Freshwater prawns are well distributed throughout the tropical and subtropical zones, there being over 100 species of the genus Macrobrachium. Table 2 summarizes data on the more important candidate species for aquaculture.
Typically prawns of this genus, though referred to as ‘freshwater prawns’ require estuarine conditions (brackish water) during the initial (larval) stages of their life cycle though some, for example, M. amazonicum, and M. dayanum, complete their whole life cycle in inland saline and freshwater lakes. Of those whose larval stages are completed in estuarine locations, the juveniles migrate upstream towards completely freshwater conditions. Large adults are often found considerable distances from the sea but always in lakes, swamps, irrigation canals, ponds and of course rivers themselves, with some connection with the sea. The larvae can survive in freshwater for the first five days but saline water is essential for survival after that (Ling, 1977). Those hatched in a freshwater environment must therefore reach coastal areas within that time. Prawns can migrate short distances over land where the vegetation is humid and are able to surmount weirs and waterfalls. Some species, such as M. rosenbergii, appear to favour turbid conditions while others are found mostly in clear water rivers (e.g. M. americanum). Of the three largest species known, M. americanum, M. carcinus, and M. rosenbergii, (Table 2), the latter has become the most favoured for aquaculture. M. rosenbergii is indigenous to the whole of South and South-East Asia, together with northern Australia and the western Pacific islands. Except where otherwise stated, the information provided in this document refers to this species (M. rosenbergii), sometimes known as the giant long-legged Malaysian prawn and later dubbed (in the USA) the “Hawaiian prawn” or the “blue lobster”. This species will be referred to in this text from now on simply as ‘prawns’.
Though broodstock can be obtained from natural waters, in practice most hatcheries rely on the capture of berried (egg carrying) females from prawn farm ponds. The natural seasonality of broodstock which is associated with the onset of the rainy season, is therefore not a problem for culture. Breeding prawns in captivity is relatively simple but it is not now normal practice for hatcheries to maintain broodstock of prawns because of the ready availability of berried females. Only hatcheries in countries where the species has been introduced are likely to hold a captive broodstock and then only until adult female progeny of the first parents become available from ponds.
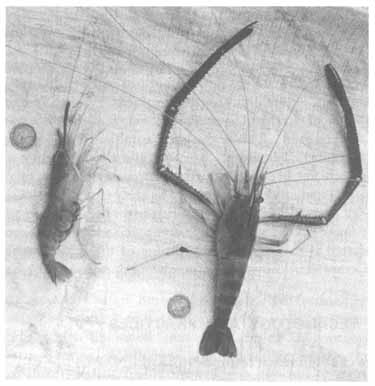
A large male prawn with a berried female.

Freshwater prawn farming provides a profitable alternative to rice in irrigated areas.
Table 2. Summary of data on commercially important freshwater prawns
(Macrobrachium spp.)1
| Species | Geographical Habitat | Local Habitat | Maximum Recorded Size (total length in mm) | ||
| Salinity2 | Bottom3 | Male | Female | ||
| M. acanthurus | Atlantic America: N. Carolina to S. Brazil plus the West Indies | FW + BW | M | 166 | 110 |
| M. amazonicum | Atlantic America: Venezuela to Paraguay | FW | 150 | ||
| M. americanum | Pacific America: Baja California to N. Peru plus Cocos and Galápagos Islands | FW (larval BW) | S+R | 250 | 193 |
| M. carcinus | Atlantic America: Florida to S.E. Brazil | FW (larval BW+SW) | S+R | 233 | 170 |
| M. dayanum | Indo-West Pacific: Bangladesh, India, Pakistan | FW | 92 | 84 | |
| M. lanchesteri | Indo-West Pacific: India, Malaysia, Thailand | FW+BW | 55 | ||
| M. malcolmsonii | Indo-West Pacific: Bangladesh, India, Pakistan | FW+BW | 230 | 200 | |
| M. nipponense | Indo-West Pacific: N. China to Annan, Japan, Taiwan | FW+BW | 86 | 75 | |
| M. rosenbergii | Indo-West Pacific: N.W. India to Vietnam, Philippines, New Guinea and N. Australia | FW+BW+ sometimes SW | M | 320 | 250 |
| M. tenellum | Eastern Pacific: Lower California Mexico to N. Peru | FW+ sometimes BW | R, S + M | 150 | |
| M. vollenhovenii | Eastern Atlantic: W. Africa from Cape Verde Islands and Senegal to S. Angola | FW, BW+ sometimes SW | 182 | ||
2 FW = freshwater
BW = brackishwater
SW = saltwater
3 R = rock or stones
S = sand
M = mud
Mature male prawns are considerably larger than females and the second walking legs are much larger and thicker. In both sexes of Macrobrachium rosenbergii these second periopods are of equal length and are clawed; the predominant colour of the adult animals is blue, sometimes brown. The male ‘head’ (cephalothorax) is larger than the female but the latter has a wider abdomen. In the male the genital pores are between the bases of the fifth periopods while those of the female are at the base of the third periopods. The pleura of the female are longer; this and the width of the abdomen form a brood chamber where eggs are carried until hatching occurs. The egg mass is orange-coloured in the early stages and, as the eggs become eyed, becomes grey-black. Further details of caridean (and penaeid) prawn biology are given by Wickens (1976). The morphology of Macrobrachium rosenbergii is summarized in Figure 1.
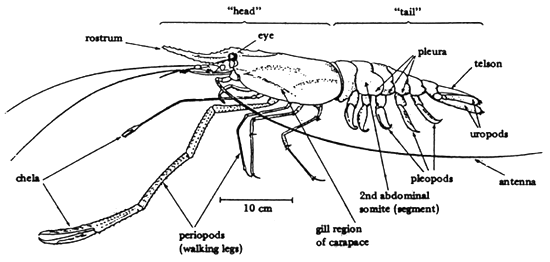
Figure 1. Some anatomical features of the freshwater prawn.
Drawing based on Forster and Wickins (1972)
Source: New and Singholka, 1985
Successful mating takes place between hard-shelled males and soft-shelled ovigerous females which have just completed their pre-mating moult. Copulation results in the deposition of a gelatinous mass of semen between the walking legs of the female and is followed within a few hours by egg laying and exterior fertilization. The eggs are transferred to a ventral brood chamber, held in place by a thin membrane between the pleura and ventilated by vigorous movement of the pleopods. Mating takes place under natural conditions at all times of the year with some environmentally related peaks. Fully mature female M. rosenbergii may lay 80,000– 100,000 eggs per spawning but the first broods are often only 5,000–20,000 eggs. In the laboratory, pre-mate intermoults have been frequently observed to be as short as 23 days and the ovary often ripens while eggs are being carried in the brood chamber. Fecundity of animals maintained in the laboratory is greater than that of animals from ponds.
Large, obviously healthy, well pigmented females are selected to supply prawn eggs for hatcheries. Normally they are caught when the egg mass is brown. After the eggs hatch, the females are discarded, either being returned to ponds or marketed. Broodstock females can be transported in buckets, aerated tanks or, if the rostrum is blunted, in inflated plastic bags, depending on the distance from the ponds to the hatchery. Berried females may be kept in separate tanks, in tanks mounted above hatchery tanks, or in the hatchery tanks themselves. No special care or diet is required since the animals are normally taken from ponds and discarded after the eggs hatch.
About three 10–12 cm (rostrum to telson) females are required to stock each cubic meter (m3) of larval tank water volume; this results in a post-hatching larval stocking density of 30–50 per litre, allowing for physical losses, cannibalism and hatching rate. Each female of this size carries 10,000 to 30,000 eggs. Berried females are quarantined for thirty minutes by immersion in aerated water containing 0.2–0.5 ppm of copper or 15–20 ppm of formalin. During the two or three days between stocking and egg hatching, the adult females are sometimes not fed (to maintain good subsequent larval water quality). However, broodstock can be easily maintained for longer periods on their natural diet (which is omnivorous and includes grain, seeds, algae, small molluscs and crustaceans, and fish flesh) or on ‘artificial’ pelleted feeds such as are used for rearing prawns to market size in ponds.
