by
Anand Tunsutapanich 2
1. INTRODUCTION
Techniques for culturing aquatic animals in freshwater, brackishwater and salt water have been improving rapidly. The feed for aquatic animals such as fish, shrimp, crab, etc. during their larval stages is therefore of vital importance. Up to now, the feeds used are planktonic plants and animals, e.g., diatoms, rotifiers, copepods, etc. Which are reared in the hatchery or collected from natural breeding grounds. These practices are greatly handicapped by difficulties in obtaining sufficient quantities of these foods. Artemia salina is one of the most important feeds. It has to be imported in large quantities from abroad. It is imported as dry Artemia cysts preserved in vacuum packed tins in such a way that they will last for years. Subsequently, these cysts can be hatched whenever deemed necessary in the required quantity. However, the cost of imported cysts is extremely high.
An experimental, research, training and development project for freshwater prawn culture at the Chacheongsao Fisheries Station has been in operation for over 3 years. This project requires an enormous quantity of Artemia for its work. The Station has developed methods of utilizing Artemia to the fullest by conducting research in techniques for its preservation and establishing technical procedures for a new local Artemia rearing industry to replace the necessity of importing cysts.
This report will deal briefly with the value of Artemia production and includes the techniques used and results of experiments on cyst production in Thailand salt ponds.
2. OBJECTIVES
Our experimental work was designed to:-
find ways and means of establishing a rearing industry for Artemia in Thailand for local use;
utilize existing salt ponds to the fullest in order to upgrade the standard of living of the salt farmers;
gain experience in the culture of Artemia.
1 Translation of a report to the Department of Fisheries, Thailand
2 Fisheries Biologist, Chacheongsao Fisheries Station, Bangpakong,
Chacheongsao, Thailand.
3. LITERATURE REVIEW
General biology and characteristics of Artemia
Artemia are commonly called brine shrimp. The scientific name is Artemia salina. They are grouped in the Phylum Arthropoda, Class Crustacean, Sub-Class Branchiopoda, Order Anostraca, Family Artimidae. Bagatova, et. al. (1979) reports that newly hatched larvae measure about 0.45 mm in length, 0.17 mm in width and 0.01 mg in weight. Sorgelcos (1977) states that female Artemia have to undergo 15 moults before reaching maturity. The thorax and the body are long and narrow. Artemia are filter feeders. When searching for food the filaments of the antennae and antennules sweep about actively and when a piece of food is found it is picked up and brought to the mouth by the swimmerets.
The functions of thoracic legs are varied as follows:-
Telepodites - responsible for filtration of food
Endopodites - responsible for movement
Exopodites - the membranous parts of which is responsible
for respiration
Following the tenth moult a modification which helps in distinguishing the difference between male and female Artemia takes place. The second pair of antennae of males are in the form of hooks and expand in size. These hooks have a contribution to play in copulation. In the female Artemia, the second pair of antennae will reduce in size. These organs also function as feelers.
Fully grown Artemia measure about 0.8 – 1 cm in length, depending on the strain. Artemia are stalk - eyed. The antennae consist of sensory nerves. The gastro intestinal tract runs parallel to the body.
General distribution
Sorgeloos et. at. (1975) listed various natural habitats from a total of more than 40 places in all areas of the world (Table 1). Additionally, Bagotova et. al. (1979) states that Artemia is widely distributed in the area of Ghenicheskoyo Lake which has a surface of 1,000 hectares and is situated in the Herson area of the USSR.
Feeding habits and food characteristics
Masters (1975) and Sorgeloos (1977) report that Artemia is a filter feeder. Therefore it is omnivorous in food habit. Its food consists for example of yeast, diatoms, algae, bacteria and other micro-organisms.
