
in Asia
| NACA-SF/WP/89/13 | July 1989 |
 | Site selection criteria for marine finfish netcage culture in Asia |
UNDP/FAO REGIONAL SEAFARMING DEVELOPMENT AND
DEMONSTRATION PROJECT
NETWORK OF AQUACULTURE CENTRES IN ASIA
National Inland Fisheries Institute
Kasetsart University Campus
Bangkhen, Bangkok
Thailand.
Hyperlinks to non-FAO Internet sites do not imply any official endorsement of or responsibility for the opinions, ideas, data or products presented at these locations, or guarantee the validity of the information provided. The sole purpose of links to non-FAO sites is to indicate further information available on related topics.
This electronic document has been scanned using optical character recognition (OCR) software. FAO declines all responsibility for any discrepancies that may exist between the present document and its original printed version.
Table 1. Summary of Criteria for Site Selection of Marine Finfish Netcage Culture
Table 2. Conditions in fully developed seas
Table 3. Cyanobacterial toxins
Table 4. Some of the known toxic dinoflagellates
Table 5. Toxicities of various animals, fungal, bacterial and blue-green algal toxins
by
Seafarming Project
RAS/86/0241
Netcage culture is a popular method of rearing finfish along the coastline. This new technology utilizes little physical facilities, less space, low initial investment and is moderately inexpensive to operate. Another advantage is the easy and fast harvesting of live fish which fetch higher price in the market.
There are two general types of cages, floating and stationary. A floating cage is made up of a floating unit from which a single or a series of netcages are suspended. Some of them are mobile and can be easily towed away. A stationary cage, on the other hand, is tied to fixed poles at their corners. In Asia, finfish like grouper (Epinephelus tauvina), seabass (Lates calcarifer), snapper (Lutjanus spp.) and siganid (Siganus spp.) are cultured in commercial scales in tropical countries such as Singapore, Thailand, Malaysia, Philippines, Indonesia and Hong Kong. While other finfish like red sea-bream (Pagrosomus major), black sea-bream (Sparus microcephalus), yellow tail (Seriola quinqueradiata), flatfish (Paralichthys olivaceus) etc., are cultured in temperate waters, such as in China, DPRKorea, ROKorea and Japan.
Proper site selection for marine netcage culture is of paramount importance as it may considerably affect construction costs, operating costs, growth and survival rate of the fish, and the period of usefulness of the cages. Although floating cages can be usually towed away, sometimes it is not economical to do so.
Site selection criteria also serve as a technical guideline for the production of seafarming resources atlas, rules and regulations, which are necessary for planning seafarming development programme in each country. The guidelines considered in this paper are broad and general, which may have to be modified to suit local conditions and species to be cultured in each area.
Prior to the establishment of a cage culture system, an extensive knowledge of the environment of the site is required by studies and analyses of field survey works, existing data provided by government, available literature and consultations with the local people. As mentioned above, there are two types of marine finfish netcage culture which are commonly practiced in the Asian region. One is the floating type which has the advantage of being movable and having more potential in deeper waters. The other is the stationary type which is suitable for small-scale farmers due to its low construction cost. Expectedly, not all of the technical criteria of site selection would apply similarly to both forms. The criteria for site selection of marine finfish netcage culture are summarized in Table 1.
1. UNDP/FAO Regional Seafarming Development and Demonstration Project, RAS/86/024.
Table 1. Summary of Criteria for Site Selection of Marine Finfish Netcage Culture.
Parameter | Acceptable standard | ||
| Topographical criteria | |||
Height of wave | - stationary cage | < 0.5 m | |
| - floating cage | < 1.0 m | ||
Wind velocity | - stationary cage | < 5 knots | |
| - floating cage | < 10 knots | ||
Depth | - stationary cage | min > 4, max < 8 m | |
| - floating cage | min > 5, max < 20 m | ||
| Physical criteria | |||
Current velocity | min > 10, max < 100 cm/sec | ||
Suspended solid | > 10 | mg/l | |
Water temperature | - tropical species | 27–31 | °C |
| - temperature species | 20–28 | °C | |
| Chemical criteria | |||
Dissolved oxygen | - pelagic fish | > 4 | ppm |
| - demersal fish | > 3 | ppm | |
Salinity | 15–30 | ppt | |
Ammonia-nitrogen (NH3-N) | < 0.5 | ppm | |
Hydrogen ion index (pH) | 7.0–8.5 | ||
Nitrate (NO3-N) | < 200 | mg/l | |
Nitrate (NO2-N) | < 4 | mg/l | |
Phosphate | < 70 | mg/l | |
Chemical Oxygen Demand (COD) | < 3 | mg/l | |
Biological Oxygen Demand (BOD) | < 5 | mg/l | |
| Biological criteria | |||
Bacteria count (E. coli) | < 3000 cell/ml | ||
Cages should be sited in sheltered areas protected from strong wind and wave. Strong winds such as those generated by a typhoon will destroy any structure projecting above the water while waves will bear on any object on and under the water. Normally, storms in tropical countries can be classified into three types: 1) cyclones or typhoons (3–15 m. wave height); 2) tropical storms (1–8 wave height); and 3) depressions (0.75–5 m wave height) (Malcolm, 1987). Meteorological records in the area will provide an indication of extreme condition of the weather. The information on the long-term frequency, direction and speed of surface wind obtained from meteorological records can be modified for prediction of the height of the wave (Table 2). The size of windgenerated waves is determined by (i) wind velocity, (ii) the duration of time that the wind blows, and (iii) the distance of open, unobstructed water across which the wind blows (fetch) (Bascom, 1964). Generally, the wind velocity should not exceed 5 knots for stationary cage and 10 knots for floating cage. In relation to the wind speed, the height of the wave in a suitable area should preferably not exceed 0.5 m for stationary cage and 1.0 m for floating cage. Waves are also created from the wake of passing vessels, hence culture site should be at some distance from navigation routes. In case of stationary cages at the mouth of river, creek and canal such as in southern Thailand, the Port Authority has to limit the speed of the vessel instead of removing the cage out of navigation traffic.
