Transport of hatchlings, fry and fingerlings of culturable species is a common necessity in aquaculture. Often large adult breeders have also to be transported for facilitating seed production. Indeed in certain parts of the world large level of transport of commercial fish in live condition from the areas of capture to the markets is a part of a highly organized industry, but we shall confine here to the transport of brood fish and fish seed, particularly the latter for culture purposes.
Transport of fish seed in earthern pots, taken either as head loads or on slings from seed collection centres to spawn* markets and to nurseries for stocking is an ancient practice in certain parts of the world. These traditional methods often entail heavy mortality during transport. Recently improvements have been made in the techniques of live fish transport with the knowledge of the basic physiological requirements of fishes in different stages of their life history (hatchling, fry, fingerlings, juveniles and adults) and also of the causes of mortality of fishes during transport (e.g. Berka, 1986).
While the empirical knowledge already available may not be ensuring very high survival, it is important that we do not fully discard it for it can still be improved upon and applied especially in rural aquaculture, when high technology is difficult to be applied. Modern developments in transport technology are from two levels; one is from an understanding, as mentioned, of internal physiological mechanisms of the fish and the optimal requirements, ensuring maximum survival of fish under transport and the other is from a study of the environmental parameters of the medium in which fish are transported. It is difficult to separate the two levels, but a synthesis of the two is in the study of the autecology of the fish and application of methods to ensure increased survival, by the control of ambient conditions (eg. ambient oxygen, pH, ammonia) and by ensuring more suitable physiological condition (eg. conditioning fish fry, starvation before transportation) of the fish to be transported.
Fish transport technology has developed from the transport of simple earthern pots, as already referred to, to transport in polythene bags under high pressure of oxygen and use of anaesthetics and chemicals. Under anaesthesia fish can be transported without water even, provided the skin and gills are kept moist under low temperature. The cryopreservation of fish sperm for use at any convenient time can be referred to here, though this would concern seed production more directly than live seed transport.
Several factors, as those given below, may be responsible for mortality of fish in transport:
* used here synonymously with hatchling/young fry.
It will be noted that items, 1 – 3 concern ambient conditions and items 4 – 7 concern the fish per se (the internal machinery). We shall now deal with the latter, especially the array of internal changes in fish due to hyperactivity and handling stress.
Live-transport of fish seed and brooders entail handling often involving chasing with or without net, catching and transfer from the nursery/farm ponds to the equilibration (conditioning) system and thence to the transport container. The stress of this pre-transport handling can itself be high, sometimes causing injury and immediate death; the stress of transport itself can be distinguished from the handling stress.
The severity of the transport stress would depend on the duration of transport and the physico-chemical characteristics of the medium/ containers of transport.
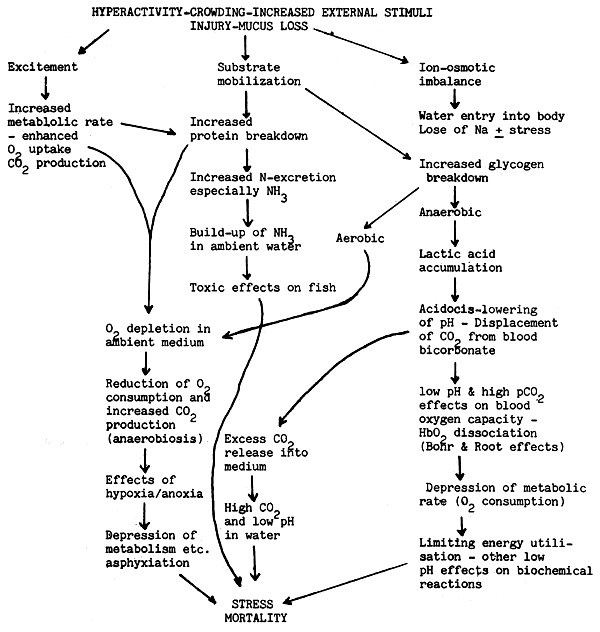
The physiological effects of handling including hyperactivity can be mentioned first. Depending on the severity of handling the effects can last over a day even. Hyperactivity or increased physical activity and handling and concomittant excitement cause immediate depletion of the labile energy store in muscle, namely glycogen, mostly through the anaerobic pathway leading to the production of excess lactic acid. The lactic acid causes ‘acidocis’ and consequent lowering of pH, which results in the liberation of CO2. The acidic pH and increased pCO2 in blood would cause reduced loading of oxygen in the blood (Bohr and Root effects), which would seriously impair the energy yielding mechanism of the fish and can cause collapse of the fish. Acidosis of the blood would affect the other bio-chemical reactions as well and can also cause fatigue collapse.
The surviving fish quite often would accumulate an oxygen debt, which under the crowded condition of the transport carrier, coupled with low oxygen conditions can again lead to the collapse and death of the fish. Therefore it is imperative that the fish caught for transport should be given sufficient time in high oxygenated water under optimal conditions for Conditioning of fish before transportation. (see flow chart “Effects of Handling/ Transport on Fish”).
EFFECTS OF HANDLING/TRANSPORT ON FISH
In the transport carrier itself it is a matter of deciding the optimal capacity of the container under defined conditions eg. with or without oxygen at high pressure. Again here the size- metabolism relation should be understood - the smaller the organism the higher the metabolic rate (O2 consumption, CO2 production and nitrogen excretion etc). We shall refer to this aspect again.
