
| NACA-SF/WP/89/12 | June 1989 |
 | Gracilaria culture in China |
NETWORK OF AQUACULTURE CENTRES IN ASIA
National Inland Fisheries Institute
Kasetsart University Campus
Bangkhen, Bangkok
Thailand.
Hyperlinks to non-FAO Internet sites do not imply any official endorsement of or responsibility for the opinions, ideas, data or products presented at these locations, or guarantee the validity of the information provided. The sole purpose of links to non-FAO sites is to indicate further information available on related topics.
This electronic document has been scanned using optical character recognition (OCR) software. FAO declines all responsibility for any discrepancies that may exist between the present document and its original printed version.
2.1 Influence of environmental factors on spore release
2.1.3 Specific gravity of seawater
2.2 Influence of environmental factors on germination and growth of spores
2.2.3 Specific gravity of seawater
2.3 The effect of environmental factors on growth of Gracilaria thallus
2.3.1 Specific gravity of seawater
3.1.1 Outdoor collection of spores
3.1.2 Indoor collection of spores
3.2.1 Mud flat or intertidal culture
Plate 1. A number of Agarophyte species
Plate 2. A number of Agarophyte species
Table 1. Main Agarophytes in China
Table 4. The effect of temperature on carpospore disc size (um) after germination
Table 5. The effect of temperature on tetraspore disc size (um) after germination
Table 6. The effect of light intensity on the spore disc growth (um) after germination
Table 7. The growth rate of Gracilaria in different water depth
Table 8. The photosynthetic intensity of Gracilaria in different water layers
by
Chen Jia Xin 1
A number of agarophytes, including Gracilaria, Gelidium and others, have been used in China as marine vegetables or traditional medicinal herbs for over 1000 years. However, these seaweeds have became an industrially important raw material for processing agar only some decades ago.
Agarophytes in China are mainly represented in three genera of Rhodophyta, Gracilaria, Porphyra and Gelidium. Among >30 species of agarophytes Gracilaria verrucosa, G. tenuistipitata, Gelidium amansii, Porphyra haitanensis are the major ones. (Table I, Plates 1 and 2). In addition, other red seaweeds, such as Pterocladia sp. Gelidiella sp. and Ceraminum sp. are often collected and mixed with the main agarophytes mentioned above and used for producing agar.
Table I. Main Agarophytes in China.
| Genera | Species | Genera | Species |
|---|---|---|---|
| Campylaephora | C. crassa | Gracilaria | G. rubra |
| C. hypnaeoides | G. tenuistipitata(*) | ||
| Ceramium | C. boydenii | G. verrucosa(*) | |
| C. japonicum | Grateloupia | G. turuturu | |
| C. kondoi | Gymnogongrus | G. flabelliformis | |
| Gelidium | G. amansii(*) | Hypnea | H. boergesenii |
| G. crinale | H. cerviconis | ||
| G. divaricatum | H. charoides | ||
| G. pacificum | Pachymenia | P. carnosa | |
| Gracilaria | G. articulata | Pachymeniopsis | P. elliptica |
| G. bursa-postoris | Porphyra | P. haitanensis(*) | |
| G. chorda | P. yezoensis(*) | ||
| G. gigas(*) | Pterocladia | P. tenuis | |
| G. lichenoides(*) | Solieria | S. mollis |
* Commercially important species
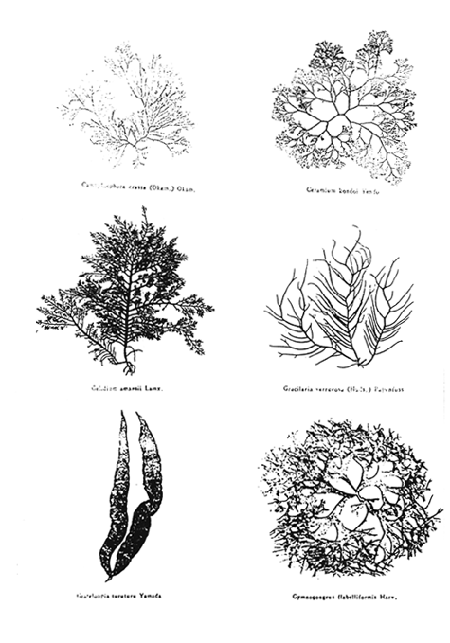
Plate 1. A number of Agarophyte species.

Plate 2. A number of Agarophyte species.