Table 2. Conditions in fully developed seas (modified from Bascom, 1964).
| Wind velocity (knots) (ms-1) | Fetch length (nautical (km miles) | Time (hr) | Average height (m) | H3significance height (m) | H10 Av. of the highest 10% (m) | Period where most of the energy is conc. (S) | ||
|---|---|---|---|---|---|---|---|---|
| 10 | 5.1 | 10 | 18.5 | 2.4 | 0.27 | 0.43 | 0.55 | 4 |
| 15 | 7.7 | 34 | 63.0 | 6 | 0.76 | 1.07 | 1.52 | 6 |
| 20 | 10.3 | 75 | 138.9 | 10 | 1.52 | 2.44 | 3.05 | 8 |
| 25 | 12.9 | 160 | 296.3 | 16 | 2.74 | 4.27 | 5.49 | 10 |
| 30 | 15.4 | 280 | 518.6 | 23 | 4.27 | 6.71 | 8.53 | 12 |
| 40 | 20.6 | 710 | 1314.9 | 42 | 8.53 | 13.41 | 17.37 | 16 |
| 50 | 25.7 | 1420 | 2629.8 | 69 | 14.63 | 23.77 | 30.18 | 20 |
The usual depth of a cage is 2–3 m, hence it is necessary to allow sufficient depth under the cage in order to maximize water exchange, avoid oxygen depletion, accumulation of uneaten food, faeces and debris, disease infection, and build up of some noxious gases such as H2S generated by decomposition of the deposited wastes. In turbid water, silt will tend to accumulate in the cage preventing good water exchange. The minimum and maximum depth of the cage can be calculated as follows:
| D′ | = | M - T + H′ |
| D′ | = | minimum depth at lowest low water during spring tide |
| M | = | measured depth |
| T | = | tidal height at the time when M is taken |
| H′ | = | minimum tidal height at lowest low water during spring tide |
The clearance for a floating cage should be at least 2–3 m at the lowest low water of spring tide. But a stationary cage is allowed 1–2 m minimal clearance to minimize the costs of fixed poles. Also, because fixed cages are usually placed in the mouth of rivers, creeks and canals where the water flow is stronger than in the open sea. In summary, the selected sites for floating and stationary cages should be at least 5 m and 4 m, respectively at the lowest low water of spring tide.
On the other hand, the maximum depth of the floating cage should preferably be less than 20 m, otherwise investment and maintenance costs will be higher as longer anchoring ropes and heavier anchor blocks are required. The maximum depth of a stationary cage should also not exceed 8 m since it is difficult to find sufficiently strong supporting posts longer than 8 m. The maximum depth can be calculated as follows:
| D" | = | M - T + H" |
| D" | = | maximum depth at highest high water during spring tide |
| H" | = | maximum tidal height at highest high water during spring tide |
A firm substrate, with a combination of fine gravel, sand and clay presents an ideal site for cage culture. The design of the cage is directly influenced by the type of substrate present at any given site. For example, floating netcages over rocky substrates require more expensive anchoring blocks, but have better water exchange rate. On the other hand, stationary cages easily set up in a muddy substrate with the use of cheaper poles are not suitable for high stocking density due to their low water exchange rate. In general, sloping areas from the shore leading to flat bottoms are suitable for cage culture because the waste build-up at the bottom is easily eliminated.
Tidal currents bring fresh oxygenated water to and remove waste from the cage. A large tidal range generally indicates better conditions for high stocking density of fish. On the other hand, strong currents will generate excessive strain on the raft anchoring system or fixed poles, distortion of the nets and cage structures, slow growth of fish caused by too much expense of energy in swimming against the current, and food losses. If the fish is unable to swim against the current, the stress will occur, from their being impacted on one side of the net. It would be therefore necessary to reduce the stocking density of fish. The direction of current is also a major criteria for positioning a raft. To minimize the strain on the anchoring system resulting from strong currents, the rectangular raft should be in a direction parallel to the current. This is opposite to the weak current areas where a cage needs to be positioned against the current for a better water flow.
