birds that become infected. The consequences of these infections will vary with the strain of virus and the species of host. Chickens are the most important host for Newcastle disease virus. However, village flocks are not limited to domestic chickens. We must be aware of the possible role of other avian species.
Ducks deserve special consideration. Ducks are reported to be readily infected with Newcastle disease virus and to be capable of spreading the virus. There are few reports of clinical Newcastle disease virus in ducks, but we must be wary. There are several accounts indicating a high rate of carriage of Newcastle disease virus by ducks. For example, in a village situation in Indonesia Kingston and Dharsana (1979) found that the virus persisted for one year in a flock of only 300 ducks. In work with which the author has been associated in Vietnam, strains of Newcastle disease virus that are virulent for chickens have been isolated from ducks. Some isolates of duck plague virus (a herpesvirus) have been contaminated with Newcastle disease virus. It would be useful to determine whether the two viruses act synergistically in dually infected ducks. These findings were with Pekin ducks. Even less is known of Newcastle disease in the Muscovy ducks that seem to be more common in Africa. At a recent IAEA/FAO meeting in Morocco, it was observed that in Tanzania Newcastle disease is a greater problem in chickens in villages where ducks are also kept.
Turkeys can also be infected with Newcastle disease virus, but are apparently not very susceptible to disease. Certain strains of Newcastle disease virus have become adapted to pigeons in some countries and cause disease in both pigeons and chickens. Accounts of the deaths of pigeons in African villages during out-breaks of Newcastle disease are not unknown. Veterinary authorities in Africa give very diverse views on Newcastle disease in guinea fowl. Opinions vary, from these birds being entirely refractory to guinea fowl being highly susceptible and a danger to commercial chickens. From China there has been a report of a strain of Newcastle disease virus adapted to, and causing disease in, geese (Linchuan and Hong, 1998).
Newcastle disease virus can infect mammals. Human infection occurs and, at least with virulent strains of the virus, causes severe conjunctivitis. There has been an isolation of Newcastle disease virus from a pig in Indonesia.
There may be more unusual hosts for Newcastle disease virus. Again from Indonesia there has been an account of the apparent replication of Newcastle disease virus in rice field crabs (Kingston and Dharsana, 1977).
The virus-host interaction
Newcastle disease virus reacts with avian hosts in various ways. When nonimmune domestic chickens and highly pathogenic strains of Newcastle disease virus meet, the common sequel is an acute disease with mortality close to 100%. Surviving birds will often have defects that are attributed to neural lesions. The birds will often be paralysed or have twisted necks. This severe disease, attributed to strains of Newcastle disease virus that we call velogenic, occurs in both village and commercial poultry. It is the only form of the disease that is of consequence in village chickens. The syndromes attributable to viral strains of lesser virulence would not be noticed in village flocks.
In commercial chickens, strains of Newcastle disease virus of moderate virulence (mesogenic_strains) cause lower rates of mortality in mature chickens, but severely deplete egg production. Strains of low virulence (lentogenic strains) cause little mortality except in young birds, but do reduce egg production. These strains also interact with other pathogens, especially respiratory pathogens, to produce severe clinical disease.
Most Newcastle disease vaccines are living vaccines, drawn from the ranks of lentogenic or even mesogenic viruses. Their use may alter the epidemiology of Newcastle disease. We now also recognise avirulent strains of Newcastle disease virus that cause no clinical signs when they spread between chickens of any age by natural routes. Lentogenic, mesogenic and avirulent strains also circulate naturally in some countries, modifying the epidemiology of Newcastle disease.
Most strains of Newcastle disease virus spread by the respiratory route, infected chickens producing aerosols of infectious virus. Faecal excretion also occurs. With some strains this is probably the most important route of spread, with infection being by ingestion.
There is little evidence for the spread of Newcastle disease virus through the egg (true vertical transmission), though transmission of virus on the shell of infected eggs is well recognised. Embryos inoculated at about 9 days of incubation with avirulent viruses (that do not kill embryos by this route) have been allowed to hatch. The chicks are infected (French et al., 1967). However the same viruses given by yolk sac route at 4 days of incubation kill the embryos (Kim and Spradbrow, 1978). The real experiment, with collection of eggs from viraemic hens, seems not to have been done.