For influence of ambient oxygen, carbon dioxide, pH, ammonia, temperature etc, on fish, refer earlier discussions under “Environmental Factors” (Site Selection & Species Selection for Aquaculture.)
Spawn, fry and older fish for long transportation have to be prepared or conditioned. This is again based on the physiological principles explained. The fish seed and broodfish are kept starving usually in a cloth ‘hapa’ or other containers in a quiet corner of the fish pond or in relatively quiet water in a canal or river for a period of time before transferring them to the transport carrier.
The advantages of conditioning fish thus are:
Eventhough fry are not fed usually during conditioning, some (eg. Alikunhi, 1957) are of opinion that fry can withstand the rigours of transport better if they are fed with animalcules like cladocerans. Since small-sized animals have a greater metabolic rate and are likely exhaust the energy store quicker such feeding may be useful for a long transport, but by and large fish can stand starvation for long periods and it is advisable not to feed fish under conditioning and transport.
It must also be mentioned that the recovery and well-being of the fish under conditioning would indeed help increased survival, but the rigours of transport are different and these can again caused stress and strain on fish and cause mortality as already explained.
As referred to, the most common method of conditioning is to store fry in a cloth ‘hapa’ in ponds or in a still part of a river or canal. According to Saha and Chaudhury (1956), the depth of water where a conditioning enclosure is to be installed should be 30 to 35 cm. The period of conditioning depends on the size and health of the spawn (hatchling), fry and fingerlings. Jagannathan (1947) stated that fry need 48 to 72 hours of conditioning. Saha and Chaudhury (1956) agree with this observation and suggest that the period of conditioning can be applied to fingerlings in general.
Alikunhi (1957) remarked that about 6-hours conditioning is required for fry, whereas Srivatsava and Karamchandani, (1964) suggest that fry (8 – 23 mm) require 24-hours conditioning for transport in a limited volume of water (2 ml per fry). The fry were seen to survive even when oxygen concentration was 0.88 ppm during transport. It appears that in general a conditioning period of 6 – 24 hours would suffice for all species of fish - longer conditioning period should be given specially in cases where stress due to capture and subsequent handling is high. A point often ignored is that there is considerable ion-osmotic stress on all fish due to handling and in freshwater fish considerable amount of salt from the body is likely to be lost due to capture and handling. Addition of a little amount of NaCl in the ambient medium especially in the case of tilapias, might be of advantage, but the system has to be sufficiently modified since conditioning is often done in open waters.
Various types of conditioning containers are used, namely boxes made of wire meshes, bamboo or cane wicker work; barrels or boats with perforated bottoms; temperary enclosures made of netting or bamboo matting, cloth ‘hapa’ etc - any of these materials available conveniently may be used (see below).
The temperature of the conditioning water should not be high - it should be preferably on the lower side of the optimal thermal range for the species.
During conditioning and transportation fry and fingerlings should not be handled with bare hands - the slime over the fish body should remain in tact. The loss of slime and scales would render the fish disadvantageous in maintaining osmotic balance and also fish are likely to get infected.
Transport carriers are of two types: (a) open system comprising open carriers, with or without artificial aeration/oxygenation/water circulation and (b) closed system having sealed air tight carriers with oxygen.
The simplest transport carrier is the earthern vessel, such as the traditional “Hundi” used in Bengal in India. The earthern hundi is now being replaced by aluminium vessels which are unbreakable, but the earthern hundies have the advantage that they keep the temperature of the water inside cool by means of evaporative cooling. The earthern vessels used in Bengal are of 2 types, the smaller one of 20 cm diameter and 23 litres capacity carried as a head load or on a bamboo sling; and the larger are of 23 cm diameter and 32 litre capacity, used for transport by railways. The earthern vessels are filled with water of the same source as that of the fry. About 50,000 carp spawn are released in the small vessel and 75,000 in the larger (Saha and Chaudhury, 1956). In traditional transport in India, about 60 g of finely pulverised red soil is sprinkled over the water surface in the transport vessel and the vessels are shaken periodically during transport. The addition of red soil coagulates the suspended organic matter and keeps down the zone and extent of pollution (Basu, 1951). During transport, the bottom sediments are periodically removed by mopping them up with a rough cloth rope - the water is also partially renewed depending on the need. The addition of red soil and change of water permit transport of fry upto a duration of 30 hours. Besides pulverised earth other absorbant substances such as activated charcoal and ‘Amberlit’ resin can be also used as these absorb carbon dioxide, ammonia and other substances from the medium (Saha et al, 1955).
As already mentioned the improved metal containers are better than the earthern carriers only because they are not breakable. The metal containers used are round vessels with a wide mouth, which can be closed with perforated pressed-in lids, the larger type being 53 cm in diameter at the base, 20 cm at the mouth and 38 cm high. Any variation to this size should be equally functional. To prevent denting and perhaps more to effect insulation, wooden covers are used on the metal containers; often the vessel is crated and kept wet during the journey. Some workers are of opinion that some air space should be left above the water in the container (Jagannathan, 1947); while this may facilitate better exchange of respiratory gases between air and water, constant splashing of water during transport is likely to injure the fish carried. From this fact is the genesis of splashless fish carriers of fry. Mammen and others, discussed under ‘closed system’.
