
A Prototype Warm Water Shrimp Hatchery
| NACA/WP/85/18 | September 1985 |
 |
Technology Series No.2 A Prototype Warm Water Shrimp Hatchery |
by
P. Kungvankij1, L.B. Tiro, Jr.,2 B.J. Pudadera, Jr.,2
E. Borlongan2, E.T. Tech2, T.E. Chua1
Regional Lead Centre in the Philippines
Aquaculture Department, Southeast Asian Fisheries Development Center
1 Food and Agriculture Organization of the United Nations
2 Aquaculture Department, Southeast Asian Fisheries Development Center
NETWORK OF AQUACULTURE CENTRES IN ASIA
Bangkok, Thailand
September 1985
Hyperlinks to non-FAO Internet sites do not imply any official endorsement of or responsibility for the opinions, ideas, data or products presented at these locations, or guarantee the validity of the information provided. The sole purpose of links to non-FAO sites is to indicate further information available on related topics.
This electronic document has been scanned using optical character recognition (OCR) software. FAO declines all responsibility for any discrepancies that may exist between the present document and its original printed version.
The completion of the life cycle of many commercially important warm water shrimps in captivity, such as the tiger shrimp (Penaeus monodon) and the white and banana shrimps (P. indicus and P merguiensis), has greatly enhanced mass production of shrimp fry under hatchery conditions. Hatchery-bred fry have been found to show comparable growth and survival rate in grow-out shrimp ponds with those collected from the wild. The shortage, and inconsistent supply of wild fry coupled with increasing areas of shrimp farms have made the shrimp hatchery an indispensable facility to meet the growing fry requirement of the shrimp industry.
Although various techniques have been developed, existing techniques in shrimp seed production as a result of more than 50 years of research studies have yet to be refined, packaged and tested in different environment conditions into an appropriate technology that can be disseminated to the shrimp growers.
Shrimp hatchery technology that has been established in developed nations may not be economically appropriate to developing nations. Hence, testing of any developed technology is essential to ensure its technical and economic viability in the country concerned.
A successful hatchery usually depends on a number of conditions; among the more important ones are right choice of site, effectiveness and efficiency of operational management.
In designing a shrimp hatchery, it is essential to consider the production target of a species or a number of species and the level of financial inputs. In the case of the tiger shrimps, the production target is very much limited by the supply of the spawners. This limits the production capacity of the hatchery. In the case of the white or banana shrimps for which spawners are easily available, tank capacity can be much higher. This prototype hatchery, however, was especially designed for the seed production of warm water shrimps especially the tiger shrimps, white and banana shrimps. The design of the prototype hatchery takes into consideration the technical advantages of the large and small tank hatchery systems to maximize hatchery facilities for the economic production of the target species. In essence, the prototype hatchery has the flexibility of operating at the desired level of operation dictated by the availability of spawners.
The present technology series attempts to describe the prototype warm water shrimp hatchery established and operated by SEAFDEC Aquaculture Department in collaboration with FAO since 1982. The techniques used in the operation of the hatchery have been refined, repeatedly tested and are now packaged for further testing in different environmental conditions elsewhere in the Philippines and other tropical countries.
The hatchery is sited near the seashore where relatively clean, clear and unpolluted seawater is easily available by means of a mechanical pump. In the tropical climate where temperature fluctuation is relatively small, the hatchery can be operated all year round.
The size of the hatchery is dependent on the production target. The number and sizes of various holding tanks. Water and air delivery systems can be computed and designed accordingly. The basic facilities in a shrimp hatchery include (a) holding tanks (b) filtration systems (c) water, air and power supply systems.
The warm water hatchery is so designed to provide a production capacity of 10 million shrimp fry which is sufficient to stock at least 2000 hectares of traditional shrimp farms (5000/ha) or no less than 50 hectares of modern shrimp farms using high stocking rate (e.g. 20/m2).
Holding tanks
The holding tanks of the shrimp hatchery are used for various purposes such as for maturation, spawning, rearing and storage. The number and size for the various types of holding tanks of the hatchery are computed based on the production target per hatchery run. The hatchery in the tropical conditions can be operated all year round with 7–8 operational runs a year. As the annual production target is approximately ten million twenty five day-old postlarvae, the tank facilities should be designed to provide a production capacity of 1.4 million postlarvae per run.
Research studies have shown that:
A nursery tank can produce 3000–5000 twenty five day-old postlarvae per cubic meter of water with 50% mortality from P1 to P25.
A larval rearing tank can produce 30,000–50,000 one day-old postlarvae per cubic meter of water with a survival rate of 30–40% from nauplii to a day-old postlarvae in 10–12 days from hatching.
A wild spawner produces an average of 400.000 eggs.
An ablated spawner produces an average of 200.000 eggs.
Optimal stocking rate in maturation tank is 5–6 individuals/cubic meter.
The ratio of tank capacity for larval food to that of shrimp larvae is 1:10.
The size and number of the various holding tanks are computed as follows:
| Type of tank | Shape | Total Capacity (tons) | Unit Capacity (tons) | Number of Units |
| Maturation | circular | 48 | 12 | 4 |
| Spawning | circular | 2.5 | 0.25 | 10 |
| Larval rearing | circular | 30 | 2.5 | 12 |
| Nursery | rectangular | 400 | 40 | 10 |
| Live food culture | circular | 30 | 1 | 30 |
| Water storage | circular | 50 | 1 | 1 |
The detail specifications for each category of the holding tanks are illustrated in Figs. 1 to 5
Water storage and filtration tank
When filtered water is used, the water storage tank should at least 10% of the capacity. Hence, approximately a 50-ton water storage holding tank equipped with sand and gravel filtration system will be needed (Fig. 6). A reversing filter is used and can be easily maintained by backflushing.