Claus et. al. (in press) fed (Spirulina sp. and Scenedesmus sp. to Artemia with satisfactory results. Oleinikiva et. al. (1979) gave a list of various types of plankton being utilized as food of Artemia as follows:-
Green algae for example: Scenedesmus quadricanda, Ankistrodesmus pseudomicrobiles, A. arwatus, Crucigenia cuadrata, Lambertia ocellata, Occystis spp., Phacotus spp., Cladophora spp., Dunaliella salina, Asteromonas granus.
Diatoms for example: Nitzchia closterium, N. sigma, N. tenuirostris, Thalassionema nitzchoides, Amphora spp., Navicula spp., Blue-green algae, Oscillatoria spp., Euglena, Trachelomonas spp.
Helfrich et. al (1973) reported that Artemia was a filter feeder and that its food was composed of particulate substances for example, single cell phytoplankton, yeasts and bacteria. From natural habitats where Artemia is found and the water is of high salinity, only a few types of algae are found, for example, Dunaliella salina, D. viridens, Stephanoptera gracites, S. oracillus, Platymonas spp., (Giber 1975 Provosalietal, 1959; Carpelan, 1964), but D. salina or S. gracilis are the most abundant. A large quantity of D. salina were found in Christmas Island where the water is extremely salty. The temperature ranged between 24°C - 30°C.
Wit and Pravit (1972) reported that the feed of Artemia should consist of yeast because it was most efficient. It was most conveniently fed by mixing it in freshwater first, prior to mixing with the hatching media. They also stated that yeast should be added daily or every two days. Artemia was able to live well at a temperature of 30°C. Artemia survived within the range of 10 – 37°C. Filter feeders obtain their food by filtering organic matter or minute organisms from a current of water passing through some part of their system.
Differences in the characteristics of male and female Artemia and in mating procedure
According to Sorgeloos (1977) the hooked graspers of the male Artemia change into an organ that contributes to copulation and can be expanded since they are muscular. Their interior parts are provided with tufts of long setae which consist of sensory nerves. The penis is located at the posterior of the thorax. The hooked graspers of female Artemia are smaller than those of the male and consist of sensory nerves. There is a pair of ovaries located parallel of the digestive tract near the posterior of the thorax. The fully developed and ripe ovaries will be extruded through the two oviducts to a pair of brood pouches or uterus.
In the precopulation of Artemia, the male begins mating by holding between its hooked graspers the uterus area and the posterior area of the thorax of the female. In doing this, the couple may swim around together for a long time. The copulation of Artemia is performed rapidly. The male which is in an embracing position bends its abdomen forward to reach the ventral thoracic region of the female and then inserts its penis into the open entry of the uterus. The fertilized eggs may then grow to the gastrula stage. Later on the shell may become a cyst which subsequently leads to the diapause and the eggs will continuously develop until they become nauplii. This is called viviparous reproduction. Whether females will reproduce oviparously or viviparously can be determined by the colour of the eggs in the uterus. It the colour is white, nauplii will be produced if the colour is brown, cysts will be produced.
Helfrich, et. al. (1975) stated in his experimental summary that it took a week for Artemia to reach maturity. If fed with yeast mixed with vitamins (Teramoto, et. al. 1961), Artemia reached sexual maturity within two weeks as also found by Bowen, (1965) and Bowen, et. al. (1966). According to Gilchrist (1960) Artemia will reproduce at all times and are able to produce eggs or nauplii at the rate of 200 very 5 days or more often. The temperature of water most appropriate for Artemia to live in is within the range of 9 – 35°C and the water should have a salinity range from 5 – 150 ppt. (Webber, 1969).
Sorgeloos (in press) refers to the report of Moreis and Arfzelvze, (1967) and Bruggeman, et. al. (1967) that the chorion of artemia was hard and had a dark brown colour. The chorion is a compound of lipoprotein and haemotin or haemoglobin. The chorion is divided into two sub-layers the critical layer and alveolar layer. In the alveolar layer there are a number of cavities which enable the Artemia eggs to float.