Hatching occurs naturally under estuarine conditions and, in the hatchery, egg hatchability is greater in brackish water than in fresh water. Berried females may be placed into water ranging from 0‰ to 12‰, after disinfection and without acclimatization, where hatching occurs. In addition to being euryhaline the adult prawns are tolerant of a wide temperature range (18–34°C), but fare best between 28°C and 31°C. Optimum pH is 7.0–8.0. Water should preferably have a dissolved oxygen level at 70 per cent of saturation but prawns can tolerate DO2 levels as low as 1 ppm (Avault, 1987).
Malecha (1983a) argues that the current practise of selecting many (because of their ready availability) small (< 40 g) females from ponds for hatchery use does not take advantage of the natural fecundity of the species; worse, there is no control on the genetic worth of the parents used. Doyle et al., (1983) suggested that simple control of broodstock age could lead to genetic progress, which had been minimal in the history of prawn culture to date. However, control of broodstock age is difficult when ponds are stocked and harvested on a continuous basis. Malecha (1983a) suggested that separate broodstock ponds should be kept, the sex ratio be manipulated to one male to four or five females and that females should be reared to 100 g to increase production of larvae per animal. Possible hereditary traits which could be exploited are discussed later in this paper.
Methods of detection of mature females as early as seven days before the pre-mating moult, based on the external examination of gonadal development and on the reproductive behaviour of dominant males have been developed by Sagi and Ra'anan (1985). These techniques assist in broodstock management for studies on breeding, artificial insemination and genetics. Other techniques have been developed to aid future genetic studies with prawns. These include methods for increasing hatchability and spawning frequency through artificial incubation (Balasundaram and Pandian, 1981; Pandian and Balasundaram, 1982), artificial insemination (Sandifer and Smith, 1979; Chow, 1982) and spermatophore cryo-preservation (Chow et al., 1985). Progeny have resulted from matings of surgically sex-reversed prawns and F2 generations created (Malecha et al., 1987b). Interspecific hybrids have been reported in the literature, including M. asperulum x M. shokitai (Shokita, 1978), whose offspring were sterile, M. rosenbergii x M. malcolmsonii (Sankolli et al., 1982), and M. nipponense and M. formosense (Wickens, 1976).
Egg incubation takes place during attachment to the parent female; females with eyed or nearly-ripe eggs (‘brown-egged females’) are incubated within the larval rearing tanks or in separate tanks. The eggs are slightly elliptical, having a long axis of 0.6–0.7 mm, and become grey-black 2–3 days before hatching. Prawn eggs (M. rosenbergii) hatch within three weeks (usually 19 days) of being laid. Most eggs within one brood hatch within 48 hours, mainly at night, but females are often left in the incubation or larval rearing tanks for 4 days (Malecha, 1983a). Once hatched, larvae are dispersed by rapid movements of the pleopods of the brooding female; these same pleopods are active throughout the incubation period to maintain high dissolved oxygen levels in the water close to the eggs.
The first-stage larvae are about 2 mm long and are planktonic, active tail-first swimmers. There are ten more stages before metamorphosis, which occurs 16–28 days after hatching. Hatching larvae are released into brackish water in the range 11–15 ‰ in Hawaiian hatcheries; 12‰ is normal in Thailand. Optimum water temperatures are 26–31°C while temperatures below 24°C or above 33°C cause retarded development or mortality respectively. Other desirable water quality criteria include pH 7.0–8.5, maximum nitrite (NO2-N) and nitrate (NO3-N) levels of 0.1 ppm and 20 ppm respectively, maximum total hardness of 100 ppm (CaCO3), and low iron and manganese content.
Larval tank size and design is important for ease of maintenance rather than because of biological requirements. Many different systems and frequencies of water exchange are used to keep ammonia levels low. Many Hawaiian hatcheries stock first-stage larvae at 60/litre (although some, who split the stock into two portions to reduce biomass loading as they grow, stock at levels up to 160/litre) (Malecha, 1983a). Thai hatchery management results in a stocking density of 30-50/ litre but aims to achieve 100,000–200,000 postlarvae from each 10 m3 larval rearing tank (10–20/litre). Acceptance of low survival levels is linked to the ready availability of berried females. Hawaiian hatcheries expect 50–70 per cent survival which would result in a postlarval production of 30/litre.
The brackishwater necessary for prawn hatcheries is normally obtained in coastal hatcheries by mixing filtered seawater with well or dechlorinated tap water. Inland hatcheries operate by trucking seawater, brine or rock salt to the site and appropriately diluting this with freshwater (Tunsutapanich, 1981). Toxic levels of ammonia in the larval rearing water are normally avoided by water exchange (up to 50 per cent per day) and sometimes also by the use of biological filters or ‘green water’ (see below). Recirculation systems for prawn hatcheries have also been devised (Singholka and Sukapunt, 1982; Ong, 1983; Chavez Justo and Ramirez Ochoa, 1983). No special photoperiodic routine is necessary for larval culture but the lighting should have the same spectral quality as sunlight. Tanks where the ‘clear water’ technique is used should be 90 per cent covered or housed indoors to prevent the ‘sun-cancer’ effect.
Prawn larvae must eat continuously to survive but do not actively search for food (Moller, 1978). Food density in the water table is therefore critically important and must be maintained regardless of the density of prawn larvae. In commercial practice, feeding is combined either with a ‘green-water’ or a ‘clear-water’ rearing system. In the former case a bloom of phytoplankton (mostly Chlorella spp.) is encouraged by fertilization of the water which is used during part or all of the larval rearing cycle to replace that removed during tank cleaning and water exchange routines. Prawn larvae passively ingest phytoplankton cells but do not digest them. However, the ‘green-water’ technique is claimed to increase post-larval production by 10–20 per cent (Malecha, 1983a) over clear-water systems because of the former's maintenance of good water quality, specifically regarding pH and ammonia levels. Green-water is not essential (New and Singholka, 1985) and few of the hatcheries in Thailand use this system because it is more complex and has an inherent risk of failure (phytoplankton ‘crashes’). Hummel et al., (1987) found that the presence of algal blooms and high pH levels were associated with increased postlarval mortality. In vitro tests showed mass mortalities at pH 9.5 and above. Mortalities were higher in ‘green-water’ than in ‘clear-water’.
A wide variety of live and dead feeds are used in combinations which are different in every prawn hatchery. The feeding regimes used in some Hawaiian and Thai hatcheries are summarized by Malecha (1983a) and New and Singholka (1985). The principal feeds are newly-hatched brine shrimp nauplii (Artemia sp.), fish flesh and egg custard. Brine shrimp nauplii are normally provided on the first day after hatching although most prawn larvae do not feed until the second day; they rely on their embryological food reserves during this time. Artemia nauplii density is not normally allowed to fall below 1/ml and is increased to about 5/ml at feeding times in Thailand (New and Singholka, 1985) but Hawaiian hatcheries tend to use densities of 5–15 nauplii/ml (Malecha, 1983a). Artemia are provided twice a day until about day 5; from then until the prawns metamorphose they are provided only at the evening feeding.
‘Dead’ or prepared feed is also essential for successful prawn larval rearing. Most Hawaiian hatcheries hand feed fish flesh (either fresh tuna or frozen pollack) several times per day (Malecha, 1983a). Cooked egg custard is also used as a larval food but is inadequate as a sole food. In Thailand (New and Singholka, 1985) egg custard is used as a base for a prepared larval feed, which also includes mussel flesh. The feeding of this prepared feed commences on day 3 in increasing weaning quantities together with Artemia nauplii. By day 5 the prepared feed is given 4–5 times during daylight hours, Artemia nauplii being reserved for the final, evening, feed.