Larger containers mounted on motor vehicles have also been in use. In some of these a semi-rotatory pump has been added producing sprays of water over the water surface in the tank, through a delivering tube with two rows of holes at 45° to each other. Fish fry have been transported in such motor vans (semi-insulated) over a distance of 500 km with mortality less than 5%. Several other adaptations of open transport carriers mounted on motor vehicles are also in vogue.
In spite of being cheaper, open packing system for transport of fish seed is going out of fashion mainly because it involves continuous vigilance and frequent renewal of water during long journeys. It is obviously not worthwhile or economical to transport bigger fingerlings and adults in small packing units. For this purpose, truck-mounted open tanks with mechanical aeration and water circulation (as the one explained) have been in use successfully (Hora & Pillay, 1962; Patro, 1968; Berka, 1986). Galvanised iron drums of 180 L capacity, and open canvas containers (1 m × 1 m × 1.25 m) have been used successfully in transporting breeders. The galvanized iron drums are provided with an opening of 48 cm × 30 cm along the main body of the container for introduction of fingerlings and larger fish. The drums is often lined inside with foam rubber to avoid injury to the buffetting fish during transport. Recently plastic pools (250 litres and other sizes) have also been used for transporting fish.
In China and South-East Asia efficient open containers have been developed, some of traditional design, for the transport of fingerling and adult fish. In Indonesia water-tight tar-coated, plaited bamboo baskets capable of accomodating 10,000 fry of 5 cm in size, are used.
It is estimated that one litre of water is required for every 250 g of adult fish weight, for whose transport oval casks of 150 litre capacity are used in Indonesia. In China, baskets and tubs, some as large as 2 metres high and about 2 metres in diameter are reported to be in use. Water is kept agitated continuously and foul water bailed out periodically. On over-night halts fry are liberated in a net fixed in a pond and fed. Sixty percent survival on journeys about a week is considered acceptable. Large live-fish boats (eg. Pituyas for transporting milk fish in Philippines) of special design with holds devided into several compartments provided with holes and other devices to allow continuous inflow and circulation of fresh water from outside, are also in use. Some of these boats have a loading capacity of 10 million fry. Similar boats designed for free circulation of water in their holds where live fish are held, are prevalent in Indonesia and Philippines as well.
In this system the water surface is exposed to compressed air or pure oxygen introduced to fill the zone over the water surface in the carriers which are sealed air-tight (see Fig. 4.1 to 4.10). This practice has been in vogue in Western countries for many years and now is accepted practice all over the world. Sealed metal containers, rubber and plastic bags have been used for the purpose. In a metal container of galvanized iron (45 × 35 × 35 cm) with two airtight openings, one to let in oxygen and other to let out water, 100 – 200 fingerlings of 7 – 10 cm, or 30 – 40 fingerlings of 13 – 20cm length, can be transported for a journey of 12 hours (Khan, 1946). Eighteen litre tins with airtight screw-capped lids for filling and provided with tubes for drawing in oxygen and letting out water, have been used in CIFRI, Barrackpore, for transporting 1000 fry of 1 – 2 cm length during a 20-hour journey.
Polythene bags of various dimensions (74 cm × 46 cm or 65 × 45 cm - thickness 0.0625 mm) are now widely used in fish fry and fingerling transport. In this method the bag is first put into a tin or any rigid box of 18 – 20 litre capacity and the bag is filled upto ⅓ of its capacity (6–7 litres) with water and the required number of seed is put into it and the bag is inflated with oxygen in high pressure from a cylinder, upto ⅔ of the bag. The upper 10 – 15 cm of the bag is twisted, bent and tied securely airtight with a string. Carp hatchlings numbering 20,000 – 40,000, 300 – 600 fry (30 – 40 mm) and 40 – 70 fingerlings per bag depending on the distance are packed and transported in this manner (see Table 4.I). The mortality reported varies from nil to 5%. Use of plastic bags is illustrated in Figs. 4.1 and 4.2. See also Figs. 4.3 – 4.10 for closed system transport.
For transport of larger fish (large fingerlings, brooders) large closed-system carrier tanks have been designed. Water being splashless in the tank is of special advantage, as explained, as the fish would not injure themselves much. Fry (1951) designed a smaller galvanized splashless carrier - the design here is simple and mainly consisted in the use of a rubber tubing (cycle tyre) which can be inflated on top to fill the space and also addition of ice cubes on top of the carrier to control temperature.