In general, maximum current speeds can be calculated from Vmax = (AH/B)10-4 m s-1, A = surface area, B = cross-sectional area, H = range of tide in metres. However, current velocity is still greatly influenced by local topography, runoff from the land, prevailing winds, contours of sea bottom and the cross-sectional area of the site. In practice it is more convenient to measure than calculate the velocity.
The most appropriate time for measuring the maximal current velocity is at 1–2 hrs after the peak of high water during spring tide. Current velocity is generally stronger at falling tide than at rising tide except that there are other factors involve such as storms, etc. The maximal current should be ideally less than 50 cm/sec and should not exceeding 100 cm/sec. If the maximum current is less than 10 cm/sec, it will cause poor water exchange, especially during neap tide, for intensive culture of fish.
Turbid water which is normally caused by freshwater run-off during rainy season is not suitable for cage culture. Organic and inorganic solids are suspended in the water column as a result of soil erosion. Run-off also brings some heavy metals leached from the catchment area as well as other industrial effluents. It also reduces salinity at the site. Suspended solids in turbid waters with strong current from freshwater run-off will also stir up already sedimented material from the usually soft muddy bottom of estuarine areas causing more solids to deposit on the nets. These sediments act as a substrate for the growth of fouling organisms, which prevent proper water circulation. In addition, suspended sediments tend to clog fish gills which may lead to mortality from asphyxiation or cause gill epithelial tissues to proliferate and thicken. The presence of suspended solids also relates to some disease such as “fin-rot” caused by Mycobacteria (Herbert and Merkens, 1961; Herbert and Richards, 1963). The visibility of fish to the feeds will also be reduced which may lead to feed loss and impair fish growth.
Suspended solids in a suitable site for netcage culture should not exceed 10 mg/l. But its effects also depends on the exposure time and current speed. In estuarine site during flood periods, the turbidity can be higher than 100 mg/l but the exposure time is only at low tide and the current is also rapid enough to prevent the sedimentation of solid matters.
The change in water temperature will affect fish metabolism and activity, oxygen consumption, ammonia and carbon dioxide production, feeding rate, food conversion, as well as fish growth. Water temperature normally changes with climatic condition, with a wide range occurring in temperature areas. Solar radiation is also important with regard to heat transfer to the top layers of the water column. Since low water movement causes mixing in neap tide, it may be found that water temperature is higher than normal in shallow areas. Temperature change in coastal areas is mainly influenced by land runoff, i.e. colder in winter/cold season and warmer in summer. Strong wind also affects temperature change by bringing up the colder water from the bottom to the surface and reducing the heating up of surface waters.
The optimum water temperature for cage culture depends on the cultured species: 27–31°C for most tropical species and 20–28 °C for most temperate species. In the Asian region the annual temperature range fluctuates from 20–35°C in tropical countries and from 2–29 °C in temperate countries. Although some fish can survive in such temperature range, growth is usually inhibited. The best solution is to select fast growing species (not more than 8 months) and avoid having the culture period running into the months with unsuitable temperature.
Various species of fish differ in their requirements with regard to water quality in terms of chemical parameters. Most chemical changes in seawater along coastal areas are affected by the discharge from rivers or canals. Hence, water sampling should be conducted at low water, during neap tide which is usually the time when the water quality is poorest. The natural tolerance of each species should also be studied for assessment of suitable site.
The problem of dissolved oxygen for netcage culture is not as serious as in pond culture due to current movements. At night, during neap tide, planktonic algae play an important role on the depletion of dissolved oxygen due to cessation of photosynthesis. In conjunction with oxygen consumption of fish at high stocking density in the cage, and limited water circulation caused by excessive fouling, this can severely lower the dissolved oxygen content of the water surrounding the cage. In the case of cage culture in shallow areas, benthic organisms and sedimented wastes may also reduce the oxygen level. Solubility of oxygen in water declines with increasing temperature and salinity. Hence depletion of DO always occurs during night time at neap tide in summer.
Oxygen consumption for each species of fish varies, with pelagic fish like rabbit fish, snapper and seabass requiring more than demersal species such as grouper. In general, dissolved oxygen should preferably be around 5 ppm or more and never less than 4 ppm for pelagic fish or 3 ppm for demersal species.