Artificial feed particle size varies from 0.3–1.0 mm depending on larval size and feed is kept suspended by vigorous aeration. Recently a number of compounded inert feeds have been introduced for fish and crustacean larvae. Commercial live feed substitutes and enhancers for crustacean larval rearing are being produced by several companies now, including Aquafauna Bio-Marine Inc. (California), Frippak Feeds (England), Artemia Systems (Belgium) and Nippai (Japan). The microencapsulated larval feed pioneered by Jones et al., (1974; 1979) has been further developed and commercially marketed under the trade name Frippak. The value of modifying the higher unsaturated fatty acid (HUFA) content of Artemia nauplii (reared to larger size for feeding late stage prawn larvae) has been demonstrated (Sorgeloos et al., 1986) and a commercial enrichment diet for Artemia is marketed by Artemia Systems. Artificial (i.e., ‘off-the-shelf’) diets have not become standard in Macrobrachium hatcheries, which generally still prefer the use of Artemia nauplii and egg-fish diets prepared on-site, with or without ‘green water’.
Postlarval prawn production rates vary widely, and have been reported as about 10–20 per litre of larval tank water in Thailand (New and Singholka, 1985), 30/litre in Hawaii (Malecha, 1983a), and up to 50/litre using highly intensive, antibiotic-aided techniques (AQUACOP, 1977). The use of antibiotics, and sometimes sulpha drugs, in larval rearing is commonplace though often unpublished. Massive blooms of Zoothamnium, Epistylis and hydroids, which are harmful to the larvae, occur if hatchery hygiene is inadequate or incoming water is poorly treated. Water treatments and tank disinfection include the use of formalin, chlorine, potassium permanganate, malachite green and copper sulphate.
Newly metamorphosed postlarval prawns are about 7 mm long and swim and soon begin to crawl like adult animals. They are tolerant to salinity variance and can be rapidly transferred from the larval brackishwater to completely freshwater. Once about 90 per cent of the larvae have metamorphosed they are acclimatized to freshwater over 2–3 hours, harvested by dip nets or finally at the tank turn-down drain and transferred to holding tanks before distribution to nursery or rearing ponds. In holding tanks densities of up to 5,000 postlarvae/m2 can be successfully maintained for one week or 1,000–2,000/m2 for one month. While in holding tanks prawn postlarvae will quickly accept the same feeds as are used for pond rearing. Floating catfish feeds or expanded pet feeds are sometimes used to provide a feed where most of the animals still live (close to the water surface) during the first few days after metamorphosis.
Postlarvae may be transported short distances (up to one hour's journey) in aerated tanks at 750/litre but inflated plastic bags in insulated containers are necessary for longer journeys. 125–250 postlarvae/litre is optimal. Survival rates during transport are better if the temperature is reduced, the water is slightly brackish and the postlarvae are at least 7 days post-metamorphosis. In shipping experiments, 17 g prawns were successfully shipped at 19–20°C in oxygenated water for 42 hours at 12–15 g/litre (Smith and Wannamaker, 1983). 6 g juveniles could be shipped at 18 g/litre for 24 hours or 9–11 g/litre for 48 hours. In this experiment neither brackish water (2–8 ‰) nor mesh material to increase surface area had any beneficial effect on shipping survival. Adults packed in an unrestricted manner, rather than those immobilized in mesh, had substantially higher survival rates. In Thailand postlarvae are transported in plastic bags at a shipping density of 300–400/litre (Boonyaratpalin and Vorasayan, 1983). If the temperature is low (e.g. at night) transport time can be as long as 24 hours. 7 day-old postlarvae survive shipping better than those only 1 day-old (Harrison and Lutz, 1980); shipping in brackish water (15‰) also improved the survival of postlarvae. Survival rate is more closely related to dissolved oxygen level than to any other water quality parameter (Smith and Wannamaker, 1983). In the latter study, 90 per cent survival of postlarvae was typically obtained if they were shipped at 187–225/litre for up to 24 hours or at 112–187/litre for 24–48 hours. Shipment water was sometimes fresh, sometimes brackish and temperature was 18–23°C. Sometimes tris (hydroxymethyl aminomethane) or other compounds are added to buffer the water and clinoptilolite may be added to freshwater shipments to absorb ammonia.
A cost study of pilot larval prawn hatchery production in Tahiti in 1976 predicted that postlarvae could be produced on a commercial scale at US$ 12/1,000 (AQUACOP, 1979) there and cheaper elsewhere. The initial growth of freshwater prawn farming in Thailand and Hawaii was stimulated by the availability of free postlarvae from state hatcheries. Table 3 gives examples of the commercial availability of prawn postlarvae. Nursed juveniles are expensive (US$ 125/1,000) while early postlarvae are one-fifth of that price.
Table 3. Cost of postlarval Macrobrachium rosenbergii raised in commercial hatcheries1
| Date | Hatchery name | Location | Price per 1,000 (US$) | Notes |
| 1980 | Government hatcheries | Thailand | 13 | |
| Private hatcheries | Thailand | 15–20 | Depending on pl. size | |
| 1981 | Aquatic Farms | Hawaii | 12 | |
| 1982 | Various | Hawaii | 16–25 | |
| 1983 | Various | Hawaii | 25 | |
| 1983 | ? | Honduras | 17 | |
| 1984 | Prawns of Hawaii | Hawaii | 17 | |
| 1985 | Aquaculture Production Technology | Israel | 15 | |
| 1985 | Various | Hawaii, Jamaica, | ||
| Honduras and California | 17–30 | |||
| 1986 | Amorient Aquafarm | Hawaii | 25 | |
| 1986 | Medina Fish Farm | Texas | 25 | |
| 1986 | Government hatchery? | India | 25–30 | |
| 1987 | Blue Lobster Farms | California | 25 | Nursed juveniles US$ 125/1,000 |
While many farmers stock postlarval prawns directly into the final rearing ponds from hatchery holding tanks (i.e. 1–4 weeks old), others prefer to use an intermediate, nursery, phase. A 2–3 month nursery phase is used in Honduras (Wulff, 1982a), South Carolina (Liao, et al., 1982) and elsewhere. In Israel, improved management procedures have included stocking 2 g juveniles (Cohen, et al., 1983) into rearing ponds. Smith and Sandifer (1979) projected that 1 ha of nursery ponds, stocked at 1,500 prawns/m2, was sufficient to produce enough small juveniles (0.5 g) to stock 198 ha of grow-out ponds. The use of nursery ponds is particularly useful where climatic conditions prevent continuous culture to market size. Prawn growth and survival in nursery ponds has been shown to be similar whether a 48 per cent protein sinking trout feed or a fertilizer regime (including a 13: 13: 13 inorganic fertilizer and chicken manure) were applied (Mulla and Rouse, 1985).
Further improvements have been made by over-wintering prawns for early stocking in grow-out ponds when the environmental conditions are suitable. Also, artificial habitats have been designed to increase the stocking density feasible in intensive prawn nursery systems (Smith and Sandifer, 1979). Malecha (1983b) describes two-phase (nursery and grow-out), and three- and four-phase systems in commercial use in Hawaii. In the latter two cases ‘grow-out’ ponds are, once or twice respectively, drain harvested and the stock split into two portions for restocking into existing and new ponds before the final grow-out to the harvesting of marketable prawns by selective culling.
Normally the final grow-out stage for producing market-size prawns takes place in earthen ponds but, in Thailand, commercial pen culture in Songkhla Lake has also been practised since 1983 (Kulkeo et al., 1985). By 1985 there were over 38 ha of pens in cultivation. Average production was 1,075 kg/ha. Most Thai prawn production originates from ponds and average production was more than 780 kg/ha in 1986 (Janssen, 1987). In Hawaii, average unit productivity is greater, at 1,449 kg/ha in 1984 (Lam and Wang, 1986) but total national production is low. Competitive advantages for Thailand in a future global prawn market were seen in a comparative study by Shang (1982). Although Hawaiian unit productivity was more than twice as great as that in Thailand, the cost of production per kg of marketable prawns in Thailand was less than half that in Hawaii's intensive systems.
