
| NACA/WP/86/34 | November 1986 |
 |
Histopathology of Microsporidia Infection in Indian Major Carp, Labeo Rohita (Hamilton) |
Central Institute of Freshwater Aquaculture
Dhauli, Kausalyagang, Bhubaneswar
NETWORK OF AQUACULTURE CENTRES IN ASIA
BANGKOK, THAILAND
Hyperlinks to non-FAO Internet sites do not imply any official endorsement of or responsibility for the opinions, ideas, data or products presented at these locations, or guarantee the validity of the information provided. The sole purpose of links to non-FAO sites is to indicate further information available on related topics.
This electronic document has been scanned using optical character recognition (OCR) software. FAO declines all responsibility for any discrepancies that may exist between the present document and its original printed version.
R. K. DEY and DILIP KUMAR
Unit for Ichthyopathology and Fish Health Protection,
Freshwater Aquaculture Research and Training Centre of Central
Inland Fisheries Research Institute,
Kausalyagang, Via Bhubaneswar,
Orissa, 751002. (INDIA)
Extensive renal lesions caused by microsporidian infection were recorded for the first time in the kidneys of Indian major carp, Labeo rohita (Hamilton). Intracellular developmental stages of the parasite caused displacement of the host cell nuclei and ultimately destroyed the renal epithelial cells releasing the parasite into the tubular lumen. In extreme cases, the tubular lumen was found to be completely filled by the microsporidian parasite and there was severe degeneration and necrosis of the renal tubules. Proliferation of haematopoetic tissue in between the renal tubules was observed. The release of the microsporidian parasite into the renal interstitium provoked granulomatus inflammatory reactions.
Intensification of carp culture practices requiring high density
stocking, supplementary feeding and fertilization (along with
facilities for aeration and other technological interventions) in
ponds and tanks have brought in important
The present observations describe histopathological studies on a case of microsporidian infection in Indian major carp, Labeo rohita (Ham.), which probably forms the first report on the subject from India. The parasite was identified as Microsporidia sp. on the basis of its spore characters and the intracellular developmental stages found in the didneys of the infected carps both in squash preparations and histological sections.
During the routinge sampling programme on health monitoring at the FARTC ponds as well as ponds under its ‘Lab to Land’ programme, a case of microsporidia infection in Indian major carp, Labeo rohita (Ham.) was recorded in a village pond. The infected specimens of carp were brought to the laboratory in live condition and thorough pathoanatomical and histopathological observations were made. Macroscopical inspection of the infected specimens including examination of the fins, skin, mucous and blood for ectoparasitic forms was followed by examination of fresh tissues of liver, kidney, gill, heart, brain and intestine under squash preparations. Tissue samples from the internal organs were also fixed in Bouin's fluid for histopathological studies. Paraffin embedded sections were cut by a Rotary microtome and stained by haematoxylin and eosin (H & E). The histological preparations were examined under light microscope and photomicrographs of the stained preparations were made. Correlations were made between the squash preparations of kidneys and the stained microtomic sections of the same for the presence of microsporidian parasites. This corelation is needed especially in the case of myxozoan parasites inhabiting the renal tubules (Lome and Dykova, 1981).
About 70 percent of L. rohita fingerlings (105 mm/30 gm) stocked alone in a pond (0.1 ha) during January, 1982 showed the clinical tail rot symptoms when sampled. The infected carps, when brought in live condition to the laboratory, were all highly emaciated and a number of haemorrhagic lesions were seen on their body surface. Dorsal lobe of the tail fin was found to be lost in all the specimens while all other fins were intact. No ectoparasites or parasitic cysts were found in any part of the body. However, when squash preparations of the internal organs were prepared and examined, the kidneys of the infected specimens showed the presence of a granular material in the tubular epithelium and in some cases in the lumen of the tubules of trunk kidney.
These observations were also confirmed by histological examinations which showed that the parasites localized themselves within the tubular epithelial cells showing intracellular developmental stages of the parasite (Fig. 1). Most of the tubules were invaded by the parasite and the degree of renal lesions depended on the stages of intracellular development of the spores. The earliest histological changes due to the microsporidian infection were found in the renal tubularepithelium where the parasite started developing intracellularly as red stained masses under H & E stain which increased in size gradually and contained the microsporidian spores under various stages of development.
The intracellular developmental stages of microsporidia caused displacement of the nuclei of the host cells gradually rendering their cytoplasm to a thin layer around the parasites. Ultimately, the infected epithelial cells were seen to be destroyed releasing the parasite into the tubular lumens. In extreme cases, the tubular lumen was found to be completely filled by the microsporidian parasite causing severe degeneration and necrosis of the renal tubules (Fig. 2). Hyaline bodies were also seen in some tubular cells as well as in the interstitial tissues. Some renal tubules were observed to be partially degenerated and necrosed containing granular detritus materials inside the tubular lumen. Gromerulii were also affected showing oedema and degeneration of capillary walls. There was proliferation of haematoO poetic tissue in between the tubules and depletion of mature and submature blood cells from the haematopoetic tissues. In places where the intracellular development of the parasites caused rupture of the basement membranc of the host cells, the parasites got released into the renal interstilium thereby provoking a granulomatus inflammatory response.