Salinity controls osmotic pressure which greatly affects the ionic balance of fish. In coastal area suitable for cage culture, marked changes in salinity are usually caused by fresh water runoff from land. Surface salinity is usually lower than bottom salinity, in areas where the water is not mixed by the current. This causes the formation of haloclines at mid depth which also prevents vertical transfer of dissolved oxygen. Selecting sites at the mouth of rivers which have a large catchment area should be avoided. The sudden change in water salinity at these sites, as well as the long exposure to freshwater, may cause considerable mortality in cultured fish. Many farmers avoid culturing fish during the rainy season because it is impossible to predict how long the freshwater will stay. Although the water from open sea can reach the site during the high tide, sometime during heavy rain, the water from open sea drops in salinity due to heavy influx a large volume of freshwater.
The suitable salinity for optimal growth of fish depends on the fish species as shown below:
| Species | Salinity range (ppt) | Optimal Salinity (ppt) |
|---|---|---|
| Seabass (Lates calcarifer) | 0–33 | 15 |
| Grouper (Epinephelus sp.) | 10–33 | 15 |
| Rabbit fish (Siganus sp.) | 15–33 | 25 |
| Snapper (Lutjanus sp.) | 15–33 | 25 |
For most tropical species, the optimal salinity is normal strength seawater; they can not tolerate low salinities such as 10–15 ppt. Suitable site for cage culture should thus be with salinities between 15–30 ppt so that cultured species can be changed according to market demands.
In shallow water cage culture, the ammonia level in water caused by the decomposition of uneaten food and debris at the bottom, can affect the fish. Normally in coastal area, sewage discharge and industrial pollution are the main sources of higher level of ammonia in seawater. The level of ammonia-nitrogen in the water should be less than 0.5 ppm. The suitable time for measurement of ammonia level should be during neap tide when water current is slow.
Extreme values of pH can directly damage gill surfaces, leading to death (McDonald, 1983). Normally, seawater is alkaline with pH values of 7.5– 8.5. At this level, water also acts as buffer to prevent pH changes caused by other factors. An exceptional case is in estuarine areas where seawater is mixed by freshwater influx during heavy rain. The pH of freshwater may have great variation from 3 to 11 caused by acid rain or limestone rocks. In estuarine area, phytoplankton population, for example Chlorella spp., may elevate pH value in water due to its waste. However pH is also important because it affects the toxicity of several common pollutants such as ammonia cyanide and heavy metals like aluminium (Malcolm, 1987).
The suitable pH for most marine species is from 7.0 to 8.5. Sampled water should be analyzed immediately or stored in a refrigerator.
The excessive amount of nitrite in water becomes toxic to fish due to oxidation of iron in haemoglobin from ferrous to ferric state (Tiensongrusmee, 1986). It will cause hypoxia in fish because haemoglobin molecule can not bind with oxygen. Nitrate can also cause methemoglobinemia, but it is not as strong as oxidation by nitrite. Nitrate also serves as fertilizer for phytoplankton which could bloom excessively. For a suitable area, nitrite level should not exceed 4 mg/litre while nitrate level should be below 200 mg/litre.
The total phosphate content in natural water may range from 0.01 to more than 200 mg/litre (Tiensongrusmee, 1986). An excessive level of phosphate in water will trigger an over-bloom of phytoplankton which causes the depletion of oxygen level in water. A good site for cage culture should have phosphate level not higher than 70 mg/litre.
Organic load in water comes from the death of phytoplankton after blooming, uneaten food and fish waste in the cage, sewage discharge and animal waste discharge, and industrial effluents. This high organic load will cause bacterial infection in fish and lower oxygen level in water. The organic content in water is measured by Chemical Oxygen Demand (COD) which should be 3 mg/litre or less for a suitable site (Chou, 1988).
Most heavy metals are occasionally released with industrial discharges without treatment, especially in developing countries. High level of heavy metals can accumulate in cultured fish to such a degree that it becomes toxic to human when ingested. Hence the suitable site for cage culture should be as far from industrial areas as possible. The important heavy metals and their acceptable safe limits in water for cage culture are as follows:
| Manganese (Mn) | < 1.0 ppm |
| Iron (Fe) | < 1.0 " |
| Chromium (Cr) | < 1.0 " |
| Tin (Sn) | < 1.0 " |
| Lead (Pb) | < 0.1 " |
| Nickel (Ni) | < 0.1 " |
| Zinc (Zn) | < 0.1 " |
| Aluminium (Al) | < 0.1 " |
| Copper (Cu) | < 0.01 " |
| Cadmium (Cd) | < 0.03 " |
| Mercury (Hg) | < 0.004 " |
Apart from the organic matter in domestic sewage, there are other pollutants such as detergents, various toxic substances (cyanide, sulphide, chlorine, formaldehydes, phenols, oil, etc.) affect cage farming. Agricultural wastes, other than animal waste, such as insecticides and herbicides often spill into the culture site and may be accumulated by fish or cause their mortality. Oil spills from tankers or shipyards have caused a number of problems for cage culture in Singapore, ROKorea, Japan and Hong Kong. For analysis of above pollutants, it would require extensive sampling and sophisticated methods in laboratory. Risks can be reduced by selecting site for cage culture as far as possible from large industrial areas to avoid pollution.