Under tropical conditions the growth rate of individual Macrobrachium rosenbergii can be rapid. Panicker and Kadri (1981a) reported increases of up to 100 g in stagnant earthen ponds in 9 months and of up to 120 g in 7 months in cement ponds supplied with running water in India. Average weights of 142 g were reported after a year's rearing in Bangladeshi ponds with a survival rate of 82 per cent (Khan et al., 1980). Prawns of 43 g or 54 g mean weight were obtained after 120 days under Israeli climatic conditions when 0.5 g juveniles were stocked at 3.5/m2 under monoculture or polyculture (with carp) conditions respectively. Willis and Berrigan (1977) gave experimental data of the average growth rate (to batch harvest) of prawns in earthen ponds at an average water temperature of 27°C (range 20.5–30.5°C). Juveniles stocked at an average weight of 0.8 g reached 43.3 g in 167 days while postlarvae stocked at 0.06 g reached 28.2 g in 170 days. Survival rates were 79 per cent and 88 per cent respectively. Survival rates are usually reckoned to be about 50 per cent in commercial farming practice.
The growth rate of prawns is sexually dimorphous, mature males being considerably larger than females, having longer and thicker second periopods and more substantial cephalothoraxes. Older juveniles and adults of both sexes are typically blue or brownish in colour. Postlarval populations have a homogenous distribution curve at first which changes to a skewed curve because some individuals grow faster than the rest (Cohen et al., 1981). In a mature population three male morphotypes can be distinguished by claw colour, relative size within the population and the ratio of claw length to body length. Early maturing blue claw (BC) males cease to grow and lose their dominance to the rapidly growing orange claw (OC) males which will eventually transform to form new, larger BC males, following a ‘leap frogging’ pattern. This process causes increasing size variation in the male populations (Ra'anan, 1987). Conversely, early maturing females retain their position as the largest female animals. Early maturity of fast growing females coupled with their reduced growth rate results in an increasing size homogeneity in the female population.
When Thai grow-out ponds are stocked with postlarvae (1–4 weeks after metamorphosis) stocking rates vary from 5/m2, for ponds which will be batch harvested within 8 months, to 16–22/m2, for ponds to be managed under a continuous cull-harvesting system (New and Singholka, 1985). Sorting prawns according to size before stocking has not been found to be advantageous by Arieli and Rappaport (1982). Batch harvesting is often practised in Thailand because of locally seasonal water shortages; in temperate countries climate is the major factor affecting the type of pond management chosen. Nearly all Thai prawn farms do not use a continuous water flow-through system (Boonyaratpalin and Vorasayan, 1983). Many use a 70–80 per cent survival nursery phase, stocked at 20–25/m2 for 2.5–3 months. Prawns are then harvested and re-stocked at 3–5/m2 for a 3 month growout phase. Those farmers by passing the nursery phase stock their grow-out ponds at 5–10/m2. In both cases harvesting starts 5½–6 months after initial stocking. Cull-harvesting then takes place every 2–4 weeks and restocking occurs once per year. Only one large farm practices more frequent stocking after periodic harvests. The number stocked is normally twice the number harvested. In Hawaii the environmental conditions are ideal for the continuous stocking/harvesting system; initial stocking rates are around 16/m2 (Malecha, 1983b). Immediate post-stocking mortality, due to poor acclimatization and predation, is often severe. Subsequent stocking strategies in the continuous culture management system are discussed in detail by both New and Singholka (1985) and Malecha (1983b).
Optimal prawn growth does not require isosmotic conditions (Singh, 1980). Postlarval prawns are tolerant of wide ranges of salinity and temperature. Smith et al (1982) reported that salinities up to 10‰ at least gave results as good as completely freshwater, and Popper and Davidson (1982) reported the use of tidal water varying between 12–25‰. Nearly all commercial prawn farming takes place in freshwater however. Temperatures below 14°C or above 35°C are generally lethal and prawns grow best at optimal temperatures of 29–31°C. Akiyama et al (1982) reported marked stunting as a result of rearing temperatures of 18–22°C which predisposed the prawns to idiopathic muscle necrosis. Macrobrachium rosenbergii is said to tolerate pond dissolved oxygen levels down to 1 ppm (Avault, 1987) but levels below 25–30 per cent saturation cause stress (Malecha, 1983b) and pond managers prefer to maintain DO2 levels of 6–8 ppm. Water total hardness of below 100 ppm has been recommended by New and Singholka (1985). Vasquez and Rouse (1987) obtained best growth rates when prawns were reared at moderately high total hardness (112 ppm). Hardness levels of 225 ppm and 450 ppm had a detrimental effect on growth rate, while soft water (20 ppm) did not produce as good a growth rate as moderately hard water. Water hardness of 305–638 ppm CaCO3 has been observed to retard prawn growth and to result in encrustation with Bryozoa sp. and Epistylus sp. (Cripps and Nakamura, 1979). Laboratory experiments, using water of 65–500 ppm CaCO3 hardness confirmed this. Prawns maintained in water of the lowest hardness grew 5 times as fast in weight and 7 times as fast in length than the highest Ca group. pH 7.0–8.5 is reported to be optimal for prawn culture.

Low-lying ponds need long tail pumps for complete draining but intermediate harvesting is conducted by seining
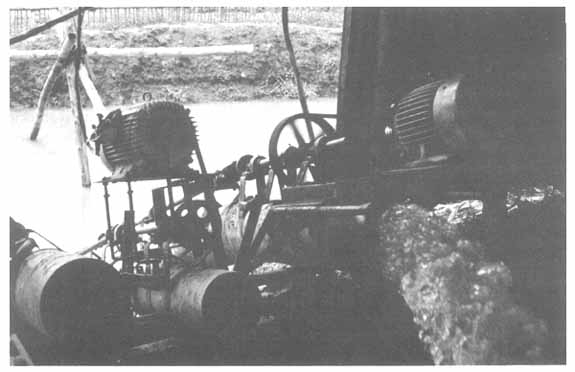
Continuous water intake is essential. In this large farm, two alternative electric pumps are backed up by a third (left) which is powered by a diesel engine (out of sight).
The time taken for prawns to reach marketable size depends not only on their uneven growth rate and on environmental conditions but also on the characteristics of the market being supplied. In continuous culture, ponds are first cull-harvested after 5–7 months and subsequently every 2–4 weeks. In this technique animals of 45 g or more are harvested at each culling. Ponds are re-stocked up to six times a year. Both batch and continuous management systems are capable of producing up to 2,500–4,000 kg/ha/yr in individual ponds although farm and average productivity is much lower. When batch cultured ponds are harvested, a wide range of prawn sizes are obtained which have to be marketed. Some are large ‘bull’ males (about 50 per cent of the males) and some are slower growing males which would have grown on had the ‘bulls’ been culled out. In addition there are females, both egg carrying and not, and soft shelled (newly moulted)prawns. When ponds that have been cull-harvested are finally drain harvested some ‘terminal growth’ prawns are also found. Market value depends on the size and type of prawns harvested (Table 4). The size divergence of males is much wider than of females and the small ‘bachelor’ males are smaller than any of the females, who occupy a size niche intermediary between the ‘bachelor’ and the ‘bull’ males (Fujimura, 1974). The weight of early maturing females (determined by sampling) can be used to predict the final female contribution to pond yield because the final 6 month harvesting average weight of females is similar to that of the earliest maturing females at 3–4 months (Ra'anan, 1987).
The yeild of prawns obtained by seining can be maximized by quite small improvements in husbandry. Wang and Losordo (1985) have shown that cutting the grass on pond banks, which is planted to control erosion but can hamper seining, enabled an increase of 20 per cent in prawns harvested per hectare annually. Yeild increases with increasing prawn stocking density, to an optimum level, but average size is inversely related. In a 140 day experiment, Perry and Tarver (1981) found that postlarval prawns stocked in brackish water at 2.5, 4.9 and 7.4/m2 grew to average weights of 21 g, 17 g and 12 g respectively. Corresponding yields were 408, 619 and 510 kg/ha. Marketable yield was even more markedly favoured by the lowest stocking density tested.