Benefits and nutrient value of Artemia
Helfrich, et. al. (1975) stated that in salt lakes under good management, the yield of Artemia would be high. For example, the 284 ha (700 acres approximately 1 750 rais) surface area of the salt ponds near San Francisco Bay in 1967 yielded 3 000 gallons of Artemia eggs within a 4 - month period. At present, Artemia are used for feeding larvae of species such as crab, shrimp, fish, etc. Artemia eggs are normally used in feeding larvae in United States Europe, Japan and other countries where prawn culture is practiced. The demand for Artemia has therefore increased and also its price, since the supply has not been enough to meet the demand of the world market. It had been estimated that the United States Europe and Japan alone require approximately 14 000 gallons per year of Artemia and in future the demand will increase to 50 000 gallons per year or more.
Artemia eggs are very high in nutrient value they contain 52% protein and 27% fat. Two hour old nauplii contain 50% protein, 16% fat (Brick, unpublished). Bagalava et. al. (1979) reported that newly hatched nauplii contains 50% of protein and 20% of fat. One day old nauplii contain 50% protein and 27% of fat (Cohen, unpublished). Six day old Artemia or 10 days old contain 63% of protein and 6.5% of fat (Brick, unpublished).
Artemia can be used as human food. Delya et. al. (1960) reported that in Libya Artemia is used as human food.
Helfrich et. al. (1975) and Sorgeloos (1978) reported that when Artemia eggs are suspended in brine with continuous aeration, detritus and other insoluble substances sink to the bottom of the container. On the contrary if Artemia eggs were suspended in freshwater, they themselves will sink to the bottom while the insoluble substances, egg shells, etc. will float to the surface. Taking into account the ability of Artemia eggs to float in salt water and sink in freshwater, a technique was established to separate Artemia eggs from other substances. After Artemia eggs are washed and cleaned, they are preserved in brine or dehydrated to 10% moisture. After this, Artemia eggs can be preserved in vacuum packs or under Nitrogen.
Earlier Artemia work at Chacheongsao Fisheries Station
The Chacheongsao Fisheries Station has experimented with the culture of Artemia since January 1978. These experiments and their benefits to Artemia culture can be summarised as follows:
Artemia can be fed directly with the following foods: chicken feed, rice bran, minced fish, chopped chicken dung. Artemia growth and development progresses at an excellent rate. In addition, when these foods become rotten, they turn into fertilizers which can serve to produce natural food for Artemia such as bacteria, yeast, algae and different kinds of micro organismas.
Giant freshwater prawn larvae can easily eat fully grown Artemia (measuring approximately 1 cm in length).
Artemia decapsulation has been improved by substituting the use of Sodium hypochlorite and Sodium hydroxide by Ca (OC1) 2 and CaO. This, and some modifications of technique at certain stages has reduced the quantity of chemicals required and thus reduced the cost of decapsulation from 300 baht to 18 baht per 1kg of Artemia eggs (Anand, 1979).*
There are two patterns of reproduction in Artemia: viviparous and oviparous. If Artemia are reared in low salinity most of them will produce nauplii while only a small number will lay eggs. Artemia reared at high salinity levels will mostly lay eggs and only a few of them will produce nauplii.
Stocking density has a special impact on reproduction mode: if the stocking density is small inspite of high salinity, the proportion of Artemia that produce nauplii will be greater than that which produce eggs.
4. EXPERIMENTAL PROCEDURE USED FOR CYST PRODUCTION IN SALT PONDS
Equipment required
50g of Artemia cysts: 50% San Francisco and 50% Aqua-Fauna (Brazilian strain)
One earthen pond 70 × 35 m in area (0.25 ha)
Analytical equipment for DO2 %, PH and temperature measurement
Equipment for collection and cleaning Artemia eggs
Chicken dung
Equipment for dehydration of Artemia eggs
Miscellaneous equipment.