Aeration system
Roots blower with capacity to generate an air pressure about 0.2 – 0.3 kg/m3 and a volume of 4 – 5 liters/m2/minute is adequate for tank less than 2 meters deep. At least one stand-by roots blower is needed. The airstones used are usually round and are made of Carborundum grinding stone. Such air stone can aerate 5 cubic meters of water. PVC pipes are used for air delivery. In this prototype hatchery, the capacity of root blower should be 7.5–10 horsepower with 2 inches wide outlet.
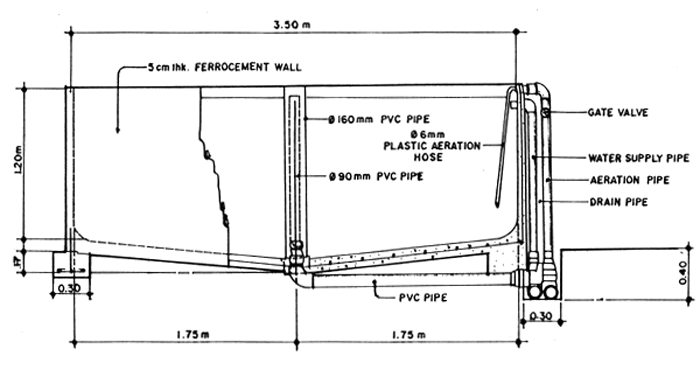
Fig. 1. Cross sectional detail of a 12-ton ferrocement broodstock tank.
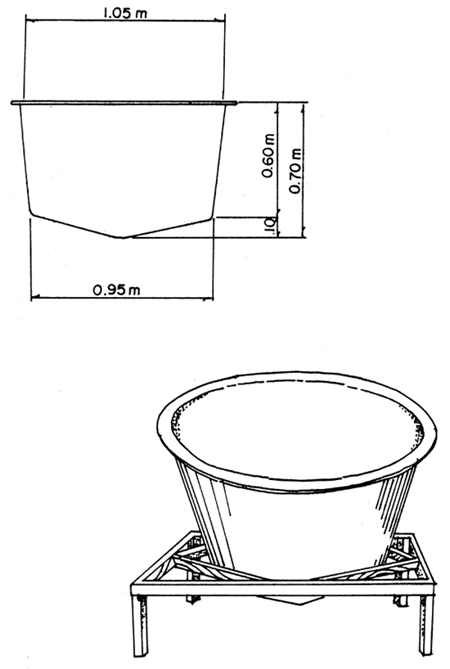
Fig.2. 250 liter fiberglass spawning tank.
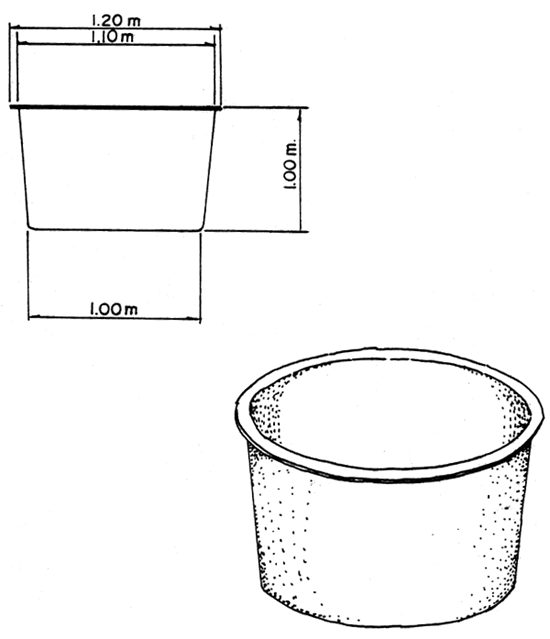
Fig. 5. 1-ton fiberglass tank utilized for algae culture
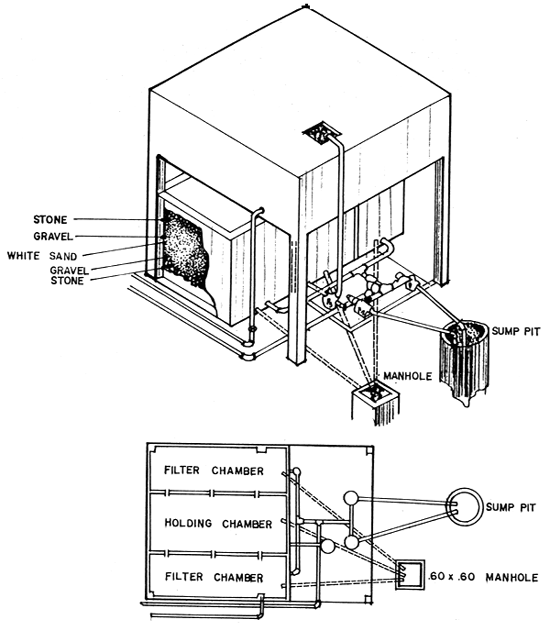
Fig. 6. Water Storage and Filtration Tank
Stand-by generator
Continuous aeration is extremely crucial in ensuring survival of larvae in high density. Any prolonged power interruption would seriously affect the culture organisms in the tank. Thus, it is essential to have a stand-by generator installed with automatic alarm or switch system whereby any power disconnection will automatically switch on the generator thereby providing the necessary power. A hatchery can be operated in an area with no supply of electricity since a marine pump and aerator can be driven directly by handy generators. However, it is more economical to have a hatchery in areas where electricity is available.