Many commercial prawn farms now employ a two (or more) phase grow-out system, especially in the USA and in ventures in other countries financed or advised by US or Israeli companies. Meanwhile research continues into methods of increasing the revenue from prawn farm ponds including polyculture, grading, monosex culture and feeding strategies.
In cage experiments Sagi et al., (1986) found that an all-male prawn population yield 473 g/m2 compared to 260 g/m2 and 248 g/m2 for mixed and all-female populations respectively. After 115 days 80 per cent of the all-male population was of marketable size, twice the proportion of the other two populations. A comparison of growth rates showed that female growth rate is inhibited in the presence of males but not vice versa. Sagi et al. (1986) expected that commercial yields could be increased by a factor of 1.7 if ponds were stocked entirely with males. Sexing of large juvenile or adult prawns is easy (New and Singholka, 1985) but sexing of juveniles of 1 g and above requires careful external examination; Janssen (1987) was unable to identify males under 3.5 cm total length. In any case segregation of prawns by hand sexing would be commercially impractical. Research into methods of producing all-male populations (perhaps by hormonal administration in feed) is underway (Sagi et al., 1986).
Malecha et al., (1987a) have described a new management system which involves mechanically grading nursed juvenile prawns and stocking them separately and, once the first animals reach harvest size, alternating seine harvests and complete drain downs until the remaining biomass is small enough to consolidate with that of other ponds. The system also involves computer-assisted pond sampling for inventory control and allows predictions on the cost and time necessary to produce an animal of a certain market value to be made. A reduction in the number of ‘pond days’ required and greater predictability of timing and size of harvests is claimed. Investigating the suppression of growth of small prawns caused by the presence of large prawns. Lam and Wang (1987) showed that although harvesting efficiency could be increased by harvesting continuous cultured ponds more frequently, it also increased stress and mortality and adversely affected growth.
Other experiments on the size-grading of juvenile prawns prior to stocking have been carried out recently (Karplus et al., 1986; 1987). The morphotypes designated by Ra'anan (1982)1 were used to examine the effect of such grading. In the second experiment of the series (Karplus et al., 1987) juvenile prawns (ca. 1.1 g) were graded into upper (32 per cent), middle (45 per cent) and lower (23 per cent) fractions. Recombined fractions were used as a control. After 97 days in polyculture with tilapia and common, silver and grass carps, the fractions differed in their male and female morphotypes. SM frequencies were 50 per cent, 32 per cent, and 8 per cent in the lower, medium and upper fractions respectively, while BC showed the reverse trend (3, 10 and 22 per cent). OC followed the same trend, 42 per cent in the lower and 63 per cent in the upper fractions. Among females, V frequencies were 94 per cent, 78 per cent, and 37 per cent in the lower, middle and upper fractions. Mature females showed the reverse trend (3 to 43 per cent for BE and 3 to 20 per cent for OP) from the lower to the upper fraction. Mean weight ranged from 32 g in the upper to 21 g in the lower fraction; since survival rates were similar, yields showed a similar differential. Net income (from prawns alone) was almost nine times greater from the upper than the lower fraction and nearly twice that of the middle fraction. The yield of fish was relatively unaffected. Net income from the reconstituted catch was similar to the middle fraction but much less than the upper fraction. However, since the weighted mean yield of the three fractions is also almost the same as the control, stocking graded weight classes did not increase overall net income. Discarding the lower fraction would waste the nursery investment in these prawns and Karplus et al., (1987) concluded that finding methods of discarding low growth potential prawns sooner by tracing the earliest stage of male morphotype differentiation (embryo, larvae or postlarvae) may preface increased profitability.
Sandifer and Smith (1977) noted that the golden short-claw type of prawns, which was almost exclusively male, was less aggressive and had 5-8 per cent more tail meat than the blue long-claw form. Genetic or environmental manipulation to increase the proportion of the short-claw form might increase the yield of marketable prawn tails. Variability in larval development time, postlarval temperature tolerance and salinity and temperature related growth were detected in stocks derived from different geographic locations by Sarver et al, (1979). Intraspecific hybrids of Macrobrachium rosenbergii races demonstrated improved growth rates (Dobkin and Bailey, 1979). Doyle et al., (1983) indicated that collecting broodstock as early as possible in the production cycle might be a simple change in management which would be effective. The earliest females in a population to mature should be selected as broodstock for a potential improvement of the genetic base because these animals are also the fastest growing females (Ra'anan, 1987). The bull-runt phenomenon in male prawns seems to be non-genetic in the sense that it may be controlled by intrapopulation environmental-social factors (Malecha et al., 1984). Male size may be a sex-linked secondary sexual characteristic under natural sexual selection, having high genetic fitness and little genetic variance.
Electrophoretic studies of gene-enzyme variation undertaken by Hedgecock et al., (1979) in natural populations of Macrobrachium rosenbergii from 11 locations from Sri Lanka to New Guinea, Palau and the Hawaiian cultured stocks, supported the hypothesis that the species has undergone considerable racial divergence and that natural populations represent a diverse genetic resource for prawn aquaculture. However Malecha et al., (1980) found no useful allometric variation in these genetic stocks and their hybrids. The ‘Anuenue’ (Hawaiian) stock had diverged little from its wild relatives. Ra'anan and Cohen (1984), comparing communally reared populations with single raised prawns, found that two principal size classes, ‘jumpers’ (exceptionally fast growing individuals) and ‘laggards’ (severely growth repressed prawns) appeared only in communal groups of juvenile prawns. They concluded that the variance of attained sizes of juveniles in a Macrobrachium rosenbergii population was more affected by interactions within the group than by genetic differences in the growth potential of individuals.
Sarver et al., (1982), reported that pond productivity in Hawaiian earthen ponds increases with time of use, at least up to 3 years, partly due to the development of a rich bottom layer and a grassy bank (providing increased shelter and nutrition). Yields of 1,790, 2,580 and over 2,900 kg/ha, for the first, second and third year of operation respectively, were quoted.
Results from experiments involving the use of substrates are somewhat conflicting. The introduction of artificial submerged substrates (made from plastic net and pipes), which added 10–50 m2 of habitat surface to each 200 m2 pond, experimentally increased marketable yield from 2,500 kg/ha to 2,850 kg/ha in a six month crop (Cohen et al, 1983). It also increased the final average marketable weight from 35.8 g to 40.3 g and the efficiency of an early selective harvest. Though total marketable yield was increased by 14 per cent, substrates did not affect survival or the proportion of the total harvest that was marketable. Increased surface area was made available to nursery reared 1 g prawns stocked into outdoor concrete tanks in an unreplicated trial by Sandifer et al., (1982). Mean size at harvest was greater at a lower stocking density and, although total yield was less, crop value was significantly higher. In temperate zones the economic feasibility of prawn culture depends on the ability to bring the greatest number of prawns to minimum marketable size (25 g) in 6–7 months. Net substrates were found by Ra'anan et al., (1984) to markedly increase both overall survival and the percentage of marketable prawns in a replicated experiment where 0.5 g nursed juveniles were stocked at 15/m2 and reared for 184 days in 200 m2 ponds. Paddlewheel aeration was also used in this intensive system of prawn culture to maintain high DO2 levels. Mulla and Rouse (1985) showed that while the use of additional substrate (Spanish moss/ plastic coated wire mesh) gave some improvements in average size and size distribution of juveniles produced from nursery ponds, overall production was not improved. Control of predacious insects with a pesticide pre-treatment retarded natural food organisms too.
In addition to the nursery phase systems described earlier, in temperate zone countries such as Israel over-wintering postlarvae in heated facilities has been tried as a method to more fully utilize the summer growing season in ponds (Ra'anan and Cohen, 1982).