Human intervention is probably a common source of infection. The clothing and shoes of people working with infected poultry are readily contaminated. Food, water and items of equipment used on infections premises are also sources of mechanical spread of contagious virus.
Infected chickens are the usual source of infection in villages
I believe that most of the cases of Newcastle disease that we see in village chickens can be attributed to chickens that are shedding virus. These will usually be chickens that are incubating the disease. Virus excretion commences before clinical signs occur. They may however be birds that have recovered from clinical infection, or vaccinated birds. Vaccinated birds may show no clinical signs at all on challenge with virulent virus, but they will become infected and excrete the virulent virus. They can transmit the virus to other birds by contact for up to two weeks.
In many countries, seasonal outbreaks of Newcastle disease are recognised. Attempts are often made to attribute these outbreaks to the weather conditions prevalent at the time. There may, however, be more realistic explanations based on patterns of movement in chickens and changes in the volume of chicken markets.
Accordingly, the seasonal outbreaks of Newcastle disease might not be directly due to prevailing climatic conditions. Possibly the real correlation is with the planting season and the need for villagers to sell chickens to buy seed rice. The volume of chickens traded through the markets increases at this time. From Uganda it has been suggested that outbreaks that occur in the dry season are not really because the virus survives better under these conditions. This is the time of low employment in the agricultural sector. Villagers use the spare time to visit kinfolk, and usually carry chickens as gifts. Outbreaks in other countries might be related to marketing for festivals, rather than to the season. There are, for example, the outbreaks that occur before Christmas in Ghana and before Easter in Ethiopia.
In many countries veterinary workers, and indeed village people, recognise that the introduction of new birds to a flock is often associated with an outbreak of Newcastle disease. This lesson needs to be spread by extension workers, although it is not possible to restrict the entry of new chickens into village flocks.
Villagers become astute at recognising the early signs of Newcastle disease. There are two common responses. One is to salvage sick or even dead birds through the cooking pot. We cannot disapprove of this practice when so many villagers are always hungry. We can however suggest that the infected entrails are buried, and not discarded to be scavenged by other creatures. The second remedy is the market place. Markets must be a common source of Newcastle disease infection, sometimes through the random sale of infected birds, but more commonly through salvage sales.
Huchzermeyer (1993) made the interesting suggestion that Newcastle disease virus may spread amongst village chickens at night rather than during the day when ultraviolet radiation is strong. He believed that chickens that were housed at night would be more readily infected than birds that roosted in trees. With roosting flocks, the inability of sick birds to fly to branches would be an added protection. Brooding hens and hens with chickens would also segregate and protect themselves. Huchzermeyer also postulated a state of endemic Newcastle disease that would not depend on persistent carriers.
Epidemic Newcastle Disease in village flocks
In many areas, the chickens in small village flocks have no previous experience of Newcastle disease virus. They lack antibody and when the virus is introduced, it spreads quickly. Then we see the epidemic occurrence of Newcastle disease. These are the flocks where we will see adult birds dying as we walk through the village. These are the events that colour our thinking about Newcastle disease, and the attitude of the villagers to the disease. There will be few survivors. These are the events that ensure that in all languages and dialects there is a word that translates into English as Newcastle disease.
Epidemic Newcastle disease is not self-sustaining. The virus will vanish when no susceptible chickens remain, or when the chicken population contains too few susceptibles for the virus to spread. We must envisage other situations in which populations of chickens maintain endemic Newcastle disease, or in which other hosts maintain the virus.
Endemic Newcastle Disease in village flocks
When Johnston (1990) commenced to monitor village flocks very closely for Newcastle disease, he found that epidemic spread was not the only form of the disease. He noted that Newcastle disease “may be able to smoulder enzootically for several months, even years, in a typical village housing one or two thousand birds”. The losses of small numbers of chickens, against the general background mortality, will not attract attention. The author noted that monitoring processes designed to study fast-spreading epidemic disease would not be sufficient to detect endemic infection.