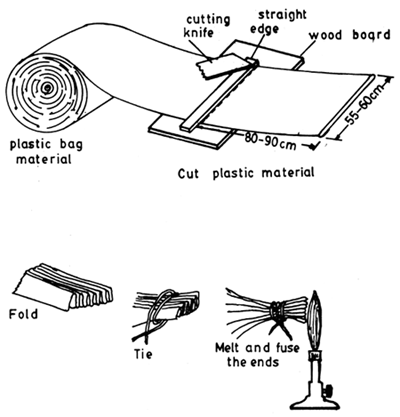
Fig. 4. 1. Procedure of closing the bottom end of a polyethylene sleeve (After Woynarowich and Horvath, 1980)
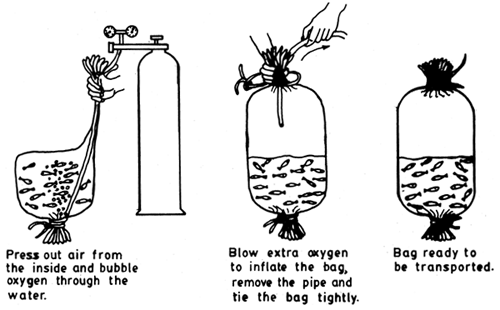
Fig. 4. 2. Procedure of filling the bag with water, stocking with the fish, displacing the air, intoducing oxygen and closing the upper end. (After Woynarowich and Horvath, 1980)
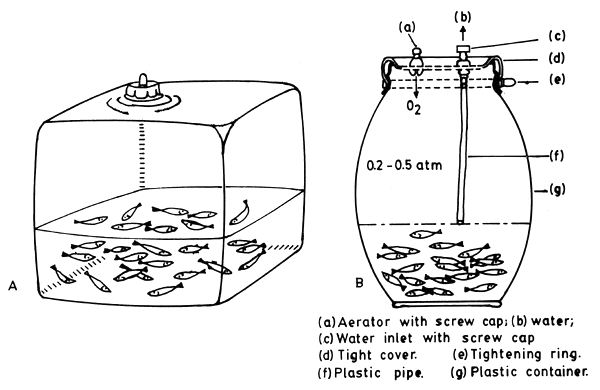
Fig. 4. 3. Sealed plastic containers
container volume 25 litres, the oxygen-inlet valve is built in the screw cap;
container volume 50–150 litres, vertical plastic p pe keeps water at the required level. (From Berka, 1986)
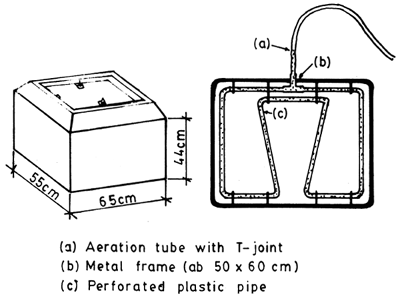
Fig. 4. 4. Small tank to be carried in a car. Container volume 100 – 150 litres; the aeration grate is adjusted to s fit the dimensions of the tank. (From Berka, 1986)
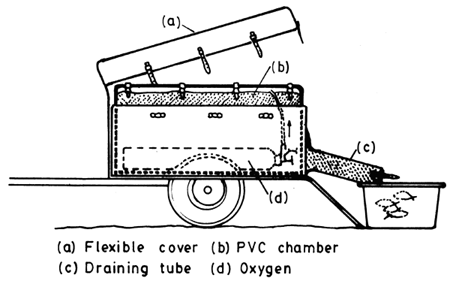
Fig. 4. 5. Passenger-car trailer for fish transport (From Berka, 1986)
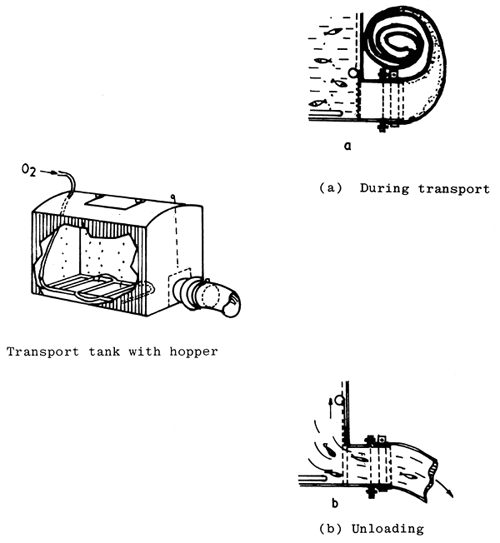
Fig. 4. 6. Transport tank with hopper. (From Berka, 1986)
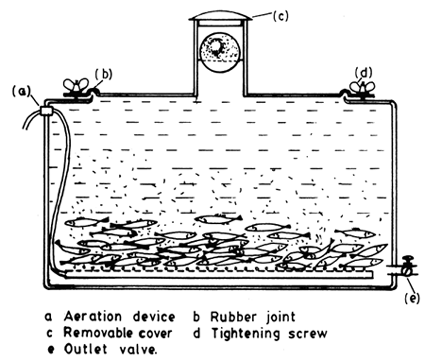
Fig. 4. 7. Specialized tank for pike-perch transport (From Berka, 1986)
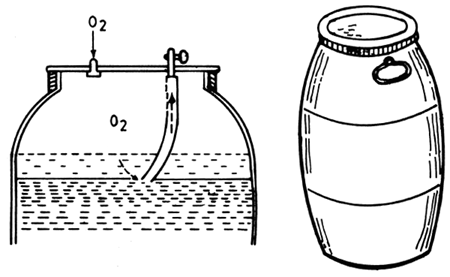
Fig. 4. 8. Plastic tank for pike-perch transport (From Berka, 1986)
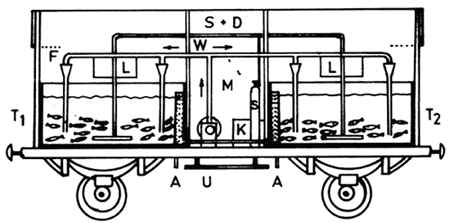
Fig. 4. 9. A fish transport tank wagon (From Berka, 1986)
M - technical space and attendant's booth, U - pump and
air compressor, T - transport tanks 1 and 2, S - oxygen
cylinders, A - tank drain, F - water aeration, L - loading
space, W - circulating water distribution system,
S + D - oxygen or compressed-air distribution system.