Comparing cage culture with ditch and earthen pond culture of prawns, Menasveta and Piyatiratitivokul (1982) found that survival was highest in cages, but productivity was the least. Pilot-scale cage culture of prawns in India showed that this technique had economic potential (Panicker and Kadri, 1981b), however. Prawns are commercially farmed in pens in Thailand.
Research or pilot-scale development studies on rearing prawns in thermal effluents or geothermal water have also been conducted, notably in the USA, Italy and the USSR. Survival and growth in thermal water is profoundly influenced by changes in temperature and dissolved oxygen (Farmanfarmaian and Moore, 1978). Dissolved oxygen is not a critical factor at low temperatures but low DO2 reduces upper temperature tolerance. In this study growth nearly doubled for every 5°C increase in the 20–30°C range, with no appreciable change in FCR. In Nevada (Aquaculture Digest, 6.3.23.) experiments with thermal effluent culture of prawns indicated that cannibalism and predation were problems and temperatures could not be maintained at optimal levels when ambient air temperatures were less than zero. A venture analysis conducted by Guerra et al., (1979), on a proposed commercial waste heat aquaculture facility to culture two species in a diseasonal sequence, concluded that Macrobrachium rosenbergii did not meet the profitability criteria for high density culture. However, the technical feasibility of rearing prawns in outdoor raceways supplied by geothermal water in ambient air temperatures as low as -20°C in the winter was demonstrated by Johnson (1979). In the USSR, studies have shown that Macrobrachium rosenbergii can be raised up to 60 g on natural food alone in cages in a water cooling reservoir or in earthen ponds at water temperatures of 25–33°C. Productivity was extrapolated at 1,000 kg/ha (Khmeleva et al., 1985). In 1978 the US Department of Energy gave a substantial grant to a private company in California which had initiated a geothermal prawn farm in 1976 based on artesian wells. No information was available (Aquaculture Digest, 8.2.3) on productivity but a financial analysis indicated that 50 acres was the minimum farm size potentially profitable. A summary of the possibilities for geothermal prawn culture was prepared by Hayes and Johnson (1980).
Prawns have shown to be a suitable candidate for commercial polyculture. They have been reared with many species of fish including mullet (using Macrobrachium acanthurus) (Martinez-Silva et al., 1977) in Colombia, grass, silver and bighead carp in Thailand (Tunsutapanich et al., 1982), silver carp in Hawaii (Weisburd et al., 1987) tilapia in the USA (Stickney, 1980; Valentin, 1988), Chinese carp (Fitzgerald and Nelson, 1979, and Buck et al., 1979; 1981), bighead and grass carp in Taiwan (Liao and Liao, 1982), Golden shiners, Notemigonus crysoleucas in Louisiana (Perry and Tarver, 1987), and channel catfish in Louisiana (Lamon and Avault, 1987) and Mississippi (Heinen et al., 1987). In the polyculture of monosex Tilapia aurea (Brick and Stickney, 1979) stocked at 2,000/ha and Macrobrachium rosenbergii at 11,000/ha compared to prawns alone at the same stocking rate, survival of both fish and shrimp was excellent over a 4½ month period and the growth rate of prawns was not depressed much by the presence of fish. In behavioural experiments with prawns, Tilapia mossambica, and the red swamp crawfish (Procambarus clarkii), Martino and Wilson (1986) found that tilapia had no apparent negative impact on survival or growth of the crustaceans. Adult prawns were, however, predacious on tilapia fry; crawfish, though aggressive, were unable to capture the fry. However, Rouse et al., (1987) found that tilapia reproduction had an adverse effect on prawn production. Rouse and Stickney (1982) concluded that prawn-tilapia polyculture had a higher net return than prawn monoculture. In polyculture experiments with common carp, tilapias and various Chinese carps, at various fish and prawn stocking densities, Wohlfarth et al., (1985) showed that the growth and survival of fish and prawns was independent. Prawns were influenced only by their stocking rate which correlated positively with yield and negatively with individual growth. Neither the species of polycultured fish used, fish stocking rate, or the differences in feeding and manuring regimes used influenced prawn production. Cohen and Ra'anan (1983) had reported that carp and tilapia growth was strongly affected by the number of tilapia stocked and by the feeding-manuring strategy used in polyculture with prawns. Costa-Pierce et al., (1985) found that biological control of phytoplankton dynamics and maintenance of DO2 concentrations at high phytoplankton standing crops by Chinese carps appears to be a viable method of water quality control in semi-intensive prawn aquaculture. Zachritz (1986) has described a two-stage polyculture pilot plant system, using water hyacinth (Eichornia crassipes) in the first stage and a combination of Azolla and Macrobrachium rosenbergii in the second stage to provide tertiary treatment to municipal wastewater. Polyculture of microphagous tilapia species combined with bottom feeding prawns is proving very productive. Little and Muir (1987) postulate that this species combination, which is non-conflicting in terms of feed and living requirements, would be well suited to a ‘Taiwanese’ system for intensive tilapia production.
Huner et al., (1983a) reported that crayfish (Procambarus spp.) were harvested in ponds used to culture prawns or channel catfish (Ictalurus punctatus) in Louisiana. Cohen (1984) found that a marginal crop (146 kg/ha) of prawns could be raised with channel catfish in Louisiana. This did not depress catfish yields and increased net income significantly. However, Huner et al., (1983b) had reported lowered survival and growth rates for prawns in ponds also stocked with channel catfish fry and in which resident crayfish (Procambarus spp.) were harvested. Prawns need to be at least 1 g in size before being stocked in catfish ponds to prevent excessive predation by the fish fry. This would necessitate on-site nursery production of prawn juveniles for the commercial success of such a polyculture system (Personal communication from Dale Sarver, 1984 quoted in Aquaculture Digest, 9.5.37.). In a twelve month pilot study utilizing channel catfish/prawn polyculture with rotation of red swamp crayfish (Procambarus clarkii) and rice (Oryza sativa), Cange (1984) produced 285 kg/ha of 41 g average weight prawns (stocked at 0.25 g; 7,400/ha) after 122 days. Following re-flooding the pond yielded 1,490 kg/ha of 16 g crayfish in 63 trapping days. Net profit was US$ 1,092/ha with an annual return rate of 23.5 per cent. Rice was not harvested in this experiment. Macrobrachium rosenbergii has been cultured in rice fields in Puerto Rice (Cepeda, 1982) without effect on rice production but prawn production was low (5 kg/ha) due to an inadequate harvesting technique and problems associated with rice row alignment. Some Japanese rice farmers also produce freshwater prawns (Anonymous, 1980b).
Tilapia have also been grown in cages in prawn ponds (Aquaculture Digest, 7.8.1.) and in reservoirs. Stocking of Macrobrachium rosenbergii in the largest Brazilian reservoir (Sobradinho) began in the late 1970s (Paiva and Gurgel, 1978). Stocking Macrobrachium rosenbergii into natural water bodies in Thailand has been quite effective as a source of food and revenue for local fishermen (New and Singholka, 1985).
Considerable research has been conducted into the feasibility of intensively culturing Macrobrachium rosenbergii in re-circulation systems, to economise the utilization of water and heat, in countries in the temperate zone. This research has included that sponsored by government (Forster and Wickens, 1972; Sandifer and Smith, 1975; Wickens and Beard, 1978) and by commercial companies in England and California (M.B. New, pers. comm. 1987) and in Ohio (R. Brick, pers. comm. 1986). Commercial-scale prawn aquaculture in unfavourable climatic zones, close to potential markets, was the common goal of this research. The goal has not yet been achieved, due to the as-yet unsolved problems involved in maximizing the stocking density of a substrate-dwelling, territorial species and because of the high cost of heat. The development of intensive prawn farming techniques is said to be a requisite for the use of sites with limited water availability, such as a Saudi Arabian oasis (Howlader and Turjoman, 1984). Sandifer et al., (1982), reporting experiments in large concrete tanks (173 m2) with a recirculation system stocked with 1–1.3 g animals at densities of 32–83/m2, projected that production levels as high as 10 mt/ha/yr could be achieved. However, commercial reality of this production magnitude has yet to be achieved. Up to 1980, due to the low cost and easy repeatability of experimentation, most prawn culture research had concentrated on the larval stages. A plea made by New (1982) for more attention to be paid to grow-out aspects seems to have been heeded in the last 5 years. Many papers have been published in that period on nursery production, grow-out management, nutrition and harvesting.