China is a major producer of cultured seaweeds in the world. The total annual production of cultured seaweeds is about 300,000 dry tons**. Of these, Laminaria and Porphyra account for 200,000 – 250,000 tons and 20,000 tons respectively. Gracilaria production is about 5,000 tons per annum (Shang, 1976; Chiang, 1981, Yang et al., 1981; Chueh and Chen, 1982,; Zheng, 1987). Laminaria in China is a main marine vegetable and raw material for extracting iodine, mannitol and alginate. Porphyra is also a popular marine vegetable. But in recent years some phycologists have observed that a considerable quantity of Porphyra thalli obtained from the later harvest during the season can be used for processing agar. The later croppings have less edible value.
At present Gracilaria and Porphyra are the most important raw materials for producing agar. Although Gelidium has long been considered as the best raw material for processing agar, it is not the ideal cultured species because of its slow growth and low yield. On the other hand, Gracilaria is easier to farm. It is expected that a higher output can be obtained in farming conditions. Shang (1976) reported that the average productivity of dry Gracilaria in Taiwan ponds was 7 to 12 t ha-1 year-1. A few years later Chiang (1981, p. 573) observed that 16–43 t ha-1 year-1 could be expected. So, it is believed that there will be a substantial development of Gracilaria culture in China to meet the increasing demand of agar market.
In the present review we shall first discuss some general ecological characteristics of Gracilaria sp. which are especially important to its commercial production and subsequently review the farming practices developed for the species.
Gracilaria verrucosa is the most common species in China. It grows fast and has high agar content. Following is a discussion on the influence of the various environmental factors on the release and germination of spores and growth of thallus of the spores.
Both carpospores and tetraspores are released naturally into seawater after their maturation. Experiment showed that the maximum quantity of spores is released at 8–10 am., gradually decreasing thereafter. The minimum quantity is released at 10 pm. to 6 am. of the following day, after which the next maximum will take place. Besides the diurnal changes of releasing rhythmicity, the released quantity of spores also depends on ambient environmental factors. (Zheng, 1987).
In general, a mature Gracilaria is taken out of seawater and kept in a shady spot for 2 to 4 hours (air temperature 15–25 °C), the tetraspores or the carpospores will be released if the plant is dipped in seawater again. Table II gives the different quantities of tetraspores or carpospores released at different durations of desiccation, or removal from the seawater.
** Weights of seaweed production provided refer to dry weight only
Table II. The relationship between the quantity of tetraspores and carpospores released and desiccation time.
| Time (hr.) | 0.5 | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| Tetraspores | 887 | 1011 | 3033 | 1921 | 1904 | 1904 | 782 |
| Carpospores | 201 | 226 | 686 | 683 | 458 | 477 | 421 |
Source: Zheng, 1987.
Experiments have shown that the highest number of spores are released at 20–25 °C (Table III). According to field surveys, the reproductive season of Gracilaria is between June to August in northern coastal provinces or between March to May in southern China. During these seasons, the natural seawater temperature is 20–25 °C. The experimental results coincide with natural reproductive seasons (Zheng, 1987).
Table III. The relationship between released quantity and seawater temperature.
| Temperature (°C) | 8–10 | 12–15 | 20–22 | 25 |
|---|---|---|---|---|
| Tetraspore | 88 | 518 | 2925 | 1525 |
| Carpospore | 522 | 998 | 1343 | 824 |
Source: Zheng, 1987
When mature plants were kept in seawater of different specific gravities, those in seawater of lower specific gravity would release spores earlier than those kept in water of higher specific gravity. If the plants are put in seawater with an obviously low specific gravity ( <1.005), the released spores will swell and even break up due to osmosis caused by the low salinity so that most of them would not complete normal germination. In order to obtain good results in the collection of spores, the optimum specific gravity range of 1.015 (19.0 – 20.2 ppt) to 1.020 (25.5 – 26.9 ppt) should be observed (Zheng, 1987).
Gracilaria spores just released into seawater, measure around 30 um in diameter and vary with species. Tetraspores are slightly bigger than carpospores. For example, the tetraspores of G. tenuistipitata have a diameter of 24–56 um, and carpospores 23–40 um. Soon after release, the spores will attach to substrates and start first cleavage of cells. The germination and growth of spores are also influenced by ambient environmental conditions such as seawater temperature, light intensity, salinity, etc. (Zheng, 1987).