5. TECHNIQUE
Preparation of pond
A pond located in the mangrove zone at a salt farm at Tambon Klong Tamru Amphur Muang, Cholburi Province, was chosen. The size of the pond was 70 × 35 m or approximately 1.5 rai (0.25 ha). The bund height was increased above the original level by 50 cm. The perimeter of the pond was dug out to form a furrow measuring 20 cm in depth and 4 m in width. In the pond there were a number of mangrove stumps. After the pond was well prepared, salt water with a salinity of 70 ppt was pumped up from other salt ponds until the level of water at the middle of the Artemia pond was 30 cm deep. The quality of the water in the pond was determined before Artemia cysts were stocked. A new supply of water was pumped in once a week to maintain the level of water in the pond.
Preparation of Artemia eggs
Two strains of Artemia eggs were stocked simultaneously (the San Francisco Bay strain which is widely used in aquaculture and harvested from a natural population and the Aqua-Fauna strain from Brazil which has been produced by inoculation in a country situated in a tropical zone similar to Thailand). Only one trial pond was available for the experiment so Artemia of both strains were mixed together a) to save time in evaluating which strain was the most suitable for Thailand and b) to allow copulation between strains with the hope of creating a new strain which might be more suitable for rearing in Thailand.
Five hundred grams of mixed Artemia eggs were put into a plastic container. Twenty-five litres of water with a salinity of 30 ppt were added and full aeration supplied. Incubation took 2 days according to the normal Chacheongsao Fisheries Station technique. The nauplii were put into plastic bag containing oxygen for transport and then stocked into the rearing pond.
Utilization of fertilizers
When the pond was stocked, 200 kg of chicken dung was thoroughly distributed in the pond (800 kg/ha). Subsequently, 100 kg (400 kg/ha) was added to the pond at monthly intervals.
Collection of Artemia eggs
The Artemia cysts produced in the pond floated to the surface and were blown by the wind to the corners of the pond. They could then be caught with a handnet or collected in a can and subsequently transferred to a plankton bag.
Cleaning and preservation of Artemia eggs
The Artemia eggs were collected, put on a sieve and washed with several changes of fresh water to remove soluble matters these eggs were then transferred to a container filled with water of 250 – 300 ppt. Aeration was provided continuously from a tube placed so that it discharged at a level not lower than half of the water height in the container. The Artemia eggs floated to the surface while other solid matter such as earth, sediments, and other particles sank to the bottom of the container. The residue was thrown away and the Artemia eggs exposed to the sun to dry them to a 10% moisture level.
These dry Artemia eggs can be hatched immediately or preserved in vacuum packs or under nitrogen which will maintain their quality for several years.
Evaluation of hatching efficiency of pond reared cysts
Two-hundred fifty (250) mg of dry Artemia eggs were placed in a beaker. Eighty (80) cc of water with a salinity of 30 ppt was added. Aeration was provided for 1 hour. More water was added to produce a total value of 100 ml. An automatic pipette was used to take 0.25 ml samples of the mixture, the amount of which were placed in a 5 ml plastic test tube. The volume was made up to 4 ml with water. The tubes were closed and inserted into a centrifuge and spun at 5 RPM. Samples were tested in this way after 24 hours, 36 hours and 48 hours. The number of nauplii and eggs was then counted. An average was taken on each occasion to calculate the hatching efficiency.
Experimental results
Nine days after stocking the Artemia nauplii into the rearing pond (18 May 1979) copulation had taken place. After 15 days the Artemia had produced nauplii and cysts. Reproduction of Artemia in the pond then increased rapidly (Table 2), but the quantity of cysts was too small for collection until 45 days after inoculation (25 June 1979). From then on eggs were collected every 2 – 4 days. The yield during the 45 days from 25 June to 10 August 1979 (3 months after inoculation) was 20.31 kg wet weight (Table 3).
The hatching efficiency of eggs collected from the ponds and dried was very good. Average Hatching Rates of 91.2%, 97.7% and 98.2% were obtained after 24, 36 and 48 hours respectively (Table 4).
