Carbon dioxide content in air is only 0.03%, but it is highly soluble in water unlike oxygen and one volume of CO2 dissolves in equal volume of water, the solubility being higher at low temperature (see Table 9.III). CO2 stays in free (dissolved) or bound from (bicarborate and carbonate) in water depending on the pH of the water.
CO2 + H2O⇌H2CO3⇌H+ + HCO3 H+ + CO=3
The amount of unionized H2CO3 and free CO2 can be estimated with the help of the Henderson - Hasselbach aquation, substituting 6.1 for the pk, changing with temperature) of H2CO3 thus;
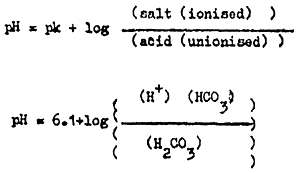
at a pH of 7.4 the proportion of free HCO3 to H2CO3 is 20:1; i.e.
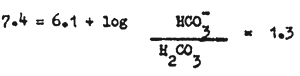
antilog of 1.3 = 20, therefore - HCO-3 : H2CO3 = 20:1
As obvious, low pH waters will have high dissolved CO2 and waters above pH 8.36, are free from dissolved CO2. The relative levels of activity (mole fractions) of free and bound CO2 (HCO-3 and CO-3) at different pH levels are shown in Fig. 9.11. Waters are classified by some authors (Birge & Juday in Welch, 1952) depending on the amount of bound CO2: Soft waters - bound CO2 level less than 5 ml/1 (25 mgCaCO3) (low calcium and magnesium); Medium - bound CO2 level, 5 – 22 ml/l; i.e. 100 mg CaCO3; Hard waters bound CO2 level over 22 ml/l. Hardness of waters will be dealt with further later.
Table 9.III: Solubility of CO2 in pure water at different temperatures
| °C | mg/litre |
| 0 | 1.10 |
| 5 | 0.91 |
| 10 | 0.76 |
| 15 | 0.65 |
| 20 | 0.56 |
| 25 | 0.48 |
| 30 | 0.42 |
| 35 | 0.36 |
| 40 | 0.31 |
Increase in free CO2 is often concommittant with a decrease in oxygen in natural waters as CO2 is an end product of anaerobic metabolism as well. Fishes in the tropics are often tolerant to high levels of free CO2 in water. Whereas the salmonids cannot tolerate CO2 levels higher than 5 – 10 mm Hg (pCO2) the carps can tolerate CO2 levels over 100 mm Hg. Again tolerance of a high free CO2 level does not mean that the fish and shellfish are able to grow well under the high CO2 condition. In waters into which sewage inflow is there the free CO2 level can be high in ponds with high photosynthetic activity the pH would go up due to the using up of the CO2 by the plants and increase in the bound form (Fig. 9.12). The levels of pCO2 should be ideally less than 5 – 10 mm Hg in fish ponds, especially if CO2 sensitive fishes are being captured.
The biological role of CO2 apart from being the end product of respiration and source for carbon fixation (photosynthesis) is that high levels of CO2 interfere with the binding capacity of haemoglobin with oxygen. Shellfishes are also subject to the same effect even though their blood pigments are different (haemocyanin). The oxygen loading capacity is decreased at high pCO2 levels and a shift of the blood oxygen (HbO2) dissociation curve to the right is effected; the phenomenon is termed “Bohr effect”. In certain fishes as shown by Root (hence called Bohr effect) high pCO2 reduces maximum blood oxygen capacity i.e. a depression of the oxygen dissociation curve is effected. Both together amplify the CO2 effect on fishes, by restricting metabolism and energy release.