Seawater supply and piping system
Seawater is drawn directly from the sea by a marine pump through PVC piping to the overhead filter tank and stored in the reservoir. If the seawater is too turbid, the water is first pumped into a sedimentation tank before passing through the filtering system. Water from the storage tank is gravity-fed to the various tanks in the hatchery (Fig. 6).
The marine pump used depends on the volume requirement, pumping time and the total head. For a medium scale hatchery, it should have a discharge rate of approximately 1 ton/minute. A marine pump with 10 HP capacity will adequately meet this requirement.
Layout
In addition to the basic tank facilities, a functional hatchery should be complemented with a small room for algal starter, storeroom, office and all other auxilliary facilities. Hatchery layout depends on the topographic characteristics of the site and the environmental conditions. The layout of the hatchery which covers a total floor area of 2,000 sq.m. is illustrated in Fig.7.
The operational activities of a shrimp hatchery can be grouped into three broad categories, viz: those of preparatory phase. rearing operation and routine management. (All activities must be efficiently executed and systematically coordinated so as to attain the set production goal).
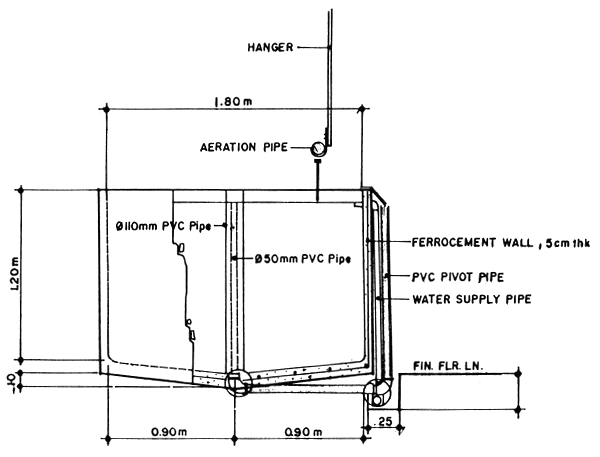
Fig.3. Cross sectional detail of 2.5-ton ferrocement larval rearing tank.
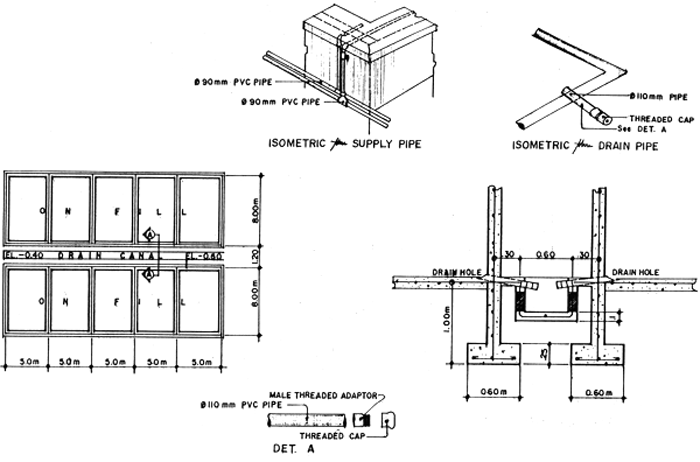
Fig. 4. Detail of 40-ton concrete nursery tanks.
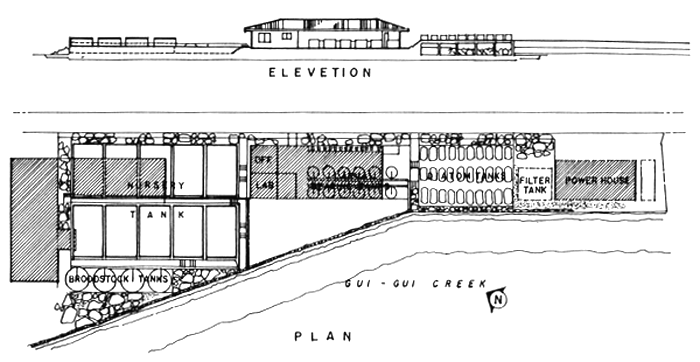
Fig. 7. Layout of medium scale hatchery.
I. Preparatory Phase
This is the initial phase of hatchery operation. All activities under this phase are as important as the rearing operation. Under this phase, all hatchery facilities must be adequately prepared for the maturation, spawning, hatching and larval rearing operation. Conditioning of broodstock is necessary prior to ablation for maturation. Proper selection of wild and ablated spawners and treatment if necessary should be done. The live food organisms must be ready in sufficient quantity when the larvae start feeding.
Facilities
For a newly constructed hatchery, tank facilities especially concrete ones must be adequately conditioned with alum (potassium aluminum sulphate) which would age the tanks and protect them from leakages. The newly constructed tanks are first filled with sea or freshwater and small pieces of alum are broadcast at a rate of 1 kg/m3 and allowed to stay for about a week until the pH of the water in the tank is fully stabilized.