Computer models generated by Johnston (1990) accorded with his field observations. The model indicated that velogenic virus would remain endemic in a group of 5 flocks, each of 80 chickens, sharing the same scavenging environment. Flocks with endemic Newcastle disease will have a mix of chickens with antibodies and chickens lacking antibodies. The problem birds are those that are clinically healthy but incubating the virus and either shedding virus or soon to shed virus. Vaccinated birds can also become infected and shed virus although they develop no clinical signs.
There are practical consequences. Village vaccine trials often involve buy-back challenge; in which vaccinated birds and control birds are purchased and returned to the laboratory for artificial challenge. Sometimes one of the apparently healthy birds will initiate the challenge before the intended challenge virus is introduced. This has occurred in the Philippines (Fontanilla et al., 1994). Unvaccinated village chickens are sometimes purchased and housed together for later vaccine experiments. One chicken excreting Newcastle disease virus can kill the whole group. This has happened in Ethiopia. It is better to obtain potential experimental chickens as one day olds and to rear them in isolation. In Tanzania the problem has been met by holding the purchased chickens together for a fortnight, and then selecting those that lack antibodies against Newcastle disease virus. Agencies supporting women's groups will sometimes purchase village chickens and vaccinate them against Newcastle disease. On one occasion, when vaccination and distribution occurred on the same day, the subsequent deaths from Newcastle disease were a tragedy to the new owners. A better approach, as practised by Dr. Robyn Alders in Mozambique, is to vaccinate and hold for a fortnight before distribution to the new owners.
Economics of Newcastle Disease control in village poultry
Until very recently, there were no methods of controlling Newcastle disease in village chickens. The conventional Newcastle disease vaccines that were effective in commercial poultry found little use in villages. The flocks were small, scattered, multi-aged and under minimal control. The vaccines were heat-labile, relatively expensive and produced in large-dose units suitable for large commercial flocks. Their application required physical control over the chickens.
The development of heat-stable vaccines that spread between chickens, such as strains V4 and I2, have changed this picture. These vaccines can be given on certain foodstuffs if necessary, allowing vaccination of near-feral flocks. These vaccines can produce substantial immunity under village conditions. This has been demonstrated in many countries. We have reached a stage where we can recommend and plan for local production and broad distribution of these vaccines.
At this stage of the project, before extensive vaccination is undertaken, we must face questions of cost benefit. There is not a lot of sound information on the productivity and the commercial value of village flocks. We seem to be in urgent need of the assistance of economists in this phase of the project.
We all know in qualitative terms the value of village chickens. Chickens, their meat and their eggs have a potential market value. The flock serves as a reserve of petty cash that can be drawn on for barter or for sale when essential items are required. Chickens serve social and ritual functions that are difficult to value. Chickens also perform waste disposal functions and provide organic fertilizer. However when we approach economists or funding bodies there is a requirement for cost benefit analysis. Few have attempted to derive monetary values for village flocks.
Johnston (1990) analysed data from 697 monthly flock monitoring visits to village flocks in Selangor, Malaysia. Outlays on the flocks were M$11.37 per month, the production M$20.46 per month, with an average monthly profit per flock of M$9.09. He cites a similar study from Sri Lanka, where the flocks are devoted to egg production rather than meat production (Veterinary Research Institute, Sri Lanka, 1990). The target population was 156 flocks, whose egg production allowed each person in each household to consume 2.5 eggs/week, which is three times the national average.
A study in Thailand demonstrated the essential wastefulness of village poultry raising. Age specific mortality rates were 25% for chickens less than 2 months old, 7% for the 2 to 6 month age group and 3% for older birds. The average time in a flock was 5.8 months. Production comprised 24.7% of all removals, but 82.2% of removals at greater than 2 months. None of the removals under 2 months of age were for productive purposes and these outnumbered the total removals of older birds. Newcastle disease was a frequent cause of death. Control of Newcastle disease and increased survival of chicks would greatly improve productivity.
Another approach used by Martindah (1991) was to measure village offtake based on hen days, to determine the productivity of adult females. In this study every 100 hens produced for sale 402 growers and 387 adults (presumably per year).