The normal measure of the degree of pollution is the Biological Oxygen Demand (BOD) which should not exceed 5 mg/l at 5 days period (Tiensongrusmee, 1986).
There are about 200 species of marine fouling organisms in the world (Lovegrove 1979). More than 34 species of algae (cyanophytes, rhodophytes, chlorophytes) coelenterates, polyzoans, annelids, arthropods, molluscs and simple chordates have been observed clinging to netcages after immersion for only two months (Cheah and Chua 1979). Colonization of fouling organism is primarily caused by silt particles deposited at the net which serve as substrate for fouling organisms. Silt particles can be more than 50% of total fouling weight (Chou, 1988). Clogging of the net by fouling organism restricts the water flow thus lowering the dissolved oxygen and waste removal in the netcage. It also increases surface area of the net which causes deformation of the cage in strong current and also increases the stress on both cage structure and anchoring system.
Rate of fouling varies with the environmental conditions and materials used. Fouling is generally more rapid in areas with low current velocities, high temperature, high turbidity (enriched water) and high salinity. Santhanam et al. (1983) found fewer fouling animals on netcages and pens sited in brackish than in marine waters. It was found that the rate of fouling of galvanized mesh and netting panels was much less than that of synthetic fibre netting panels (Milne, 1979). Different fouling organisms colonized the bamboo, oil drum and polyethylene netting parts of cages (Santhanam et al., 1983). In an area of high fouling growth, netcages would have to be cleaned and washed more often to facilitate water exchange. The additional weight of fouling will make net changing difficult and time consuming.
To minimize maintenance cost, netcage farms should be sited in areas unfavorable for the growth of fouling organisms.
Excessive blooms of phytoplankton can happen whenever the suitable condition prevails such as high light intensity, high nutrient level (organic load), warm water temperature, stagnant hydrological conditions. These conditions should be avoided when selecting cage farming. Algal blooms can affect fish, not only by damaging fish gills by clogging, but also by competing for dissolved oxygen at night. Some species of phytoplankton can produce toxins which can kill fish or accumulate in fish up to the level that becomes toxic to human. A number of marine algae groups form blooms, including diatoms, Cyanobacteria, prymnesiophytes and dinoflagellates. One diatom species, Chaetoceros convolutus has a number of prominent spines which interfere with gill function and loss of blood from injury (Kennedy, 1978). Although a few tropical marine species of Cyanobacteria are toxic (eg. Lyngba and Oscillatoria, Moore, 1982), their blooms are uncommon. In estuarine area, blooms of some freshwater species which can produce toxin, will become dominant due to the influx from river. Cyanobacterial toxins in both marine and freshwater are summarized in Table 3.
Table 3. Cyanobacterial toxins (modified from Reynolds and Walsby, 1975, and Skulberg et al., 1984).
| Species | Toxin name | Structure |
|---|---|---|
| Lyngba majuscula | lyngbatoxin A | alkaloid |
| debromoaphysiatoxin | phenolic | |
| Schizothrix calcicola | debromoaphysiatoxin | phenolic |
| Oscilatoria nigroviridis | oscillatoxin A | phenolic |
| Nodularia spumigena | nodularia toxin | unknown |
| Microcystis aeruginosa | microsystin | peptide |
| microsystin type-c | peptide | |
| Anabaena flos-aquae | anatoxin-a | alkaloid |
| anatoxin-b | unknown | |
| anatoxin-c | peptide | |
| anatoxin-d | unknown | |
| anatoxin-a | unknown | |
| Aphanizonenon flos-aquae | aphantoxin | alkaloid |
| Oscillatoria agardhii | oscillatoria toxin | unknown |
| Oscillatoria agardhii var. isothrix | oscillatoria toxin | unknown |
| Oscillatoria rubescens | oscillatoria toxin | unknown |
The most important group of toxin-producing algae is dinoflagellates (the cause of red tides). A dozen species of dinoflagellates are known to produce toxins, and only half are implicated in fish kill (Table 4). Red tides commonly occur in warm water, especially during summer months. Fish farm wastes and effluents from fertilizer plants can also generate red tide blooms due to nutrient loading. Before selecting the site for cage culture, it is necessary to inquire with the local people or concerned authorities about the occurrence of red tides in the past in that area.
The effect of toxins produced by blue green algae (Cyanobacteria) and dinoflagellates can be compared to the other toxins as shown in Table 5.
Most pathogenic or potentially pathogenic organisms spread to the cage farm with the polluted water from sewage (domestic, industrial and agricultural) and the nearby cages. For example, ‘red-boil disease’ in estuarine grouper (Ephinephelus salmoides) is produced by the bacterium, Vibrio parahaemolyticus, and is contracted following skin damage caused by handling (Wong et al., 1979). This organism is commonly found in excessive amount in sewage-polluted water. Ectoparasitic marine isopod, Nerocilia, which attacks the rabbit fish (Siganus spp.) is also more prevalent in organically enriched marine water (Chua, 1979). E. coli number in water is used as an indicator to determine the degree of pollution as well as the possibilities of disease infection in fish. A good site for cage culture should have an E. coli count of not more than 3,000 cell/ml.