For an operational hatchery, all tanks used must be properly cleaned with freshwater, dried and exposed to the sun for at least one day if posible prior to stocking. After each run, the tanks are disinfected with 12% sodium hypochloride for 24 hours. (12% sodium hypochloride, 2 liters/cubic meter of seawater).
It is important to ensure that the air from the aeration units is oil free and the air pressure is adjustable. Generally, root blowers are designed for oil free operation unlike air compressors and rotary air blowers. Air filters at the inlet and outlet pipes are therefore adequate to filter the air. The water supply system should guarantee good water quality during operation. Periodic checking during and after rearing operation should maintain the variation of the physico-chemical parameters of the water to allowable limits for living organisms. As a matter of practice, disinfection of the sand filters is often done monthly with 12% sodium hypochloride solution to reduce organic contamination due to bacterial decomposition.
Broodstocks and spawners
Sufficient broodstocks must be maintained to provide the necessary quantity of spawners for supplying the targeted quantity of seeds. When wild spawners are scarce especially for P. monodon. It is essential to develop sufficient broodstocks to supplement in time of shortage of wild spawners. A shrimp can be induced to mature by ablating one of its eyestalks (Fig. 3).
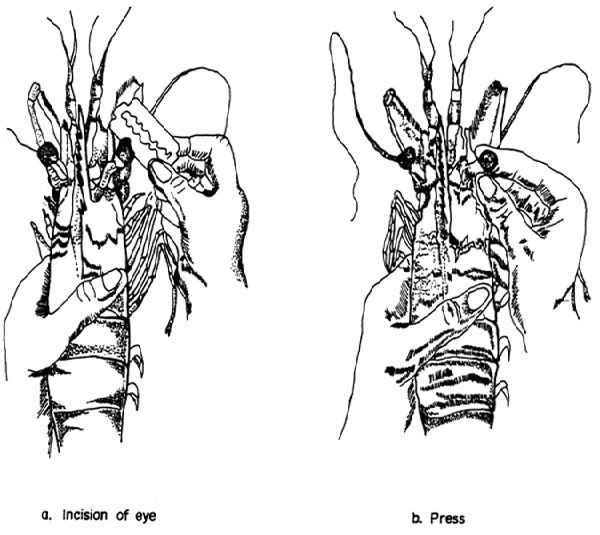
Fig. 8. Eyestalk ablation
Broodstocks collected from the wild undergo the following treatment in the hatchery:
Conditioning - they are acclimatized in holding tanks for 4–7 days. Each tank is adequately aerated with 60% of its water replaced daily. Once the animals have recovered from transport stress, they are further induced to molt by decreasing the salinity of the water by about 4–5 ppt for 2 days. The salinity is then raised to the normal level. Mating takes place soon after molting.
Induced maturation - the shrimps are ablated 2–3 days after molting or when the shell is completely hardened. They are stocked in maturation tanks at a sex ratio of 1:1. Only suitable females are used for induced maturation based on the following criteria: (a) complete appendages (b) presence of spermatophore in the thylecum of females and (c) size at least 100 grams. After ablation, the broodstocks are fed with high protein diet at 10% body weight. The diet consists of squid, mussel or oyster meat or pellet feeds. A flow through system is usually applied to maintain the water quality.
Ablated or wild females attaining maturity stages 3 and 4 (Fig. 9) are transferred to the spawning tanks which is sufficiently covered and left undisturbed till after spawning.
Larval feeds
One of the key factors ensuring success in shrimp hatchery production is the timely supply of the needed food organisms in sufficient quantity. The hatchery should have an algal culture room to maintain pure stocks of the needed live feeds such as Chaetoceros. Skeletonema. Tetraselmis. Chlorella. The algal stocks can be easily maintained in the standard culture media (Appendix 1). While a pure rotifer Brachionus sp. stock must also be maintained throughout the year.
Mass production of larval feeds must closely synchronize with the various procedures shown in Fig. 10 which should be closely followed to mass produce sufficient quantity of the desired species. The steps suggested in the figure is based on results of numerous trials and are found to be reliable procedures based on the knowledge of the growth pattern of each species and economic production cost of live foods using different quality of fertilizers. Technical procedures in the cultivation of diatoms, rotifers and the hatching of the Artemia eggs are briefly outlined in Figs. 11 to 13.
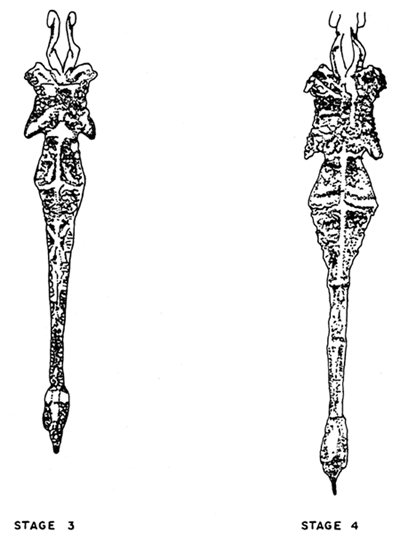
Fig. 9. Spawner with mature gonad.