Besides its realized and potential value as an aquatic farmed species, Macrobrachium rosenbergii also has potential in disease control. Lee et al., (1982) showed that juvenile prawns would prey upon the two major South American species of schistosome vector snails (Biomphalaria glabrata and B. tenagophila). This could be of use in the control of schistosomiasis in Africa and S. America especially if Macrobrachium rosenbergii were polycultured with Tilapia sp.
Semi-intensive or intensive prawn farming depends on supplementary feeding. Macrobrachium spp. are omnivorous in nature. This field observation has been confirmed by enzyme studies (Lee et al., 1980). Their diet includes aquatic insects and larvae, algae, nuts, grains, seeds, fruits, small molluscs and crustaceans, fish flesh and offal of fish and other animals. They may also be cannibalistic. In farming practice, supplementary feeds may consist of individual animal or vegetable ingredients, farm-made mixed feeds, or feeds compounded by manufacturers specifically for prawns or for other farmed aquatic or terrestrial animals (New and Singholka, 1985).
The known nutritional requirements of shrimp and prawns have been reviewed in a number of papers in the past twelve years (New, 1976, 1980b, 1980c; Biddle, 1977, Forster, 1976, Sick and Millikin, 1983, Corbin et al., 1983). Recently, a practical manual on the preparation and feeding of diets for prawns (and fish) has been published (New, 1987), in which some examples of formulations for compound prawn diets were provided.
In ponds receiving either high levels of chicken manure or of fish mealenriched pellets stocked with prawns, common carp, tilapia hybrids and silver carp, Δ C studies showed that Macrobrachium rosenbergii depended on natural feeds from the pond bottom and banks (Schroeder, 1983). This was independent of the presence or absence of feed pellets. This confirmed the findings of stomach analyses conducted by Weidenbach (1982). The latter author also found, however, that prawns additionally ingest pelleted feed when available (in monoculture, for example) and compensate for the non-availability of pellets by increased consumption of natural organisms. Fertilization is an alternative to supplementary feeding in semi-intensive prawn culture. Fair and Fortner (1981) found that ‘formula feeds’ contributed markedly to increases in prawn growth. Prawns fed pelleted feeds had twice the growth rate of prawns fed pulverized, relatively unavailable feed, and three times that of those reliant on the natural productivity induced by manuring. Prawns in ponds which were not fed or manured did not grow.
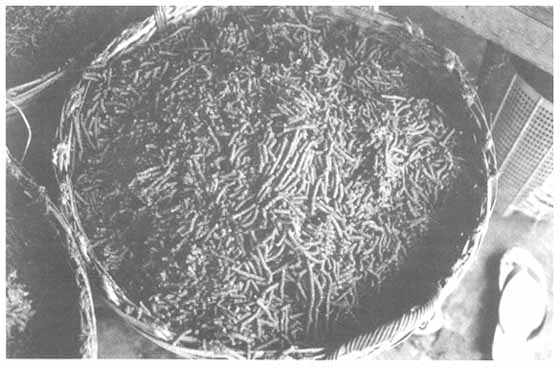
Sun-dried farm-made freshwater prawn feed. Methods of feed manufacture are described by New (1987).

A worker cleans the mincer die-plate used for moist extrusion prior to sun-drying.
Compound rations for prawns are generally cheaper than those designed for marine shrimp because the former appear to require less protein. Many commercial farms use chicken starter feeds for prawn culture. Some farms apply the feed used in daily amounts, calculated as a proportion of the biomass of prawns in the pond. Others, knowing that the feed presented also acts as a fertilizer in increasing the natural productivity of the pond, rely on water turbidity and colour as a means of determining feeding rate (New and Singholka, 1985). Feeding normally takes place once per day, in the late afternoon, but multiple feeding may have advantages (Corbin et al., 1983). Food consumption by prawns was found to be greater when feed was presented twice, rather than once or thrice, daily (Taechenuruk and Stickney, 1982). However, Mensi and Heinen (1988) found that growth rate, survival, and yield were best when postlarvae (up to 0.5 g) were fed once, rather than twice or thrice per day with a 50 per cent protein trout feed. Food conversion ratios (FCR) of 1.8–3.0:1 may be expected for dry diets while moist diets made on the farm and containing waste prawn heads, trash fish, etc. would have an FCR of 3–5:1.
The crude protein level of commercial feeds being applied to prawn farming in Hawaii (Corbin et al., 1983) varied from 23.8–38.5 per cent; lower levels may be satisfactory. Clifford and Brick (1979) concluded that optimum conditions for prawn growth were achieved with a 25 per cent dietary protein level, and a lipid: carbohydrate ratio of 1:4. Studies on prawn growth in concrete ponds (Boonyaratpalin and New, 1980), asbestos asphalt or earthen bottom ponds (Bartlett and Enkerlin, 1983) and aquaria (Antiporda, 1986), indicate favourable results at dietary crude protein levels as low as 14 per cent. Stahl (1979) had postulated that the earthen substrate in ponds appeared to supply a major growth factor lacking in the feeds applied in a simulated pond experiment. However, studies using purified crab protein (d' Abramo and Reed, 1988) and, in aquaria (Freuchtenicht et al., 1988) indicate optimum dietary protein levels of 33–35 per cent and 30 per cent respectively. In fact, the optimum dietary requirement must depend upon the other sources of nutrients available. The quantitative amino acid requirements of prawns have not yet been defined, but growth rate improvements due to amino acid supplementation of compound shrimp feeds have been demonstrated (Farmanfarmaian and Lauterio, 1980). Newman et al., (1982) proposed that the common occurrence of food regurgitation by prawns might have energetic benefits by decreasing the requirement for energy to transport unassimilable material through the entire digestive system.
Studies have been conducted on the suitability of various ingredients for use in prawn feeds. These include corn (maize) silage (Moore and Stanley, 1982) and fresh leaves (Ailanthus altissima and Malva parviflora). The latter (Harpaz and Schmalback, 1986) resulted in the elimination of moult-death syndrome and a reduction in black spot disease incidence, also increasing moulting rate and average weight, when supplementing a dry artifical diet. Nutritional deficiencies tend to result in failure to moult; moulting is the time when prawns experience the greatest physiological stress. Moist pressed brewers grains were found a useful food source for prawns when applied nine times during a 3.5–4.0 month trial in Illinois (Kohler and Kreuger, 1985). Ashmore et al., (1985) found that at a 30 per cent dietary protein level, barley seems to be more efficiently used than wheat. The lower gelatinization temperature of barley compared to wheat was thought to be the cause of this effect. Wheat provided significantly less digestible energy than maize, milo or barley. Commercial farmers are often prejudiced against the inclusion of maize in shrimp and prawn feeds, believing it to be an unattractive or indigestible ingredient. However, this may be due to the twin facts that the maize has not been ground finely enough and that yellow maize is an easily visible dietary component if any of the total feed remains unconsumed. Shrimp and prawns appear to be able to utilize complex carbohydrates better than simple ones like glucose (New, 1976). Cellulases (Noborikawa, 1978) and chitinases are available to prawns and carbohydrate can be used to reduce the optimum dietary protein level by ‘sparing’. Fair et al., (1980) showed that dietary fibre at levels up to 30 per cent did not suppress the growth of prawns fed isonitrogenous diets. These authors postulated that fibre might contribute to cost effective prawn feeds.