The setting up of a large number of cage culture units in the same area, will cause the outbreak of diseases, especially when they spread from long-established cages. Wild fish as well as some intermediate hosts of parasites can also carry some disease and transmit them to the caged fish. Fish predators include sea birds, puffer fish and some small fish which compete in feeding. Some of these predators can also carry diseases. Hence the above problems should be considered for site selection.
The culture site should be near a shore preferably with a jetty for boat connection with farms and near a good road for land transportation. Good accessibility facilitates distribution of farm products, (especially live fish), transport of feed, fingerlings, fuel, farm equipment, supplies and other necessities. The owner can visit the farm site more often to ensure proper management if it is easily accessible. There are many evidences that the production in the farm is poor because the owner leaves only one or two labourers staying at the farm in an isolated area. Fresh water is needed for daily living and washing of farm equipment. The suitable site should have above facilities close by.
In most of the developed or large scale-intensive cage farms, there are housing facilities on the floating rafts (such as in Singapore) or on the shore close to the cages which always include an office, feed store, laboratory, hatchery and dormitory. Housing facilities on the rafts or close to the cages would increase the possibility of the sewage and toilet waste being released to the water which is not hygienic. It would also minimize production costs if other facilities like power source, telephone, market and food supplies are close to cage culture sites.
Table 4. Some of the known toxic dinoflagellates. (Interested readers should also consult Proc. 3rd Int. Conf. Toxic Dinoflagellates, 8– 12 June 1985, New Brunswick, for most recent information).
| Dinoflagellate | Usual distribution | Poison |
|---|---|---|
| Gonyaulax catenella | North Pacific coasts, | Causes PSP |
| California to Japan, | Structure determined | |
| Chile, South Africa | ||
| Gonyaulax tamarensis | Coasts of New England, | Causes PSP |
| Canada, countries along North sea | Structure determined | |
| Goynaulax acatenella | Coast of British Columbia | Causes PSP |
| Poison not isolated | ||
| Gonyaulax monilata | Gulf of Mexico | Toxic to fish, but not to warm blooded animals |
| Poison not isolated | ||
| Gonyaulax polyedra | Coast of Southern California, North Sea | Poison reported but not verified |
| Gymnodinium breve | Gulf of Mexico | Toxic to fish, chicks and mice |
| Partially purified | ||
| Gymnodinium venficium | English Channel | Toxic to fish and mice |
| Gyrodinium aureolum | North Sea | Toxic to fish and mice and possibly shellfish |
| Pyrodinium bahamense var compressa | South China Sea | Toxic to fish and shellfish |
| Pyrodinium phoneus | North Sea | Causes PSP |
| Poison not isolated | ||
| Exuviaella mariaelebouriae | Japan | Causes degeneration of liver and kidney tissue |
| Partially purified | ||
| Chattonella antiqua | Japan | Toxic to fish. Causes damages to gills and probably interferes with gas exchange |
Security is an important consideration anywhere, and probably more so in the region. Since cage culture units are sited in public waters, few countries in the region have laws and regulations to protect the products of cage farmers. Hence the farmers have to keep a careful watch on them to prevent poaching, or select a site far away from the village. These will also increase the production costs in terms of guarding, transportation, and management costs. In some areas such as in Thailand, the owners will site the cages in front of their houses but this also bring in other problems like sewage discharge from village, low water exchanges due to blocking of water currents by boats, jetties and fish traps. In many countries like Philippines, Thailand, etc., a prime consideration in site selection is security.
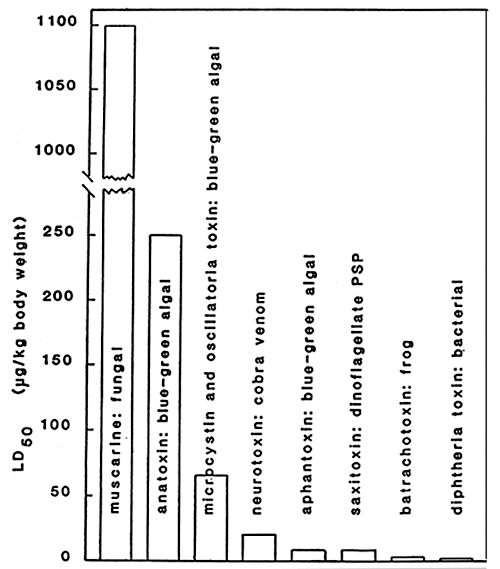
Table 5. Toxicities of various animals, fungal, bacterial and blue-green algal toxins. LD50 represents the dose, in g purified toxin per kg body weight, required to kill 50% og a given population of laboratory mice or rats by intraperitoneal injection.