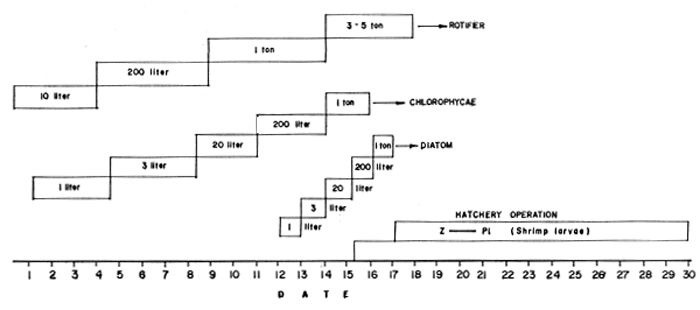
Fig. 10. Programming of Natural Food Culture for Hatchery
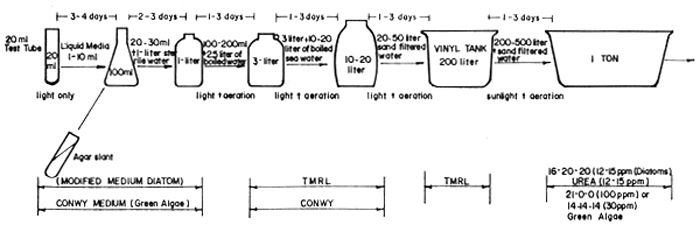
Fig. 11. Flow Chart for Mass Culture of Phytoplankton
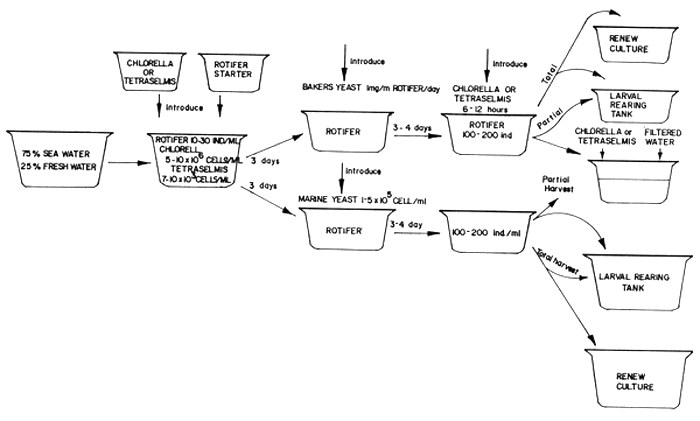
Fig. 12. Flow Chart of Rotifer Culture
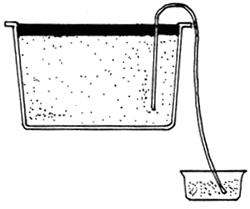 |  |  |
| A. HARVEST ARTEMIA FROM HATCHING TANK | B. PLACE IN A NEW CONTAINER | C. COVER THE CONTAINER |
 | ARTEMIA NAUPLII | 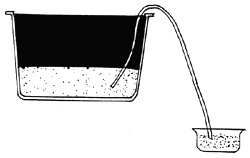 |
| D. ARTEMIA SWIM TOWARDS LIGHT SOURCE | E. HARVESTED |
Fig. 13. Harvesting of Artemia
II. Larval Rearing Operation
After spawning, the animals are removed from the tank the following morning. The eggs are then cleaned either by siphoning them into the egg collectors or draining 2/3 of the water through a filter net that effectively retains the eggs within the tank. After draining, the scum in the tank is then removed using a scoop net with mesh size bigger than that of the shrimp eggs. The tank is then filled up with water.
From the spawning tank, sample of eggs are counted to determine the number of eggs spawned per female. In normal condition, fertilized eggs hatch within 12–15 hours. The hatching rate is measured by assessing the number of hatched nauplii. Nauplii are then transferred directly into the 40-ton tanks if the number of nauplii is between 0.5 million to 1 million. They are then reared directly in the large tank up to the twenty-fifth post larval day. On the other hand, if the number of nauplii is less than 0.5 million, they are stocked in 2.5 tons indoor tanks at a density of 100–150 larvae/liter. The larvae are reared either to the third mysis stage (M3) or one-day old (P1) postlarvae. They are then transferred to the outdoor 40-ton nursery tanks for further nursing.
After hatching, the newly hatched nauplii are stocked at a density of 100–150 nauplii per liter in the 2.5-ton larval rearing tanks with fresh filtered seawater filling up to 3/4 of tank capacity. No feed is required at the nauplius stage since the nauplius still utilizes its yolk as food. However, diatom are innoculated immediately after stocking to ensure availability of feed when the nauplii molt into the Protozoea stage.
Protozoea stage
This is a critical stage of larval rearing. The larvae at this stage start feeding on external food and feed on minute and easily digested microscopic algae such as Skeletonema costatum, Chaetoceros sp. and Tetraselmis sp. The optimal feeding of phytoplankton in the tank is 50,000 cells/ml for Skeletonema or Chaetoceros and 10,000 cells/ml for Tetraselmis.
Failure in diatom bloom may happen especially during rainy months. As alternative feed, marine yeast, soya bean milk, fertilized oyster eggs or fine particles of egg custard may be used. However, the molting period of the animal may be delayed when fed with such diets.
On the first day of protozoea 1, about 100 liters per cubic meter of fresh filtered seawater is added and on the succeeding protozoea 1000 litres/m3 of water is replaced daily untilo the animal reach the mysis stage. Henceforth, approximately 30% of the water should be changed daily but the required density of food organisms must be maintained.
Mysis stage
The larvae at this stage will start feeding on rotifers (Brachionus plicatilis) or the brine shrimp nauplii (Artemia salina). The number of rotifers or brine shrimp nauplii required depends on the density of shrimp larvae being reared. Each mysis larvae consumes about 100–200 rotifers or 20–50 Artemia nauplii per day or a standard ratio of about 5 grams dry Artemia cysts is required per m3 of rearing water.