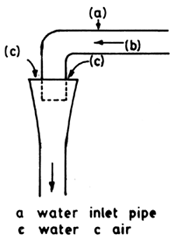
Fig. 4.10. Water aeration in the tank wagon (From Berka, 1986)
Table 4.I: Packing density of fish seed under for 12 hour journey (data from Fisheries Extension Unit, Hyderabad, India)
Number of fry or fingerling
| Size of fry or fingerling | Milk can type (metal) 40 – 50 litres | Plastic bags 16 – 18 litre capacity |
| 1 cm | 500 – 25,000 (Range) (15,000) (Average) | 1000 – 10,000 (5,500) |
| 2 cm | 500– 20,000 (3000) | 500 – 5,000 (2,200) |
| 3 cm | 200 – 10,000 (3000) | 200 – 1,000 (600) |
| 4 cm | 100 – 700 (400) | 100 – 500 (330) |
| 5 cm | 50 – 500 (250) | 75 – 300 (225) |
| 6 cm | 50 – 400 (200) | 50 – 200 (80) |
| 7 cm | 50 – 300 (100) | 25 – 100 (70) |
| 8 cm | 50 – 200 (90) | 25 – 50 (40) |
A larger model is the splashless thank of Mammen (1967) which is a modified petrol tank of 1150 litre capacity with an auto-clave type of lid. It has a built-in aeration system for supplying compressed air which works on a belt driven by the engine of the transporting vehicle.
An oxygen cylinder is also carried as a stand-by for emergency. The inside of the tank is lined by foam rubber (U-foam) which prevents physical injury to live fish, as explained earlier. A total weight of 250 kg of fish can be transported at a time, fish weighing 60 kg and 90,000 fingerlings have been transported successfully in this splashless tank. The load ratio comes to about 1 kg of fish per 4.5 litree of water i.e. 1:4.5 (Mammen, 1967; Jhingran, 1975).
Patro (1968, see Jhingran, 1975) designed a live fish carrier, which comprises of an outer cylindrical chamber of 120 cm diameter open from top and a lightly smaller inner cylinder closed from top. The top of the inner cylinder is provided with an air vent and an oxygen valve. The outer chamber serves as a storage tank and is initially filled with water and fish to be transported let in.
The inner chamber which is slipped in from the upper open end of the outer chamber, serves as an oxygen holding chamber at its top and is also lined with foam rubber. This ‘double barrel’ carrier can transport 100 kg of live fish at a time. Once filled, the oxygen supply of the carrier lasts upto 5 hours (and indeed on the rate of utilization by fish) and has a unique advantage that a constant pO2 is maintained throughout, which is determined by the weight of the inner cylinder. The whole unit is a modification of the laboratory gas supply unit, and is a good example that transports carriers can be made out of common material available and ingenuity of the users.
Berka (1986) presents guide numbers for 5 – 20 hour transport of large fish of average weight of one kg with a proper oxygen supply (Table 4.II). He also indicated in the table modified figures for fish of lesser weights down to 100 g. Berka, (1986) also presents graphic information of the various equipment used in fish transport (Figs. 4.1 to 4.10).
In any carrier system the factors which basically determine sucessful transport of fish are:
i, ii, and v are closely linked - here of utmost importance is the size-metabolism relation, the metabolic rates increasing logarithmically with decrease in fish size. If a size-metabolism relation of the species in question is available, the total O2 requirement (i and ii) and CO2 and NH3 build-up (v) can be estimated. Items iii and iv would decide the total oxygen available: while (ii) can be directly measured (iv) can be theoretically or experimentally determined. If the oxygen available is sufficiently high, item (v) becomes less important and in short journeys can be ignored. One concern should be in working out models and functional systems for the specific fish of our interest.
Table 4.II: Guide numbers for 5–20 h transports of fish with average body weight of 1000 g with a proper oxygen supply (From Berka, 1986)
| Amount of fish (kg) in 1000 litre water at | ||||||||
| Fish species | 0–5 | 5–8 | 8–10 | 10–15 | 15–20 | 20–25 | 25–28 | 30°C |
| Common carp and tench | 700 | 600 | 450 | 400 | 350 | 280 | 220 | 180 |
| Grass carp | 750 | 650 | 500 | 450 | 400 | 310 | 250 | 200 |
| Silver carp | 300 | 250 | 200 | 150 | 100 | 80 | no suggestion | |
| Bighead carp | 700 | 650 | 500 | 450 | 400 | 300 | 220 | 180 |
| Sheatfish | 800 | 700 | 600 | 500 | 400 | 320 | 250 | 200 |
| Pike-perch | 250 | 200 | 150 | 120 | 100 | 80 | no suggestion | |
Notes: (For Table 4.II)
Transport guide numbers of fish with 1000 – 1700 g body weight can be increased by 10–15%. Numbers given can be decreased in the following day:
20–30% if the body weight is about 500–1000 g
30–50% if the body weight is about 200–500 g
50–60% if the body weight is about 100–200 g
60–80% if the body weight is under 100 g.