The lesson seems to be that it is difficult to measure the productivity of village flocks, and extremely difficult to place a monetary value on this production. Yet it is necessary to value the enterprise if the response to vaccination is to be measured. Simple census data suffices for villagers and for simple virologists. Villagers will see every chicken saved as a profit, and will probably value the vaccine in chicken equivalents. I believe that in Bangladesh, villagers are prepared to pay a few percent of the potential final price for the chicken to have it vaccinated. Where vaccination programs are introduced it will be necessary to devise methods of cost recovery if production and delivery of vaccine is to be sustainable.
Granting bodies are more likely to demand a commercial evaluation of village vaccine projects. This is not to be encouraged for these data are difficult to generate. Few are known to me. James (1991) described a theoretical framework for achieving these ends. The project requires vaccinated and control villages and observation for one year or for a number of whole years. Inputs and outputs would need to be recorded at intervals of one or two weeks. Any change in flock size would be recorded, and increase in flock size would be regarded as reinvestment.
Johnston (1990) carried out an examination of the probable economic impact of oral vaccination on whole countries, using both computer modelling techniques and field data. He estimated that the early ACIAR projects in five countries would generate a flow of net benefits of Australian $389 million.
It should be possible to calculate the cost of thermostable Newcastle disease vaccine made under basic conditions in provincial laboratories. A single egg should yield 5 ml of infected allantoic fluid, the equivalent of 5,000 doses of vaccine. There should be minimal costs in diluting and storing this vaccine, which should have a shelf life of about two weeks.
References
Fontanilla, B.C., Silvano, F. and Cummings, R. (1994.) “Oral vaccination against Newcastle disease of village chickens in the Philippines”, Prev. Vet. Med., 19, 39–44.
French, E.L., St. George, T.D. and Percy, J.J. (1967). “Infection of chicks with recently isolated Newcastle disease viruses of low virulence”, Aust. Vet. J., 43, 404–409.
Huchzermeyer, F.W. (1993). “Why is velogenic Newcastle disease endemic in some countries and not in others?”, Zimbabwe Vet. J., 24, 111–113.
James, A. (1991) Proceedings, Newcastle Disease Vaccines for Rural Africa. Rweyemamu, M.M., Palya, V., Win, T. and Sylla, D. (eds.) PANVAC. Debre Zeit, Ethiopia, 185–190.
Johnston, J. (1990). Health and Productivity of village Poultry in Southeast Asia, ACIAR Working Paper No. 31, Canberra: ACIAR, 61 + appendices.
Kaleta, E.F. and Baldouf, C. (1988). “New castle disease in free-living and pet birds”, in D.J. Alexander (editor), Newcastle Disease, Boston/Dordrecht/London: Kluwer Academic Publishers, 197–246.
Kingston, D.J. and Dharsana, R. (1977). “Mortality in crabs in the presence of Newcastle disease virus”, Vet. Rec., 100, 433–444.
Kingston, D.J. and Dharsana, R. (1979). “Newcastle disease virus infection in Indonesian ducks”, Philippines J.Vet.Med., 18, 125–130.
Kim, S.J. and Spradbrow, P.B. (1978). “Some properties of lentogenic Australian Newcastle disease virus”, Vet. Microbiol., 3, 129–141.
Linchuan, W. and Hong, Y. (1998). “Identification of the infection of paramyxovirus type 1 in goose”, Proceedings, Fourth Asia Pacific Poultry Health Conference, Melbourne, November 1998, 135.
Martindah, E. (1991). Estimation of production parameters for village chicken production in Bogor District, West Java, M.Sc. Thesis, University of Reading, U.K, 77.
Thitisak, W., Janviriyasopak, O., Morris, R.S., von Kruedener, R. and Srihakim, S. (1989). “A poultry health and productivity profile- diseases and control measures”, Proceedings, International Seminar on Animal Health and Production Services for Village Livestock. Khon Kaen, Thailand, 409–415.
Veterinary Research Institute, Sri Lanka (1990). Annual Report.