The water temperature in the trial pond varied from 27 – 47.5°C during the experiment while the salinity ranged from 70 – 145 ppt. The level of dissolved oxygen in the water ranged between 4 – 6 ppm. and the pH was between 7.8 and 8.2 (Table 2).
6. CONCLUSION AND DISCUSSION
This experiment has shown that it is possible to rear Artemia cysts in Thai salt ponds. The harvest of Artemia cysts was excellent, with 20.31 kg (10.14 kg dry weight) being produced in a 45 day period. This initial work will lead to finding ways and means to increase and production of Artemia eggs in the future.
The experiment was conducted partly in the summer and partly in the rainy season. The latter had an effect on salinity. It is therefore more advisable that future experiments be started in the winter season so that the harvesting period can be longer.
In subsequent small scale experiments, salt water obtained from the salty soils found in the north-east of Thailand, were there are underground salt deposits, has been used for Artemia rearing. The results were satisfactory. Therefore there is a possibility that Artemia rearing could be used to increase the income, not only of salt pond farmers, but also for people in the north-east of Thailand who own underground salt deposit land. All the fresh water resources which have become highly saline because of the development of salt ponds from underground salt deposits at Botabue, Mahasarakram Province, for instance, could also be utilized for Artemia rearing.
7. ACKNOWLEDGEMENTS
The author wishes to express his gratitude to Mr. Somsuk Singholka, Chief of the Chacheongsao fisheries Station, for his support, his contribution of valuable advice and his assistance in solving problems for this project experiment to Mr. Vidhaya Charoenphol for his close cooperation and participation in convincing the salt pond farmers about the objective of the experiment and for persuading them to lend the site used in the experiment also to Khun Ruangpoeng and Khun Puud Suknoi, the owners of the salt pond.
The author also wishes to express his gratitude to all those government officials and officers in the Chacheongsao Fisheries Station and those in the Freshwater Fisheries Division, as well as friends, who contributed valuable advice and assistance either directly or indirectly in making the smooth and rapid progress of this work possible.
Table 1: List of natural habitats of Artemia
| Continent | Country | Natural habitat |
| Africa | Algeria | Two un-named areas |
| Kenya | Filmenteita | |
| Tunisia | Tunis | |
| America | Argentina | Lapampe |
| Ituzaingo | ||
| Brasil | Cabo Firo | |
| Salinas Perynas | ||
| Canada | Chaplip Lake | |
| Little Manitou Lake | ||
| Mexico | Lake Mawe | |
| Yavares, Sonora | ||
| Peru | Callao | |
| Chilca, Lima | ||
| Puerto Rico | Boyueron | |
| U.S.A. | San Francisco Bay | |
| Great Salt Lake | ||
| Mono Lake | ||
| Moss Landing | ||
| Soap Lake | ||
| Los Angeles area | ||
| Venezuela | Gulf of Curacao |
Table 2: Salinity, pH, DO2 and Artemia density in the experimental cyst rearing pond.