During this stage, the tank bottom is already filled with dead organisms and must be siphoned out daily. Once the larvae reach the first day of postlarval stage (P1), they can be transferred to the bigger nursery tanks.
One day before transferring the postlarvae to the outdoor tanks, the nursery tanks should be first filled up with fresh filtered seawater to allow adequate blooming of diatoms. The postlarvae is stocked at a density of 15–20 larvae/liter.
The initial water level in the 40-ton nursery tanks during stocking is 100 cm. The nauplii density is usually about 20–50/liter. Immediately after stocking, diatom starters are innoculated to ensure bloom of the desirable species. Technical grade fertilizers can be used directly to enhance algae growth. The fertilizers used are:
| KNO3 | 3 ppm |
| Na2HPO4 | 0.3 ppm |
It is pertinent to monitor the types and density of algae in big tanks to ensure that the optimal density is maintained.
During the protozoea stage, about 10–20 cm of fresh filtered seawater is then added daily. However, the amount of water added is dependent on the diatom growth. When diatom density is below the desired level in the culture tank, more cultured diatoms and fertilizers are added to accelerate algal bloom. On the other hand, over-blooming of algae should also be controlled by shading or by draining out a portion of water and replenish with fresh seawater.
Mysis stage
The same operational procedure for rearing of mysis stage used in small tank system is employed when conducting in the outdoor nursery tanks. After the water level in nursery tank has been filled to its full rearing capacity, approximately 30% of the water is changed daily.
Postlarvae stage
Early postlarval stages (P1-P6) are fed with brine shrimp nauplii at a rate of 100–200 per postlarvae per day.
Once the postlarvae reaches the sixth day (P6), they are fed with finely minced mussel or cockle meat or larval pellet feed while gradually decreasing dependency on brine shrimp nauplii which eventually stopped at P9. Beyond this stage, the larvae are fed solely on minced mussel or cockle meat or artificial diets 3 to 4 times daily. In the meantime, polyethelene nets were provided as a substrate for the larvae.
Good water quality should be strictly maintained especially during this phase of larval rearing. While 30–40% of the rearing water is being changed daily, efforts must be made to ensure saturation of dissolved oxygen concentration in the water and low concentration of ammonia (0.1 ppm). Hence, regular siphoning of tank bottom to remove excess feeds, metabolic wastes and dead algae is an important and routine hatchery function. When 50% of the rearing water has been drained, continuous flow-through of fresh seawater is maintained for 2–3 hours. Such flow-through operation enables the suspended (solid) particles to be drained out and water clarity maintained.
Sometimes, when over-blooming of diatom becomes uncontrollable or the sediment accumulation at the tank bottom is too thick, which deteriorate water quality rapidly, it becomes necessary to transfer the larvae to another well-prepared tank.
Some operational procedures have become a routine management activity. It is highly important to ensure no drastic fluctuation of environmental conditions. Efforts must be made to maintain stable condition in the tank and the water quality maintained within the allowable limits:
| * Salinity | 30–32 ppt |
| * Dissolved oxygen saturated | |
While hatchery techniques are gradually being perfected hatchery operation is still highly dependent on the experience and skill of the hatchery operators/technicians. Mechanical control of diatom blooms, for example, has yet to be developed and is still dependent on the technical experience of the hatchery operators. Regulation of optimal food density at different stages of larval development remains an important operational strategy. Supplementary feeds in time of shortage of natural food organisms become a management decision and the types and quality of supplementary feed used varies with the operational situation. Fertilized oyster eggs, soybean cake, soybean curd, egg custard and encapsulated egg yolk are possible supplementary feeds but they should not be used as a total substitute (Fig. 14). The importance of maintaining diatoms should be emphasized in regulation and maintenance of water quality if supplementary feeds are the only option.
Management is a very important operational function of a hatchery. Some of the operational procedures have become daily routines. In shrimp hatchery, such routine activities include (a) maintenance of water quality (b) regulation of feeds and feeding schemes at different stages of larval growth and (c) monitoring of the environmental parameters in relation to survival rate and growth.
Maintenance of water quality
Good water quality is essential throughout the hatchery operation. It is also very important to minimize drastic fluctuation of environmental parameters such as water temperature, salinity or dissolved oxygen content of water.