Drugs and chemicals are either used as tranquilizers and sedatives or as antiseptics and antibiotics. General listing of anaesthetics for fish has been given by several workers (eg. Bell, 1964). Sedatives are generally used for:
reducing metabolic rates, mainly oxygen consumption and excretion of carbon dioxide and ammonia.
reducing excitability of fish and injury, and
convenience in handling fish.
The sedatives and drugs have to be used very carefully, for slight increase in dosage and/or exposure time can cause irretrievable loss of fish. Pioneering work in this field has been done by MacFarland (1960). Durve (1975) and Durve and Dharma Raja (1974) screened several common anaesthetics for possible use in transport of mullet fingerlings. MacFarland categorised the behavioural responses of fishes to anaesthetica (Table 4.III). He classified 4 definite levels of stages in anaesthesis. Fish for transport should not go beyond his stage 1, where reactivity to external stimuli (visual and tactile) is reduced and there would be a reduction in random activity and depression of metabolism. Stage II is ideal for handling fish for experimental work, injections etc, but in stages beyond which recovery to normality becomes difficult. As already referred to, fish anaesthetized properly can be carried without water, provided the skin and gills are kept moist, and the ambient temperature is cold. Successful lifts have been done with salmonids, but there are no reports on tropical fish transport in this fashion.
Different drugs have been studied for use as anaesthetic for transport of fish. They are sodium amytal, chloral hydrate, tertiary amyl alcohol, methyl paraphenol (Dormison), tricaine methane sulphonate (MS 222 Sandox), urethane, quinaldine, hydroxy quinaldine, novocaine, amobarbital sodium, barbital sodium etc (Table 4.IV).
Table 4.III: Behavioural responses of fishes in relation to different levels of anaesthesia (From MacFarland, 1960)
| Definite levels of anaesthesia | Behavioural response of fish | |
| Stages | ||
| 0 | Normal | Reactive to external stimuli equilibrium and muscle tone normal |
| I (1) | Light sedation | Slight loss in reactivity to external stimuli (visual & tactile) |
| I (2) | Deep sedation | Total loss in reactivity to external stimuli except strong pressure, slight decrease in opercular rate. |
| II (1) | Partial loss of equilibrium | Partial loss of muscle tone, react only to very strong tactile and vibrational stimuli, rheotaxis present, but swimming capacities seriously disrupted, increased opercular rate. |
| (2) | Total loss of equilibrium | Total loss of muscle tone, react only to deep pressure stimuli, decreased opercular rate - below normal. |
| III | Loss of reflex | Total loss of reactivity, respiratory rate very slow, heart rate slow. |
| IV | Medullary collapse | Respiratory movements cease, followed several minutes later by cardiac collapse. |
Table 4.IV: Dosages of drugs used in transport of live fish (tropical (modified from Jhingran, 1975)
| CHEMICAL | RECOMMENDED DOSE | AUTHOR- REMARKS |
| Sodium amytal | 21 – 28 ppm | in ambient water - reduced metabolic rate of carp fry by 30% (Saha et al, 1955) |
| 50 ppm | in water - Sreenivasan, (1962) | |
| 52 – 172 ppm | in water, depending on | |
| (0.2–0.65 g/gallon) | salinity - McFarland (1960) the drug is antagonistic to calcium and can be used only in soft water | |
| Tertiary amyl alcohol | 2 ml/gallon | more desirable than sodium amytal (McFarland, 1960) |
| Methyl paraphenol (Dormison) | 1 – 2 ml/gallon | McFarland, 1960 |
| Chloral hydrate | 3–3.5 g/gallon | McFarland, 1960 |
| Tricaine methane sulphonate (Ms 222 Sandoz) | 50 ppm | in water for Cirrhinus reba and Barbus mahicola (Sreenivasan, 1962) |
| Urethane | 100 ppm | in water for carp fingerling (Sreenivasan, 1962) |
| Thiouracil | 10 ppm | Quinaldine - 5 ppm used for |
| Hydroxy quinaldine | 1 ppm | transport of carp brood or |
| Quinaldine | 5 – 10 ppm | - Natarajan and Ranganathan (1960) |
| Quinaldone | 25 ppm | T. guineensis and S. malanotheron, O. niloticus - tests. (Sado, 1985). |
| Novocaine | 50 mg/kg fish | - injected Ruhu & Mrigal brooders (1–3 kg weight) |
| 30 mg/kg fish | - injected catla (2–3 kg weight) (Kewalramani and Gogate, 1968) | |
| Barbital sodium | 50 mg/kg fish | - injected carp brooders - to bring partial loss of equilibrium for transport |
| Amobarbital sodium | 8 mg/kg fish | - injected carp brooders - to bring partial loss of equilibrium. (Kewalramani & Gogate, 1968) |
Results obtained at ARAC on use of quinaldine as anaesthetic on tilapias based on Sado (1985) are presented in Table 4.V.; see also Idris (1984) and Onyango, (1985).