There are many large scale farms which may have conflicts with villagers. For example, they may have to hire the labour from outside the village. This always brings conflict with villagers and finally lead to poaching problem. If the site cannot be avoided from such villages, it might be a good idea to have a leader of the village be one of the partners. The conflicts may occur from the other common users of the sea, such as waves or oil leak from boats, pollution from industries, waste from other farms and oil spilt from tankers or shipyards.
Most of the countries in the region have a standard law on lease of public water for any construction and for fisheries. The land below the low water tide level is owned by the government. In some countries in the region cage farmers have to obtain licence to culture fish in cages with restrictions concerning site, species, size structure and type of developments. The government should identify the site for cage culture so as to avoid competing with the other common users of the sea and interference with local navigation regulations. This site identification should also follow the above technical criteria. Size of the farm is also important to avoid or minimize disease outbreak. Lay out plan and strength of cage structures should be approved by the government. Fish species and culture methods should also be regulated with the public interest in mind such as having the proper outputs and avoiding environmental degradation, pollution and other adverse effects
Existing regulations should be carefully studied to avoid any obstacle. Lease and licence (if any) should be applied for early enough due to the lengthy processing involved in obtaining permission in some countries caused by many government departments involved. The operations of cage culture should strictly follow the conditions required by the government such as lighting at night, pollution avoidance, etc.
Bascom, W., (1964). Climatology from satellites. Methuen, London. 418 pp.
Basa, S.S., (1987). BFAR Manual on Finfish Cage Culture.
Beveridge, M. C. M., (1987). Cage Aquaculture. Fishing News Books Ltd. 1 Long Garden Walk, Farnham, Surrey, England. 352 pp.
Cheah, S. H. and T. E. Chua, (1979). A preliminary study of the tropical marine fouling organisms on floating net cages. Malay. Nat. J., 33, 39– 48.
Chou, R., (1988). Singapore Report on Site Selection Criteria of Seafarming. UNDP/FAO Regional Seafarming Development and Demonstration Project, RAS/86/024. (Second National Coordinator Meeting, 20–23 Sept., 1988, Singapore).
Chua, T. E., (1979). Site Selection, Structural Design, Construction, Management and Production of Floating Cage Culture System in Malaysia. In: Proc. IDRC/SEAFDEC Int. Workshop on Pen and Cage Culture of Fish. Tigbauan, Iloilo, Philippines, 11–22 Feb. 1979. SEAFDEC, Iloilo, Philippines. 65–80.
Herbert, D. W. M. and J. C. Merkens, (1961). The effects of suspended mineral solids on the survival of trout. Int. J. Air Wat. Poll., 5, 46–55.
Herbert, D. W. M. and J. M. Richards, (1963). The growth and survival of fish in some suspensions of solids of industrial origin. Int. J. Air Wat. Poll., 5, 297–302.
Kim, J. S., (1988). The Present Status of Marine Culture in Korea. Seminar Report of Training Course on Marine Finfish Netcage Culture, Singapore. In: NACA-SF/WP/88/6. Lovatelli, A. and P. B. Bueno, (Eds). Seminar report on the status of finfish culture in China, DPRK, Indonesia, ROK, Malaysia and Singapore. 53 pp.
Lovegrove, T., (1979). Control of fouling in farm cages. Fish Farming Int., 6 (10, 33, 35–37.
Milne, P. H., (1979). Aquaculture in Raceways, Cages and Enclosures (Proceedings of the International Workshop on Pen and Cage Culture for Fish). Tigbauan, Iloilo, Philippines, 11–22 Feb. 1979. SEAFDEC, Iloilo, Philippines.
Mok, T. K., (1981). Selection of Suitable Sites for Cage Culture, SCS/GEN/82/34. In: Report of the Training Courses on Small-Scale Pen and Cage Culture for Finfish. 26–31 Oct., 1981, Laguna, Philippines. South China Seas Programme, Manila, Philippines.
Moore, R. E., (1982). Toxins from marine blue-green algae. In: The water environment. W. W. Carmichael (ed). Plenum Press, New York. 15–24.
Othman, K. B., (1988). Status of Finfish Culture in Malaysia. Seminar Report of Training Course on Marine Finfish Netcage Culture, Singapore. In: NACA-SF/WP/88/6. Lovatelli, A. and P. B. Bueno, (Eds). Seminar report on the status of finfish culture in China, DPRK, Indonesia, ROK, Malaysia and Singapore. 53 pp.
PPD, (1986). Primary Production Department of Singapore; Manual on Floating Netcage Fish farming in Singapore's Coastal Waters.
Renville, A., (1988). Country Report on Netcage Culture of Coastal Finfish in Riau Archipelago, Rian Province, Indonesia (Seminar Report of Training Course on Marine Finfish Netcage Culture, Singapore). In: NACA-SF/WP/88/6. Lovatelli, A. and P. B. Bueno, (Eds). Seminar report on the status of finfish culture in China, DPRK, Indonesia, ROK, Malaysia and Singapore. 53 pp.