For the prototype hatchery, the major parameters are maintained within the following allowable limits:
| * Salinity | 30–32 ppt |
| * Dissolved oxygen content | supersaturated |
| * Water temperature | 26 – 31°C |
| * pH | 7.5 – 8.5 |
| * NH3- | 0.1 ppm |
| * NH1 | 1.5 ppm |
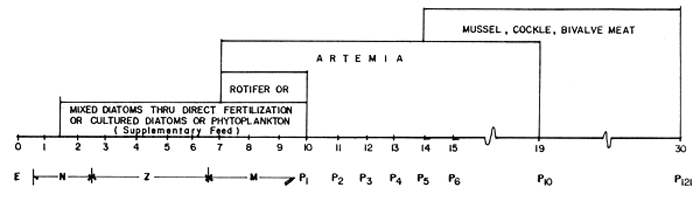
N NAUPLIUS Z PROTOZOEA M MYSIS P POST LARVAE | FERTILIZER | KNO3 | 3.0 gm./m3 | |
| NA2HPO4 | 0.3 gm/m3 | |||
| DIATOM/PHYTOPLANKTON | SKELETONEMA | COSTATUM | 40,000–50,000 Cells/ml | |
| TETRASELMIS | SP. | 10,000 Cells/ml | ||
| CHAETOCEROS | SP. | 40,000–80,000 Cells/ml | ||
| SUPPLEMENTARY FEED | FERTILIZED EGGS OF OYSTERS | |||
| SOYBEAN CAKE | ||||
| SOYBEAN CURD | ||||
| EGG CUSTARD | 10 mg / larva / day | |||
| EGG YOLK | ||||
| DRY ACETES | 10 mg / larva/ day | |||
| FRESH ACETES | 50 mg/ larva/ day | |||
Fig. 14. Feed and Feeding Scheme for Shrimp Larvae
Monitoring of environmental parameters
In any functional hatchery, regular monitoring of basic environmental parameters such as temperature, salinity, dissolved oxygen, ammonia and nutrient levels is a routine basic operational function. Equipment for determination of these parameters are easily available in the market.
While the above activities are operational routines, the environmental data should be carefully analyzed to detect development of undesirable changes of environmental conditions.
The juveniles in the nursery tanks can be harvested when they reach 21 to 25 days old. The larvae at this stage have reached not only the preferred size of shrimp growers but also are more sturdy to withstand stress due to transportation.
During harvest, the water level in the nursery tank is gradually reduced to about ⅓ of tank depth and then drained out through the outlet pipe and with a bag net installed at the tip of the pipe. The larvae that go with the water are collected at the bag net. This method is efficient enough to harvest all the larvae.
The postlarvae can be transported live in tanks or plastic bags following the standard packing and transportation procedure for shrimp larvae. Very often, they are conveniently transported in polyethelene bags provided with oxygen. The bag (60 × 40 cm) is first filled with 6–8 liters of fresh seawater and then packed with 3,000–5,000 postlarvae. They are then placed in styrofoam boxes. Temperature inside the bag can be reduced to about 20–24°C by crushed ice mixed with sawdust placed at the bottom of the box and on the top of the polyethelene bag. Under such condition, the larvae can be kept alive for more than 12 hours.
Financial analysis shows that hatchery operation is economically viable if production target can be reached (Table 1), considering the hatchery to be operating at 10 million tiger shrimp fry per year based on the existing production capability, a net income of 77 centavos per peso investment can be obtained fully demonstrating its commercial viability. Operating cost is fairly high (66.8%), almost half of which are spent on the procurement of Artemia eggs. The hatchery is fully dependent on electricity supply which constitutes 13.5% of the total cost. Since the hatchery is heavily relying on the skill and experience of the operators, salary forms about 8.5% of the total cost or 12.8% of operating cost. Interest rate forms a sizeable expenditure (20.2%) because of the high bank interest rate.
The hatchery operation is still profitable if it is operated at 80% capacity. The income over total cost is about 55%. However, when operated at half its capacity, the profit margin will go below 8.5%, rendering hatchery operation less lucrative.
| ITEM | VALUE (PESOS) | ||
| A. | Income | ||
| P. monodon larvae (PL 20–21) at 0.35 pesos/pc × 10 million | 3.500.000 | ||
Sub Total A | 3,500,000 | ||
| B. | Fixed Cost/tax | ||
| Land cost (100,000 pesos × 20% interest) | 20,000 | (1.0%) | |
| Hatchery construction 1 (1,000,000 pesos × 10% depreciation) | 100,000 | (5.1%) | |
| Equipment (500,000 pesos × 20% depreciation) | 100,000 | (5.1%) | |
| Interest (2,000,000 pesos × 20%) | 400,000 | (20.2%) | |
| Property tax (1.5%) | 1,500 | (0.07%) | |
| Sales tax (1% of income) | 35,000 | (1.8%) | |
Sub Total B | 656,500 | (33.2%) | |
| C. | Operating Cost | ||
| P. monodon spawners | 160,000 | (8.1%) | |
| Fertilizer/chemical | 20,000 | (1.0%) | |
| Feed | 15,000 | (0.71%) | |
| Artemia | 600,000 | (30.4%) | |
| Electricity | 276,000 | (13.9%) | |
| Labour | 168,000 | (8.5%) | |
| Fuel and oil | 10,000 | (0.5%) | |
| Materials and supply | 10,000 | (0.5%) | |
| Maintenance | 20,000 | (1.0%) | |
| Miscellaneous | 40,000 | (1.5%) | |
Sub Total C | 1,319,000 | (66.8%) | |
| D. | Total cost (B + C) | 1,975,500 | |
| E. | Net operating income (A - C) | 2,181,000 | |
| F. | Net income (A-B-C) | 1,524,500 | |
| G. | Income over total cost | 77.17% | |
Table 1. The projection of cost and return of a prototype warmwater hatchery based on operation at full capacity in the Philippine conditions.