To examine the germination and the growth of the spores those attached to slides for 12 hours were moved to containers with sterilized seawater enriched with 1 ml of 1 M KNO3 and 1 ml of 0.1 M KH2PO4 per litre of seawater medium, and incubated with a light intensity of 400 Lux, photoperiod of 10:14 for different periods of time (Zheng, 1989). The results are shown on Table IV and V.
Table IV. The effect of temperature on carpospore disc size (um) after germination.
| Temperature (°C) | |||||
|---|---|---|---|---|---|
| Time (days) | 7 | 10 | 15 | 20 | 28 |
| 10 | 39.6×39.6 | 42.9×42.9 | 46.2×46.2 | 46.3×46.3 | 42.9×42.9 |
| 20 | 39.6×39.6 | 45.9×45.9 | 52.8×52.8 | 59.4×59.4 | 49.5×42.5 |
| 30 | 46.2×46.2 | 59.4×59.4 | 59.4×59.4 | 56.1×56.1 | 56.1×56.1 |
| 40 | 56.9×49.5 | 62.7×59.0 | 69.3×62.7 | 75.3×75.9 | 72.6×59.4 |
| 50 | 56.9×49.5 | 66.0×59.4 | 66.0×66.0 | 95.7×82.5 | 99.0×82.5 |
Table V. The effect of temperature on tetraspores disc size (um) after germination.
| Temperature (°C) | ||||||
|---|---|---|---|---|---|---|
| Time (days) | 7 | 10 | 15 | 20 | 28 | |
| 10 | 39.6×39.6 | 36.3×36.3 | 39.6×39.6 | 36.0×36.0 | 39.1×39.1 | |
| 20 | 39.6×39.6 | 42.9×42.9 | 49.5×49.5 | 42.9×42.9 | 42.9×42.9 | |
| 30 | 46.2×46.7 | 62.7×62.7 | 62.8×62.8 | 52.8×52.8 | 52.8×52.8 | |
| 40 | 52.8×49.8 | 69.3×62.7 | 75.9×61.9 | 75.9×66.0 | 75.9×66.0 | |
| 50 | 49.5×42.9 | 66.0×59.0 | 95.7×82.5 | 95.9×85.0 | 99.0×82.5 | |
Source: Zheng, 1987.
The results illustrate that both tetraspore discs and carpospore discs need a seawater temperature higher than 15 °C for their growth. If the temperature is below 15 °C, even if all other environmental conditions are suitable for their growth, they would survive but grow very slowly (Zheng, 1987, pp. 234– 235).
Light intensity is one of the most important factors that influence the germination and growth of spores. When the light intensity is stronger, the rate of germination and growth of spores are higher within 3000 Lux. If the spores attached to slides are kept in a dark place they will die in 20 days. The experimental results are shown on Table VI.
Table VI. The effect of light intensity on the spore disc growth (um) after germination.
| Time (days) | ||||
|---|---|---|---|---|
| Light intensity (Lux) | 10 | 15 | 20 | 25 |
| 3000 | 69 | 78 | 93 | 112 |
| 1500 | 50 | 63 | 85 | 104 |
| 200 | 40 | 48 | 60 | 76 |
| DARK | poor | poor | died | - |
Source: Zheng, 1987
Although Gracilaria plants prefer to inhabit estuarine areas, their spores are unable to withstand seawater with low salinity. If the spores attached to substrate are put in seawater with a specific gravity below 1.010, (12.5–13.7 ppt) their cells swell up due to the absorption of water into the cells from ambient environment. The colour of the pigments in the cells would change from red to pale, and the spores will die eventually. Experiment proved that the optimum specific gravity of seawater for germination and growth of spores range from 1.018 (23.0– 24.2 ppt) to 1.025 (25.5–26.9 ppt) (Zheng, 1987).
The effects of environmental factors on Gracilaria thallus are similar to that on their spores, but not entirely the same. For instance, as mentioned above, spores kept in seawater with a specific gravity below 1.010, will break up and die, but the thallus can grow very well in the same conditions. In view of this, ambient environmental factors required by Gracilaria thallus must be studied.
Gracilaria is a group of euryhaline seaweeds. Under natural conditions, the specific gravity in which the plant can grow out ranges from 1.005 to 1.026 (5.2–38.1 ppt). Experiments and field surveys have shown that the optimum specific gravity is 1.010–1.020 (11.3–30.1 ppt) where freshwater regularly flows in (Zheng, 1987).