Chemicals have been used as well for water conditioning in the transport carrier. We have already referred to the use of pulverised red soil in earthern transport vessels for coagulating organic pollutants. Similarly activated charcoal ‘Permutit’ and ‘Amberlit’ resin have been used for removal of carbon dioxide, ammonia and other toxicants from the ambient medium. Various buffers such as Tris-buffer and sodium phosphate (Na2HPO4 at 1.5 mg/l) have been used for controlling pH of water during transport.
Table 4.V: Effect of different concentrations of the anaesthetic quinaldine on three species of tilapia acclimatized and tested at 27–30°C (28.8 ± 1.0°C) and various salinities between 0.25 and 8.9%.o (From Sado, 1985).
| Species | Mean standard lenth (cm) (± S.D) | Acclimation salinity (%0) | Anaesthetic concentration (ppm) | Mean time to loss balance (min) | Mean recovery time (min) | Remarks | Number of fish | Average post-experimental survival time |
| Tilapia guineensis | 4.0±0.0 | 7.8±0.7 | 1 000 | 1.5±0.4 | 45.5±3.5 | Complete collapse | 4 | 0.51 |
| Sarotherodon melanotheron | 4.0±0.3 | 7.8±0.7 | 1 000 | 2.0 | 26.5±0.5 | Complete collapse | 4 | 0.57 |
| T. guineensis | 4.1 | 8.5 | 500 | 8.0 | 40.0 | Complete collapse | 1 | 3.46 |
| S. melanotheron | 4.6 | 7.8±0.7 | 500 | 4.0±2.0 | 31.0 | Complete collapse | 2 | 2.58 |
| S. melanotheron | 10.5±0.5 | 5.4 | 250 | 2.2±0.4 | 34.4±9.4 | Complete collapse | 5 | 3.29 |
| S. melanotheron | 10.0±0.5 | 5.4 | 125 | 2.0 | 13.2±2.04 | Complete collapse | 5 | 4.01 |
| O. niloticus | 6.5±3.2 | 0.25 (Freshwater | 75 | 32.5±30.3 | 9.8±4.8 | Complete collapse | 6 | 5.54 |
| T. guineensis | 7.0±0.2 | 0.25 | 75 | 4.5±0.5 | 11.5±0.5 | Complete collapse | 2 | 5.32 |
| S. melanotheron | 7.1±2.2 | 8.9 | 50 | 29.0±5.0 | - | Partial collapse for over 12h | 5 | 11.42 |
| O. niloticus | 6.2±2.2 | 8.9 | 50 | 14.2±2.4 | - | -do- 12h | 4 | 11.59 |
| S. melanotheron | 5.8±0.7 | 8.9 | 25 | - | - | No collapse | 4 | Fish lived normally |
Commonly used chemicals and their doses are indicated below
| Methylene blue | - | 2 ppm |
| Acriflavin | - | 10 ppm |
| Chloromycetin | - | 8–10 ppm |
| Copper sulphate | - | 0.5 ppm |
| Sodium chloride | - | 3% |
| Potassium permaganate | - | 3 ppm |
A prophylactic bath of fry and fingerlings in the above mentioned chemicals is recommended while handling the fish prior to transport, for prevention and spread of diseases - pathogens and parasites. For additional information text books on fish diseases should be referred to.
Huet (1972) presents tables for water requirements of live fish and loading capacity for various volumes of water under temperate conditions - updated information is also available in Berka (1986), but here again the main concern is temperate fishes - since the ambient temperature in most of these cases are low, below 20C, these tables cannot often be applied to our local tropical conditions. We have referred to certain loading capacities of transport containers with oxygen supply. Eventhough there would be species differences some generalizations can be made. Let us now examine a simple case, say live-fish load, i.e. biomass of tilapia fingerlings of 20 g size, for 50 litres of water near air saturation, but no additional O2 supply (diffusion from air will be negligible), at an ambient temperature of 28 – 30°C, for a transport of 2½ hours. We assume that a 20g tilapia will have an average routine metabolic rate (O2 consumption) of 200 mg/kg per hour or 4 mg per fish per hour, and that the initial O2 concentration is 7 mg/litre and that the fish could safely extract 4 mg O2 from each litre. Therefore the O2 available for fish: 50 × 4 = 200 mg O2 (in 50 litres); O2 requirement of one 20 g Tilapia for 2½ hours: 4 × 2½ = 10 mg.
Total number of Tilapia which can be transported in 50 litres
of water = 
i.e. 200/10 = 20
Indeed, depending on the conditions and assumptions (actual information) available, more or less than 20 fish can be transported. It is well known that tilapia can survive longer if given access to air, as for example, in open containers and also that oxygen consumption varies with the level of excitement and handling. Metabolic rates of tilapias is depressed considerably when MS 222 is added to the medium (Lemu, 1984; Onyango, 1985).