In polyculture with Tilapia spp., prawn production was achieved through the application of swine manure as an alternative to a pelleted feed (Teighert-Coddington et al., 1987). The low level manure application, 17 kg/ha/day (dry matter), gave significantly better prawn production than the high level (51 kg/ha/ day) and was similar to that achieved through the use of pelleted feed. Survival over 105 days was three times higher when low level manure application was used. Lilystrom and Romaire (1987) showed that prawns in polyculture with channel catfish depended mainly on seston and macrophytes for growth. Using stable carbon isotope ratios (Δ C) these authors found that 68–99 per cent of catfish growth originated from the formulated feed. Stomach content analyses confirmed this. Prawns increased their ingestion of macrophytes and catfish feed as they grew, consuming less seston.
Recent studies by Heinen (1988) on Macrobrachium rosenbergii reared in individual cages on purified diets have thrown some light on the requirements of this species for vitamins and trace elements. Deletion of a mixture of fat-soluble vitamins did not significantly affect growth rates and growth rate reduction when a trace mineral mix was omitted from the diet was small. Omission of a water-soluble vitamin mix caused great mortality however and when each of ten water-soluble vitamins were omitted, lack of vitamin C proved to be the cause. One of the effects of dietary Vitamin C deficiency was failure of the animals to moult. Significant growth rate reduction occurred when pyridoxine was omitted from the diet but deletion of riboflavin significantly increased growth rates, indicating that it is possible to have a deleteriously high level of dietary vitamin B2.
Prolonged water stability was originally considered by many authors to be of primary importance for formulated feeds for all cultured crustacea (New, 1976, 1980b). Recently, however, Bordner et al., (1986), describing purified and unrefined diets developed for the study of lobster nutrition, have thrown doubts on this. Attractants have shown to be of value to crustacean diets (Meyers, 1987). The broiler chicken feed frequently used in commercial prawn farming sometimes elicits an ‘uninterested’ response from the prawns. Costa-Pierce and Laws (1985) found that trimethylammonium hydrochloride (TMAH) increased pellet ingestion by starved prawns by 30 per cent and by previously fed prawns by 38 per cent under aquarium conditions. Coprophagy is common in fish (New, 1987) and crustacea. TMAH imparts a distinctive faecal odour to formulated feed. In common with other crustaceans, Macrobrachium rosenbergii is attracted by the presence of the chemoattractants taurine, betaine, glycine and proline (Harpaz et al., 1987). Prawns were found to be responsive throughout the whole of the moult cycle, although the level of responsiveness varied, apart from the brief duration of ecdysis itself. Colour too has a role to play in feed acceptability. Meyers and Hagood (1984) reported that light coloured flaked diets were more readily taken by Macrobrachium rosenbergii larvae than dark ones.
New (1976) criticized the lack of standardization in experimental design, culture conditions, and analytical techniques which limited the value of published information on shrimp and prawn nutrition and made comparisons of the results from different laboratories difficult or impossible. In response to these criticisms, the World Mariculture Society (now re-named the World Aquaculture Society) established a Nutrition Task Force in 1977 (Castell et al., 1981) to propose guidelines for standardizing aquaculture nutrition research methodology. One of the results was the development of feed formulations for use as standard reference diets for crustacean nutritional research (Castell et al., 1985). These are now in use in many laboratories involved in such studies (Castell and Kean, 1986).
The problems of prawn culture, as of other forms of aquaculture, include predation from other aquatic species, birds, snakes and humans (theft). Methods of decreasing the losses caused by predation are summarized by New and Singholka (1985); similarly, the maintenance of good water quality and rooted weed prevention are discussed.
Disease studies on freshwater prawns were reviewed by Johnson (1982). Besides water quality deficiencies and predation, prawn deaths are caused by epibionts, such as Zoothamnium and Epistylis, fungal diseases (though less common than in marine shrimp), stress and necrotic symptoms, bacterial problems following injury to the exoskeleton and moult arrest due to poor environmental conditions or nutritional deficiencies. Vitamin C deficiency has been shown to cause mortalities due to moult failure, together with sub-cuticular lesions and blotches in surviving prawns (Heinen, 1988). Darkening of the gill cavity was considered by Johnson (1982) to be due to precipitating chemicals and nitrogenous waste products. Disease management has included salinity modification for fungal problems, the use of antibacterials, improved tank cleaning and water exchange for epibionts, mechanical water filtration, biofiltration and ultraviolet light.
Moult failures increased and moulting frequency decreased in postlarval prawns exposed to mirex at the higher levels tested, which were 0.1–100 μg/1 (Summer and Eversole, 1978). The LC50 value for prawns exposed to mirex for 24 hours was 104 μg/1. Direct damage or adverse effects to prawns were not observed to be caused by the eight species of ectocommensals noted on internal and external body surfaces of larvae or adults (Hall, 1977). These included Vorticella sp. and Zoothamnium sp. Formalin, at 20 ppm, was found to give complete control of Zoothamnium in larval Macrobrachium acanthurus (Roegge, et al., 1977). Black burn spot exoskeletal lesions of bacterial aetiology, a frequent feature of prawns reared in intensive culture, have been treated by oxolinic acid (El-Gamal et al., 1986). A natural bacterial flora which included Vibrio anguillarum and the protozoans Epistylis and Zoothamnium has been detected in larval prawns which frequently exhibit low survival rates in the early stages, but was not found in the Artemia nauplii fed to them (Colorni, 1985). Vibrios were found in brackishwater used to culture prawn larvae but not in the water from the well used to dilute sea water for this purpose (Fujioka and Greca, 1984). Vibrios were also detected in freshwater grow-out ponds.
Certain types of muscle lesion are good indicators of postlarval fitness; mortalities in the first 4 days after stocking into ponds vary from 40–87 per cent (Sarver et al., 1982). Sometimes these losses are caused by exposure to pond water of 19°C or less, or a pH of 9.5 or more. This mortality was shown to be affected by genetic background and larval history as well as recipient pond water quality. Seine harvesting used during the culling operations in a multiple stocking management system also affects stocking survival and subsequent yield. Idiopathic muscle necrosis (muscle opacity or necrosis) is thought to be caused by sub-optimal conditions (Akiyama et al., 1982) which cause marked stunting. Mid-cycle disease (MCD), a toxic period of unknown aetiology beginning on the tenth day of larval prawn culture, resulting in survival rates of 5–10 per cent instead of the normal 50 per cent to metamorphosis, has been described by Akita et al., (1981). Haemocytic enteritis (HE), a disease which affects prawns as well as marine shrimp is caused by blue-green algae (Lightner, 1982).
Like fish, juvenile prawns are susceptible to hydrogen sulphide toxicity originating from the decomposition of food and organic matter. LC50 levels for Macrobrachium rosenbergii are lower than for walking catfish (Clarias batrachus) but similar to several other species of fish (Jayamanne, 1986b).
Blooms of algae result in ecological imbalance in prawn ponds, causing oxygen deficits at night while alive and toxic ammonia levels following collapse of the bloom. Chemicals produced by the phytoplankton may also affect flavour. Blue-green algal blooms are a particular problem due to their indigestibility by prawns. Typical oxygen depletion problems associated with blue-green algal blooms are described by Jayamanne (1986a) in an experiment involving the use of paddlewheels in prawn monoculture. Stocking small numbers of ‘sanitary’ planktivorous fish, such as silver, bighead, and common carp, and tilapia, is suggested by Harimurti (1986) as a means of controlling algal bloom to maximise the profitability of prawn culture. Dense phytoplanktonic blooms can increase pond pH above 10.5 (Avault, 1979). Administering the algicide Clarosan was found to lower pH levels rapidly and, at 0.02 ppm, was not deleterious to prawns.
The toxicity of mercury to Macrobrachium rosenbergii abruptly changes at the fifth larval stage (Piyan et al., 1985). The threshold lethal concentration for stage 1 larvae was 0.041 ppm Hg while that for postlarval prawns was 0.325 ppm Hg.