The present findings on the histopathology of a case of micro-sporidian infection in Indian major carp, L. rohita have indicated distinct pathological lesions in the kidneys resulting in diseased condition of the fish and the problems created by the microsporidian group of myxozoan parasites in carp culture practice to pathological changes caused by this microsporidian parasite in the present observations were quite characteristic and worth recording. Pathological changes due to renal sphaerosprosis and mitrasporosis in goldfish were mentioned by Ahmed (1973) and Hoffman and Mitchel (1980). Lom and Dykova (1981) opined that renal lesions associated with Mitraspora cyprini and Sphaerospora sp. differed in details only, most of the changes being common to both. Histological changes varied with the stages of development of the parasite and with the intensity of infection. The earliest histological alterations in Mitraspora and Sphaerospora infections were found in the tubular epithelium where both the species developed intracellularly. Our observations on the histopathology of a microsporidian infection in L. rohita appear to corrobcirate the findings of Lam and Dykova (1981). Detailed investigations on the taxonomy and biology of microsporidian parasites in cultivated carps are in progress in our laboratory. However, the present information on microsporidian infection in L. rohita is a useful addition to the existing knowledge on the extremely small number of myxozoans with known intracellular developmental stages in their life cycles.
The pathogenicity of the microsporidian parasite involved in the present case could be precisely assessed due to the infliction of direct damages to the renal epithelium during its course of intracellular development. The release of the parasites into the tubular lumen during the last phase of development probably brought about mechanical damage affecting the normal function of the kidneys of the infected carps. However, the external clinical symptoms involving haemorrhages, fin erosion etc. may be attributed to secondary invasion by some pathogenic microbes.
The authors express their deep sense of gratitude to Dr. A. V. Natarajan, Director, Central Inland Fisheries Research Institute and to Dr. V. R. P. Sinha, National Project Director, FAO/UNDP Projects and Head, Freshwater Aquaculture Research and Training Centre for their keen interest in the work and encouragements.
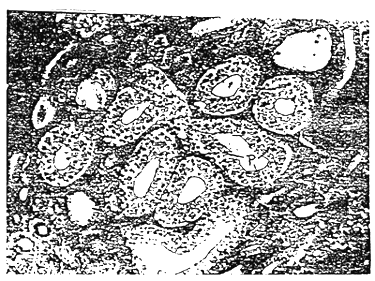
Fig. 1. Photomicrograph of a section of the kidney of Microsporidia infected Labeo rohita (Ham.) showing intracellular developmental stages of the parasite (P) (H & E stain × 120).

Fig. 2. Photomicrograph of a section of the kidney of Microsporidia infected Labeo rohita (Ham.) showing accumulation of the parasite (P) within the tubular lumen and degeneration and necrosis of the renal tubules (H & E stain × 312).
Ahmed, A. T. A., 1973. Morphology and life history of Mitraspora cyprini Fukita, parasitic in the kidneys of gold fish. Japanese Journal of Medical Science and Biology, 26: 87–101.
Burza, L., Hamory, G., Sziklai, F., 1971. Sphaerosphora - a new parasite of the gill in carp fingerlings. [in Hungarian] Halaszat, 17: 152.
Fujita, T., 1912. Notes on new sporozoan parasites of fishes. Zoologischer Anzeiger, 39: 259–262.
Hoffman, G. L., Mitchel, A. J., 1980. Exotic Mitraspora cyprini gold fish now in the U. S. Proceedings of the 1980 Meeting of the American Society of Parasitologists, San Francisco, A 223, P. 82
Leger, M. L., 1930. Sphaerospora pernicialis n. sp., Nouvelle Myxosporidie pathogene pour la Tanche. Comptes Rendus Hebdomadaires des Seances de l'Academic des Sciences, Paris, 190: 849–851.
Lom, J., Dykova, I., 1981. Pathogencity of some protozoan parasites of cyprinid fishes. In. Proceedings of an international Seminar on Fish, Pathogen and Environment in European Polyculture, Szarvas, Hungary, p. 146 – 169.
Molnar, K., 1979. Gill Sphaerosporosis in the common carp and grass carp. Acta Veterinaria Academiae Scientiarium Hungaricae, 27 : 99–113.
Molnar, K., 1980. Renal Sphaerosporosis in the common carp Cyprinus carpio L. Journal of Fish Diseases, 3: 11 – 19.
Narasimhamurti, C. C., Kalavati, C., 1972. Two new species of microsporidian parasites from a marine fish Saurida tumbil. Proceedings of the Indian Acad. Sciences, Vol. IXXVI (4) : 165–170.
Putz, R. E., Hoffman, G. L., Dunbar, C. E., 1965. Two new species of Plistophora (Microsporidia) from North American fish with a synopsis of microsporidia of fresh water and eryhaline fishes. J. Protozool., 12 (2) : 228–236.
Sprague, V., Vernicko S. H., 1968. Observations on the spores of Pleistophora gigantea (Thelohan, 1895) Swellengrebel, 1911, a microsporidian parasite of the fish Crenilabrus melops. J. Protozool., 15 (4) : 662–665.
Summerfelt, R. C., Warner, M. C., 1970. Incidence and intensity of infection of Plistophora ovarie, a microsporidian parasite of the golden Shiner, Notemigonus crysoleucas. In. A Symposium on Diseases of Fishes and Shellfishes (ed. by S. F. Snieszko), pp. 143–160. American Fisheries Society Special publication No. 5 Washington D. C.