Gracilaria is also an eurythermal plant which can grow at 5 to 30 °C. Optimum temperature varies with species. For example, the optimum temperature of G. verrucosa is 15–25 °C. They can be found during May to August in northern China, but in south China only in winter and spring. In summer, the growth of Gracilaria is almost completely stopped in the south until late autumn when the water temperature drops below 25 °C and the seaweeds will resuscitate again.
Field surveys have shown that the optimum seawater temperature of G. tenuisipitata, a species mainly distributed in Guangdong and Hainan Island, is identical with G. verrucosa. When the water temperature is 30 °C, its diurnal growth rate measures 0.1–0.2 cm/day but as the temperature continues to fall to 28 °C, the growth rate increases to 0.4–0.5 cm/day. When the temperature is from 15–25 °C, growth rate can be higher than 1 cm/day.
The growth of Gracilaria requires a high light intensity. It has been shown that growth rates of the seaweeds vary when they are planted at different depths of water (Table VII and VIII). (Zheng, 1987, pp. 238– 239).
Table VII. The growth rate of Gracilaria at different water depths.
| Depth (cm) | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 |
|---|---|---|---|---|---|---|---|---|
| Lo (cm) | 22.0 | 22.5 | 27.3 | 15.3 | 19.5 | 27.7 | 19.8 | 12.8 |
| Lt (cm) | 27.0 | 28.0 | 28.7 | 16.0 | 20.5 | 26.5 | 18.8 | 10.7 |
| Lt - Lo (cm) | +5.0 | +5.5 | +1.4 | +0.7 | +1.0 | -1.5 | -1.0 | -2.1 |
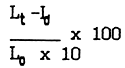 | +2.3 | +2.4 | +0.5 | +0.5 | +0.5 | -0.5 | -0.5 | -1.6 |
Source: Zheng, 1987
Lo - initial length; Lt - terminal length in 10 days;
Mean water temperature - 17 °C
Mean specific gravity of seawater - 1.020 (25.5–30.1 ppt)
Transparency of seawater - 3m
Table VIII. The photosynthetic intensity of Gracilaria in different water layer (O2 mg/L).
| Depth (a) | Control ** | 0 | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 3.5 | 4.0 |
|---|---|---|---|---|---|---|---|---|---|---|
| I * | 8.25 | 11.20 | 9.77 | 9.19 | 9.10 | 8.96 | 8.00 | 8.72 | 7.68 | 7.64 |
| II* | 8.25 | 10.90 | 10.50 | 10.50 | 9.24 | 9.10 | 8.24 | 8.16 | 8.00 | 7.12 |
| Mean | 8.25 | 11.06 | 10.23 | 9.84 | 9.17 | 9.03 | 8.42 | 8.24 | 7.84 | 7.38 |
Source: Zheng, 1987.
* I and II are same experiments run on different date.
** Seawater without seaweeds.
Table VIII illustrates that the Gracilaria can grow well at a water depth less than 1 m deep where the seawater transparency is around 3 metres. If the seaweeds are planted in seawater deeper than 3 m not only do they stop growing but start rotting as well.
Values shown on Table VIII refer to dissolved oxygen in seawater collected in a bottle from different water depths where Gracilaria is located. The figures point that Gracilaria exposed to the maximum light intensity has also more intense photosynthetic activity. As the plants are located beneath 3 m depth, their respiratory intensity will exceed the photosynthetic intensity, which explains the finding that the content of dissolved oxygen in the seawater of the experimental groups was less than that of the control group which is without Gracilaria in it.
China has more than 30 years history of farming Gracilaria. According to the specific biological characteristics of the seaweeds, different methods of collecting spores and farming thalli have been studied and practiced. This includes (1) collecting natural spores, (2) artificial outdoor collection of spores, or (3) indoor collection, (4) mud flat culture, (5) floating raft culture and (6) pond culture.
The procedure involves 4 steps: selection of site, preparation of substrate, collection of parent plants (tetrosporophyte and/or carposporophyte) and collection of spores.
1) Site selection
A suitable site for collection of spores should be an intertidal flat area with harder bottom and clear seawater with a specific gravity range of 1.010–1.025 (12.5–33.4 ppt). A large spherical dwarf dam of 20–30 cm high is made at the selected site to store seawater after ebb tide.