| Date | Salinity (ppt) | pH | Average quantity of Artemia/litre | DO2 (ppm) |
| 9 May | 70 | 8.2 | - | - |
| 10 May | 70 | 8.2 | 2 | 6 |
| 16 May | 81 | 8.0 | - | 6 |
| 18 May | 83 | 8.1 | - | 6 |
| 25 May | 90 | 7.9 | 10.1 | 5 |
| 1 June | 110 | 7.9 | 135.5 | 5 |
| 8 June | 120 | 8.0 | 160.1 | 5 |
| 16 June | 140 | 7.9 | 240.3 | 4 |
| 25 June | 145 | 7.9 | 270.4 | 4 |
| 29 June | 135 | 7.8 | 317.1 | 4 |
| 6 July | 130 | 7.8 | 334.0 | 4 |
| 13 July | 130 | 7.8 | 382.4 | 4 |
| 22 July | 120 | 7.8 | 379.0 | 4 |
| 27 July | 100 | 7.8 | 358.7 | 4 |
| 3 August | 100 | 7.9 | 397.5 | 4 |
| 10 August | 90 | 7.8 | 365.7 | 4 |
| Averages | 107.12 | 7.92 | 4.6 |
Table 3: Artemia cyst harvest weights
| Date | Weight of Artemia | |
| Wet wt. (g) | Dry wt. (g) | |
| 25 June | 1,400 | 700 |
| 29 June | 700 | 345 |
| 2 July | 1,350 | 700 |
| 4 July | 600 | 300 |
| 6 July | 800 | 410 |
| 9 July | 1,400 | 700 |
| 11 July | 980 | 480 |
| 16 July | 2,100 | 1,055 |
| 18 July | 1,900 | 980 |
| 21 July | 1,430 | 735 |
| 24 July | 980 | 450 |
| 27 July | 800 | 400 |
| 30 July | 870 | 430 |
| 3 August | 1,450 | 710 |
| 7 August | 2,050 | 1,015 |
| 10 August | 1,500 | 735 |
| TOTAL | 20,310 | 10.145 |
| Average | 269,37 | 634,06 |
Table 4: Hatching efficiency of harvested cysts
| Duration of hatching (24 hours) | Duration of hatching (36 hours) | Duration of hatching (48 hours) | ||||
| Number of | Number of | Number of | ||||
| Nauplii | Cysts | Nauplii | Cysts | Nauplii | Cysts | |
| 1. | 210 | 234 | 236 | 240 | 256 | 260 |
| 2. | 221 | 238 | 257 | 260 | 240 | 245 |
| 3. | 237 | 265 | 239 | 240 | 219 | 223 |
| 4. | 233 | 253 | 225 | 232 | 227 | 231 |
| 5. | 215 | 234 | 222 | 234 | 231 | 235 |
| Total | 1,116 | 1,224 | 1,179 | 1,206 | 1,173 | 1,194 |
| Average | 223.2 | 244.8 | 235.8 | 241.2 | 234.6 | 238.8 |
| Number of nauplii from 1g eggs: | 233.2 × 4 × 100 × 4 = 357.120 | 235.8 × 4 × 100 × 4 = 337.280 | 234.6 × 4 × 100% 4 = 375.360 | |||
| Wt. of eggs giving 1 million Artemia nauplii: | 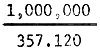 = 2.8 gram | 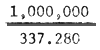 = 2.65 gram | 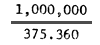 = 2.66 gram | |||
| Hatching % | 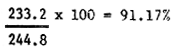 | 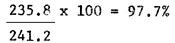 | 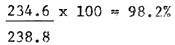 | |||
27 – 28 June 1979.
* Weigh 250 mg of Artemia cysts/100 cc of water, salinity 30 ppt; take 250 ml sample (ph 8.2)
Table 5: Temperature of water in trial pond (°C) during the months of May - August 1979
| Date (1979) | Temperature | ||
| Minimum (°C) | Maximum (°C) | Average (°C) | |
| 9 May | 27.5 | 37.0 | 32.25 |
| 10 May | 27.0 | 37.0 | 33.25 |
| 16 May | 28.0 | 40.5 | 34.25 |
| 18 May | 27.0 | 37.5 | 32.25 |
| 25 May | 28.0 | 37.5 | 32.75 |
| 1 June | 29.0 | 38.5 | 33.75 |
| 8 June | 28.5 | 39.5 | 34.00 |
| 16 June | 30.0 | 41.0 | 35.50 |
| 25 June | 29.0 | 41.5 | 35.25 |
| 29 June | 29.5 | 40.0 | 34.75 |
| 6 July | 27.0 | 39.0 | 33.00 |
| 13 July | 29.0 | 38.0 | 33.50 |
| 22 July | 30.5 | 38.5 | 34.50 |
| 27 July | 30.0 | 40.0 | 35.00 |
| 3 July | 29.5 | 37.0 | 33.25 |
| 10 July | 28.0 | 32.5 | 30.25 |
| Average | 28.6 | 38.6 | 33.6 |