Huet (1972) has described the various receptables used in live fish transport, in the lines explained already. Many of these, such as baskets and boxes (Fig. 483, in Huet, 1972) for dry transport of handy fishes such as tilapias and carps (it is common knowledge that tilapias can survive considerable time out of water - it would be advisable however to have the skin and gills kept moist; O. mossambicus fingerlings can stay alive for 1 to 3 hours out of water); bamboo baskets waterproofed with latex or tar, pairs on sling (suspended at the end of poles carried on shoulders), different types of galvanised cans (Fig. 483 & 485 in Huet, 1972); partly perforated (top) pail for transporting and releasing trout fry in seeming water; oxygen - inflated polythene bags for transporting fish; and polythene bags in card-board (carton) container (Fig. 486 - 488, Huet, 1972); have been well illustrated. Larger containers, mounted on motor vehicles are also illustrated. Large oval-shaped wooden barrels oval shape specially convenient because it would avoid rolling, with inverted pyramid type openings for pulling in fish and also keeping ice, have been in use in Europe, before newer methods were adopted. Wooden, cone-shaped vat equipped (built-in or outside) with an oxygen cylinder and diffuser, has also been used for fish transport. Small tanks mounted on wheel barrows, and also on small rail road tracks serving large fish farms in Europe have been in use -these may be provided with an oxygen cylinder especially if delicate fishes such as the trouts are to be transported. Three foot-square aluminium tanks with net suspended inside so as to avoid injury to fish by cutting on the sides have been used for open transport of fish. Motor lorries with tanks along with oxygen containers and diffussion apparatus, as referred to earlier, are also in vogue in several parts of the world (see Figs. 492, 493 and 496 in Huet, 1972).
Updated information is available in a recent EIFAC publication, Berka (1986). Techniques and equipment described herein are presented graphically in Figs. 4.1 – 4.10, referred to earlier.
We have attempted to present herein only the more important information on live fish transport. It is obvious that there is need for more information on the biology of the species and their interaction with the environmental factors (both physical and chemical). These have been dealt with in chapters 8 & 9, “Site Selection for Aquaculture” and also in handouts, on “Species Selection for Aquaculture.”
Alikunhi, K. H. 1957. Cited in Jhingran, 1975.
Bell, G. R. 1964. A guide to the properties, characteristics and uses of some general anaesthetics for fish. Fisheries Res. Board of Can. Bull. 148 : 3 pp.
Berka, R. 1986. The transport live fish. A review. EIFAC Tech. Pap., 48 : 52 pp.
Durve, V. S. 1975. Anaesthetics in the transport of mullet seed. Aquaculture 5: 53–63.
Durve, V. S., A. K. Dharma Raja., 1974. Effects of anaesthetic on the behaviour of mullet fingerlings and the scope of using these in different fishery procedures - I.J.Mar. Biol. Assoc. India, 84 : 28 –85.
Fry, F. E. J. 1951. A splashless tank for transporting fish. Canadian Fish culturist, 10: 1–2.
Gupta, M. V. and B. K. Sharma. 1974. A note on the transport of chinese carp breeders, using MS 222. J. Inland Fish. Soc. India, 6 : 99–100.
Hora, S. L. and T. V. R. Pillay, 1962. Handbook of fish culture in the Indo-Pacific Region. FAO Fish. Tech. Pap. 14. 204 pp.
Huet, M. 1972. Textbook of fish culture. Fishing News (Books) Ltd, Farnham, Surrey, V.K. 436 pp.
Jagannathan, 1947. Cited in Jhingran, 1975.
Jhingran, V. G. 1975. Fish and fisheries of India. Hindustan Publishing Corporation (India), Delhi.
Kewalramant, H. G. and M. G. Grogate. 1968. Anaesthetisation in fish - Tilapia and major carp. Proc. Indian Acad. Sci. Sect. B. 67 (5): 145 – 147.
Khan, 1946. Cited in Jhingran, 1975.
Lemu, I. A. 1983. Influence of anaesthetic quinaldine and tricaine methane sulphona te (MS 222) on Sarotherodon niloticus. M. Tech. (Aquaculture) thesis. African Regional Aquaculture Centre/Rivers State University of Science and Technology, Port Harcourt, Nigeria. 40 pp.
McFarland, W.N. 1960. The use of anaesthetics for the handling and the transport of fishes. Calif. Fish. Giaire, 46 (4): 407–432.
Natarajan, W. N. and V. Ranganathan. 1960. A note on the possibilities of using quinaldine in transporting live fish. Curr. Sci. 29: 393.
Onyango, D. A. 1985. Inflence of anaesthetic quinaldine on metabolism and survival of Oreochromis niloticus (Linnaeus, 1757). Project report , P. G. Diploma (Aquaculture). African Regional Aquaculture Centre, Port Harcourt, Nigeria. 63 pp.
Saha, G. N. et al, 1955, cited in Jhingran, 1975.
Saha, G. N. and Chaudhury, 1956. Cited in Jhingran, 1975.
Sado, E. K. 1985. Influence of the anaesthetic quinaldine on some tilapia. Aquaculture, 46: 55 – 62.
Srivatsava and Karamahandani, 1964. Cited in Jhingran, 1975.
Patro, 1968. Cited in Jhingran, 1975.
Woynarowich, E. and L. Horvath. 1980. The artificial propagation of warm water finfishes - a manual for extension. FAO Fish. Tech. Pap., 201 : 147 pp. Issued also in French and Spanish.