2) Preparation of substrate
Cheap substrates like stones, shells or broken corals can be used. These substrates have a clean surface for easy attachment of spores. If the substrate is a stone, each block should weigh about 0.5 kg, the total amount is about 600 tons stones per ha. For shell, 120 tons per ha. is sufficient. The substrate is spread on the selected site. In recent years, artificial fibers, nylon, polyethylene ropes or nets have been used to collect spores. The texture of these materials is good, but the cost is higher. Used or worn fishing nets can be used as a cheaper substrates.
3) Collection of parent plants
The parent plants must be fully mature with dull red-brown colour and without rotted spots on seaweeds body. As a fully mature plant, their sporangia are easily seen on the entire surface of the plant body.
4) Treatment of parent plants
Parent plants are washed with clean seawater to get rid of mud or other matters and allowed to air dry on bamboo curtains for 2–4 hours until irregular corrugations appear on the plant surface. At this time, the seaweeds can be spread in a selected site with substrate. The quantity of parent plant required is about 300 kg per ha.
As soon as the parent Gracilaria are planted in seawater, they release a great quantity of spores into the seawater. The spores will settle down on the substrate in one day. Under natural conditions, the germination of spores is very fast. They will turn into dish-shaped thalli in 5 days.
Another method is to put the desiccated seaweeds into a vat and stir them vigorously with a stick to stimulate release of spores into seawater. About 2 hours later, the seawater containing a huge quantity of spores can be sprayed into a selected site.
The main steps followed in indoor collection of spores are similar to those in outdoor collection. The only difference is that the spores attached to substrate will be kept in indoor tanks for rearing until they grow into young plants.
The main feature of this culture method is that the spore collection and farm sites are usually the same. In southern China, in late autumn or early winter, young Gracilaria can grow up to 5–6 cm long as they enter a period of faster growth rate. At this stage, the substrate to which young Gracilaria are attached are kept in lines on the bottom at an interval of 30–40 cm which will serve as a walk way. The routine management practice involves removal of miscellaneous seaweeds, collection of herbivorous gastropods and so on. Women and children often do it. In a normal season, 1500 kg (dry) of Gracilaria can be harvested.
The method has been adapted from kelp farming. In a suitable season, such as, January in southern China or May in the northern part, young seaweeds, both collected from other fields or reared in indoor tanks, are pulled up from substrate and inserted into ropes called “seedling rope” which are made of palm thread or artificial fiber. In each rope of 20 m length 200 pieces of seaweeds 10 cm apart can be inserted. The seedling ropes are then fixed on floating raft. Three months later, the seaweeds will reach a length of over 1 m and can be harvested. The yield can reach about 3000 kg (dry) per ha.
Pond culture of seaweeds has been adapted by Taiwan's phycologists and farmers since the 1960s. Before 1962 agarophytes were very scarce in Taiwan, and had to be imported. Phycologists and farmers tried to rear the agarophyte Gracilaria in fish pond with milkfish. Unexpected good results were obtained so that many farms began to change the main cash crop from milkfish to Gracilaria. At present, Taiwan produces 12000 tons of Gracilaria (fresh weight) annually from 300 ha of ponds (Chiang, 1981).
The stock seaweeds are cut into pieces and spread in the fish pond at a density of (5–6 t of fresh thalli) per ha. Chemical fertilizer or pig manure is applied regularly. Every 30–45 days, most parts of the seaweeds can be harvested. The rest of the body is continually left in the ponds as stock. The harvesting period lasts six months from June to November in the northern part of China.
This method is being used in the southeast region owing to lesser inputs and higher production.
Although satisfactory results have been obtained with Gracilaria through several culture facilities there have not been any great advances in production similar to Laminaria or Porphyra farming. The main reason for this is lack of balance between production cost and market price. The lower market price is inhibiting the development of farming Gracilaria.
In recent years Gracilaria farming has become a popular industry owing to the increasing demand from the agar processing industry. A main production zone for Gracilaria has appeared in Southern China including Guangdong, Guangxi and Fujian provinces where seawater is warmer and rich in nutrient suitable to growth of Gracilaria plants. Liu (1987) reported that there are 4–5 species (G. verrucosa, G. tenuistipitata, G. gigas. and G. bursa-postoris) which are qualified farming species. His experiments and investigation have shown that it is possible to obtain an average yield of 2–3 tons ha-1. He suggested that the acreage under farming of Gracilaria can be expanded to 6700 ha in Guangdong Province to produce 15000 tons (dry) of high quality Gracilaria. Technical progress and advances in farming Gracilaria will further reduce the production cost and improve product quality. The following three suggestions are made to improve the culture production and utilization of this agarophyte:
Selection of suitable species for farming. High priority should be given to selection of species with faster growth rate and higher agar content and quality.