Reynolds, C. S. and A. E. Walsby, (1975). Water blooms. Biol. Rev., 50, 437–481.
Santhanam, R., Natarajan, P. and M. D. K. Kuthalingam, (1984). Fouling problems in cages and pens. In: Proc. Natl. Seminar on cage and pen culture, Fisheries College, Tamil Nadu Agricultural University, Tuticorin. March 18–19, 1983. Tamil Nadu Agricultural University. 143–147.
Skulberg, O. M., Codd, G. A. and W. W. Carmichael, (1984). Toxic blue-green algal blooms in Europe: A growing problem. Ambio, 13, 244–247.
Teo, K. L. and F. W. Chang, (1988). The Status of Marine Finfish Culture in Singapore Report of Training Course on Marine Finfish Netcage Culture, Singapore).
The Institute of Aquaculture, Univ. of Stirling; Fishfarming and the Safeguard of the Natural Marine Environment of Scotland, 1989.
Tiensongrusmee, B. (1986). INS/81/008/Manual/1 - Site Selection for the Culture of Marine Finfish in Floating Netcages.
Wong, S. Y., Ong, B. and T. E. Chua, (1979). Isolation, identification of causative agent of “red-boil disease” in grouper (Epinephelus Salmoides) and its possible control by vaccination. In: Proc. IDRC/SEAFDEC Int. Workshop on Pen and Cage Culture of Fish. Tigbauan, Iloilo, Philippines, 11–22 Feb. 1979. SEAFDEC, Iloilo, Philippines. 81–87.
Yongprapat, W. (1988). Country Report on Netcage Culture of Seabass in Thailand (Seminar Report of Training Course on Marine Finfish Netcage Culture, Singapore). In: NACA-SF/WP/88/6. Lovatelli, A. and P. B. Bueno, (Eds). Seminar report on the status of finfish culture in China, DPRK, Indonesia, ROK, Malaysia and Singapore. 53 pp.
PUBLICATIONS AND DOCUMENTS OF THE
REGIONAL SEAFARMING DEVELOPMENT AND DEMONSTRATION PROJECT
Working Papers
NACA-SF/WP/87/1. Lovatelli, A. Status of scallop farming: A review of techniques. 22 pp.
NACA-SF/WP/88/2. Lovatelli, A. Status of oyster culture in selected Asian countries. 96 pp.
NACA-SF/WP/88/3. Lovatelli, A. and P. B. Bueno, (Eds). Seminar report on the status of oyster culture in China, Indonesia, Malaysia, Philippines and Thailand. 55 pp.
NACA-SF/WP/88/4. Lovatelli, A. Status of mollusc culture in selected Asian countries. 75 pp.
NACA-SF/WP/88/5. Lovatelli, A. and P. B. Bueno, (Eds). Seminar report on the status of seaweed culture in China, India, Indonesia, ROK, Malaysia, Philippines and Thailand. 79 pp.
NACA-SF/WP/88/6. Lovatelli, A. and P. B. Bueno, (Eds). Seminar report on the status of finfish culture in China, DPRK, Indonesia, ROK, Malaysia and Singapore. 53 pp.
NACA-SF/WP/88/7. Lovatelli, A. Seafarming production statistics from China, Indonesia, ROK, Philippines, Singapore and Thailand. 37 pp.
NACA-SF/WP/88/8. Lovatelli, A. Site selection for mollusc culture. 25 pp.
NACA-SF/WP/88/9. Lovatelli, A. and P. B. Bueno, (Eds). Seminar report on the status of finfish netcage culture in China, DPRK, Indonesia, ROK, Malaysia, Philippines, Singapore and Thailand. 56 pp.
NACA-SF/WP/88/10. Chong, K. C. Economic and social considerations for aquaculture site selection: an Asian perspective. 17 pp.
NACA-SF/WP/89/11. Chen J. X. and A. Lovatelli. Laminaria culture - Site Selection criteria and guidelines. 30 pp.
NACA-SF/WP/89/12. Chen J. X. Gracilaria culture in China. 18 pp.
Bibliography
NACA-SF/BIB/88/1. Selected bibliography on seafarming species and production systems. 20 pp.
NACA-SF/BIB/88/2. Selected bibliography on seafarming species and production systems. 52 pp.
NACA-SF/BIB/89/1. Selected bibliography on seafarming species and production systems. 49 pp.
Training manuals
Culture of the Pacific Oyster (Crassostrea gigas) in the Republic of Korea. 64 pp.
Culture of the seabass (Lates calcarifer) in Thailand. 90 pp.
Manual on seaweed farming: Eucheuma spp. 25 pp.
Manual on marine finfish netcage culture in Singapore. 275 pp.
Culture of kelp (Laminaria japonica) in China. 204 pp.