THE CULTURE MEDIA AND THEIR PREPARATION
| I. | Allen-Neison “Miguel seawater” | |
| Solution A | ||
KNO3 | 20.2 g/100 ml.H2O | |
| Solution B | ||
Na2HPO412H2O | 4.0 g | |
Cacl26H2O | 4.0 g | |
HCl conc. | 2.0 g | |
FeCl3 (melted) | 2.0 ml | |
Distilled water | 80.0 ml | |
Add 2 ml. solution A to 1 liter seawater and 1 ml. of solution B to 1 liter of seawater. | ||
| II. | Conwy Medium (Walne, 1974) | |
| A. | Sodium Nitrate | 100 g |
| EDTA, disodium salt | 45 g | |
| Boric Acid | 33.6 g | |
| Sodium Phosphate, monobasic | 20 g | |
| Ferric Chloride, 6-hydrate | 1.3 g | |
| Manganous Chloride, 4-hydrate | 0.36 g | |
| Trace Metal Solution* | 1 ml | |
| Vitamin Mix** | 100 ml | |
| Distilled water (to make) | 1000 ml | |
| (Note: use 1 ml Conwy medium/liter of seawater) | ||
| * Trace Metal Stock Solution | ||
| Zinc Chloride | 2.1. g | |
| Cobalt Chloride. | ||
| 6-hydrate | 2.1 g | |
| Ammonium Molybdate, | ||
| 4-hydrate | 2.1 g | |
| Copper Sulfate | 2.0 g | |
| Distilled water | 100 ml. | |
| (Note: acidify with IN HCI until solution is clear) | ||
| **Vitamin Mix | ||
| Vitamin B12 | 10.2 mg | |
| Vitamin B1 | 20.0 mg | |
| Distilled water | 200 ml | |
| III. | TMRL Enrichment (Liao & Huang, 1970) | |
| KNO3 | 100 g | |
| Na2HPO412H2O | 10 g | |
| FeCl36H2O | 3 g | |
| Na2SiO39H2O | 1 g | |
| Distilled water | 1000 ml | |
| (Note: Use 1 ml TMRL/liter of seawater) | ||
| IV. | Enrichment for Outdoor Cultures | |
| 16–20–0 | 12 g | |
| Urea 46-0-0 | 12 g | |
| 21-0-0 | 100 g | |
| (Note: add above nutrients to 1 ton seawater) | ||
| V. | Modified F Medium (modified from Guillard & Ryther, 1962) | |
| A. N-P Stock (500×) | ||
| Sodium nitrate | 42.07 g | |
| Sodium Phosphate, monobasic | 5.00 g | |
| Distilled water (to make) | 1 liter | |
| B. Sodium Metasilicate Stock (500 x) | ||
| Sodium metasilicate | 15.0 g | |
| Distilled water (to make) | 1 liter | |
| C. Ferric Chloride Stock (500 ×) | 1.45 g | |
| Distilled water (to make) | 1 liter | |
| D. EDTA Stock (1000 ×) | ||
| EDTA, Disodium salt | 10.00 g | |
| Distilled water (to make) | 1 liter | |
| E. Vitamin stock (1000×) | ||
| Thiamine HCl | 0.2 g | |
| B12 | 10.0 ml* | |
| Biotin | 10.0 ml** | |
| * B12 stock (primary) | ||
| - B12 | 0.1g | |
| Distilled water 1 liter | ||
| ** Biotin primary stock | ||
| Biotin | 0.1 g | |
| Distilled water | 1 liter | |
| F. Trace Metal Stock (1000×) | ||
| TM primary stock A | 1 ml | |
| TM primary stock B | 1 ml | |
| TM primary stock C | 1 ml | |
| TM primary stock D | 1 ml | |
| Distilled water (to make) | 1 liter | |
| 1) TM primary stock A solution | ||
| Copper sulphate. 5-hydrate | 1.96 g | |
| Zinc sulphate. 7-hydrate | 4.40 g | |
| Distilled water (to make) | 100 ml | |
| 2) TM primary stock B solution | ||
| Sodium molybdate, 2-hydrate | 1.26 g | |
| or | ||
| (Ammonium molybdate, 4-hydrate) | 0.907 g | |
| Distilled water (to make) | 100 ml | |
| 3) TM primary stock C solution | ||
| Manganous Chloride | 36.00 g | |
| Distilled water (to make) | 100 ml | |
| 4) TM primary stock D solution | ||
| Cobalt Chloride | 2.00 g | |
| Distilled water (to make) | 100 ml | |
NOTE: Dispense 2 ml each of solutions A, B, & C/liter of seawater.
Dispense 1 ml each of solutions D, E, & F/liter of seawater.
List of equipment
| Unit | Unit Cost+ | Total | |
| 1. Marine pump, 3" × 4" 10 Hp | 2 | US$4,000 | US$ 8,000 |
| 2. Air blower, 2" 10 Hp | 2 | 2,500 | 5,000 |
| 3. Microscope | 1 | 2,500 | 2,500 |
| 4. Refractometer | 1 | 1,000 | 1,000 |
| 5. Spectrophotometer (Spectronic 20) | 1 | 2,000 | 2,000 |
| 6. pH meter | 1 | 1,000 | 1,000 |
| 7. Refrigerator, 10 cubic feet | 1 | 1,000 | 1,000 |
| 8. Freezer | 1 | 1,500 | 1,500 |
| 9. Thermometer | 5 | 10 | 50 |
| 10. Gas stove and tank | 1 | 200 | 200 |
| 11. Glassware | 1 | 450 | 450 |
| 12. Top leading balance | 1 | 1,500 | 1,500 |
| 13. Haemacytometer | 1 | 100 | 100 |
| 14. Tally counter | 4 | 25 | 100 |
| 15. Submersible pump | 2 | 400 | 800 |
| TOTAL | US$25.000 |