Adequate measures should be taken to protect natural stocks of Gracilaria to prevent over-exploitation of natural stock.
Polyculture of finfish and shellfish with Gracilaria offers several advantages. For example, the seaweeds belonging to autophytes can improve the quality of water contaminated by cultured animals; Gracilaria is a superior fodder for abalone which has a higher price in the world market. Gracilaria farming can supply abalone farms with the abalone food, while abalone farming would also help to promote agarophyte farming.
Chiang, Y. M., 1981. Cultivation of Gracilaria (Rhodophycophyta, Gigartinales) in Taiwan, Proc. Int. Seaweed Symp., 10:569–574.
Chueh, C. T. and C. C. Chen, 1982. Seaweed economics. In: R.T. Tsuda and Y.M. Chiang (Editors). Proceedings of Republic of China United States Cooperation Science Seminar on Cultivation and Utilization of Economic algae. Univ. of Guam Marine Laboratory, Mangilao, Guam, pp. 9–16.
Liu, S. J., 1987. Distribution, present production status and future of Gracilaria in Guangdong Province. Sci. and Tech. of Fisheries., 1:14–15.
Shang, Y. C., 1976. Economic aspects of Gracilaria culture in Taiwan. Aquaculture 8:1–7.
Yang, S. S., Eang, C. Y. and H. H. Wang. Seasonal variation of agar produced in Taiwan area. Proc. Int. Seaweed Symp., 10:737–742.
Zheng C. K., Wang, S. J. and S. J. Liu, 1987. Phycoculture. Shanghai House of Science and Technology Publication. pp. 225–254.
PUBLICATIONS AND DOCUMENTS OF THE
REGIONAL SEAFARMING DEVELOPMENT AND DEMONSTRATION PROJECT
Working Papers
NACA - SF/WP/87/1. Lovatelli, A. Status of scallop farming: A review of techniques. 22 pp.
NACA - SF/WP/88/2. Lovatelli, A. Status of oyster culture in selected Asian countries. 96 pp.
NACA - SF/WP/88/3. Lovatelli, A. and P. B. Bueno, (Eds). Seminar report on the status of oyster culture in China, Indonesia, Malaysia, Philippines and Thailand. 55 pp.
NACA - SF/WP/88/4. Lovatelli, A. Status of mollusc culture in selected Asian countries. 75 pp.
NACA - SF/WP/88/5. Lovatelli, A. and P. B. Bueno, (Eds). Seminar report on the status of seaweed culture in China, India, Indonesia, ROK, Malaysia, Philippines and Thailand. 79 pp.
NACA - SF/WP/88/6. Lovatelli, A. and P. B. Bueno, (Eds). Seminar report on the status of finfish culture in China, DPRK, Indonesia, ROK, Malaysia and Singapore. 53 pp.
NACA - SF/WP/88/7. Lovatelli, A. Seafarming production statistics from China, Indonesia, ROK, Philippines, Singapore and Thailand. 37 pp.
NACA - SF/WP/88/8. Lovatelli, A. Site selection for mollusc culture. 25 pp.
NACA - SF/WP/88/9. Lovatelli, A. and P. B. Bueno, (Eds). Seminar report on the status of finfish netcage culture in China, DPRK, Indonesia, ROK, Malaysia, Philippines, Singapore and Thailand. 56 pp.
NACA - SF/WP/88/10. Chong, K. C. Economic and social considerations for aquaculture site selection: an Asian perspective. 17 pp.
NACA - SF/WP/89/11. Chen J. X. and A. Lovatelli. Laminaria culture - Site Selection criteria and guidelines. 30 pp.
Bibliography
NACA - SF/BIB/88/1. Selected bibliography on seafarming species and production systems. 20 pp.
NACA - SF/BIB/88/2. Selected bibliography on seafarming species and production systems. 52 pp.
NACA - SF/BIB/89/1. Selected bibliography on seafarming species and production systems. 49 pp.
Training manuals
Culture of the Pacific Oyster (Crassostrea gigas) in the Republic of Korea. 64 pp.
Culture of the seabass (Lates calcarifer) in Thailand. 90 pp.
Manual on seaweed farming: Eucheuma spp. 25 pp.
Manual on marine finfish netcage culture in Singapore. 275 pp.
Culture of kelp (Laminaria japonica) in China. 204 pp.
