Productivity of broodfish and seasonality of their production depends very much on the broodfish management methods.
Though the African magur population can be maintained in extremely high density, in areas where adequate protein-rich complete food is not available, the best way is to follow the semi-intensive culture systeme. Like other fish species for semi-intensive broodfish management dens stocking is not suggested. The biomass of fish should not exceed 50–70 % of pond carrying capacity. This biomass will not exhaust the population of different fish feed organisms. Viveen et al. (1986) suggested 1 broodfish/m2 stocking density for semi-intensive management. According to his suggestion broodfish should be fed with artificial food containing 35–40% digestible protein at the daily rate of 1 % body weight.
Considering limited availability of well balanced feed in Bangladesh, the suggested stocking density is approximately 300 kg/bigha of ripe broodfish or maturing broodfish. In addition to magur, 150–200 kg silver carp broodfish should be maintained in the same pond to control water quality.
To avoid wild spawning in pond environment the two sexes are suggested to be maintained in separate ponds during breeding season. Approximately 70 male fish are required for fertilization of eggs stripped from 100 females of same age group.
Feeding of broodfish at the suggested stocking density is essential. Development of fish depends mainly on animal protein supply. The best course is to feed the broodfish with food containing high percentage of animal protein, especially to facilitate the multicycle reproduction of fish within same season.
Considering the lack of animal protein in Bangladesh and the fact that the animal blood is held as a slaughterhouse waste here, the cheapest way might be to feed the fish with similar food than that was developed for carp fingerling production by FAO Project BGD/87/045 (Feed type I) (Peteri and Nandi, 1990). If no blood is available, only then the expensive fish meal be used (Feed type II). The suggested ratio of feed ingredients in broodfish feed are as follows:
| Feed type I. | Animal blood 40% |
| Mustard oil cake 20%* | |
| Wheat bran 40% | |
| Feed type II. | Fish meal 20% |
| Mustard oil cake 25 % | |
| Wheat bran 55% |
(*: Mustard oil cake is mixed in feed in dry powdered condition.)
Based on protein content of the above mentioned components (See Table 6), these feeds contain approximately 36% protein, out of which ⅓ is animal origin.
Table 6 Chemical composition of food ingredients (expressed in %) available in Bangladesh (After Ghol, 1981.)
| Food ingredients | DM | CP | EE | NFE | CF | A |
| Mustard oil cake | 90 | 39 | 11 | 28 | 12 | 10 |
| Rice bran | 92 | 10 | 10 | 46 | 19 | 14 |
| Rice husk | 92 | 4 | 1 | 29 | 44 | 22 |
| Wheat bran | 90 | 16 | 4 | 63 | 10 | 7 |
| Fish meal | 94 | 66 | 4 | - | - | 30 |
| Cattle blood | 20 | 95.7 | 0.2 | 4.1 | ||
| Duck weed | 7 | 35 | 5 | 41 | 9 | 10 |
DM - Dry matter
CP - Crude protein
EE - Ether extract
NFE - Nitrogen free extract
CF - Crude fibre
A - Ash
It is suggested that the feeds be supplied in wet condition. Considering the high wet content of blood, feed type I becomes dough type after mixing the feed ingredients. For proper mixing of feed type II, water should be added to dry components at ⅓ quantity of total volume of feed.
The best method of feeding is to feed the broodfish on feeding trays. For 300 kg broodfish in one bigha pond, two feeding trays are suggested. Using these feeding trays the daily feed consumption can be regulated easily. Recommended rations for mature broodfish and juvenile broodfish at different temperature are given in Table 7.
Table 7 Recommended feed rations for mature and juvenile broodfish at different water temperature
| Water temperature | Mature broodfish | Juvenile broodfish |
| < 20°C | 2% | 4% |
| 20–25°C | 5% | 10% |
| < 25°C | 8% | 12% |
Some authors (Viveen et al., 1986; Janssen, 1987) have suggested stocking of tilapia in the broodfish pond for improving the animal protein supply of magur broodfish. By Kutty's experience (personal information), the African catfish did not consume the sharp-fin tilapia fry voraciously unless the population was underfed. Consequently, the tilapias could be competitors for food, not as the protein source for broodfish especially if the tilapia biomass is high. (Similar phenomenon was observed in Bangladesh fish pond where the tilapia and magur were stocked for market size fish production.) However Kutty observed that very low density of catfish in tilapia ponds yielded better results by reduction of tilapia through catfish predation.
For breeding purpose, the male fish is killed for obtaining sperm. Consequently the male broodfish are eliminated soon. So yearly recruitment of new fish is necessary from market size fish populations. A special method of operation of male fish was developed locally to retain the male population which reduced the required recruitment rate. The female broodfish should be changed in every third or fourth year to avoid the difficulty of handling of very big females. The stock may be exchanged to avoid inbreeding problems also.
Presumable only few farms will be established to produce the only magur in Bangladesh. But the present government and private farms may produce 1 or 2 lakh of African magur fingerling in addition to fingerlings of other fish species. For production of such a number of fingerlings, 10–20 female and 15 male broodfish are sufficient and no separate African magur broodfish ponds will be required in majority of farms. These fish can be stocked together with the broodfish of other species. Advantage of co-stocking of silver carp in magur pond has already mentioned earlier. However presence of other fish species may hamper food supply of magur, because carps will eat significant portion of expensive magur food. It is recommended to avoid stocking of magur broodfish in ponds where market size African catfish are produced.
The best pond for magur broodfish is the small size fish ponds with plain bottom. It will be easier to harvest the broodfish from such pond without dewatering. As African magur can escape from the pond using first rays of pectoral fins as leg, it is advisable to select such broodfish ponds which have steep slope of dykes.
The broodfish, like all other age groups of African catfish after development of accessory breathing organ, are strongly resistant to diseases when food supply is sufficient. But sometimes a special disease known as “crack head disease” and some bacterial diseases can break out when the organic load of the environment is very high. Partial exchange of water and/or light liming are the best remedies for these problems.
Strong bacterial infection can break out in cold season in stressed populations after transportation, or after sampling. Antibiotics (Terramycin, Furazolidone or Chloramphenicol) mixed in fish food at the rate of 50 mg/kg daily and fed for ten days usually can control the infection. It is advisable to decrease the daily food quantity during treatment and mix some trashfish into the feed for increase its acceptability.
The wounds of body surface originated from harsh treatment of fish heals up and fish recovers easily in warm period and no special treatment is necessary. Though they are strong and resistant, any rough treatment of broodfish should be avoided. Testing of ripeness of female with strong pressure on belly is strictly forbidden, as it may cause harm to ovary.
As mentioned earlier, under controlled conditions in concrete tanks the broodfish can be used for breeding throughout the year, and can produce same quantity of eggs at consecutive breedings (10–17 % of the body weight) Janssen (1987). Janssen (1987) recommended tanks with 1–1.5 m3 water volume for broodfish rearing. Stocking rate of tank is 100 kg/m3. Water exchange must be at least 0.2 liter/kg/minute. The most favorable temperature is 25–27°C. Oxygen content of outlet water should exceed 3 mg/liter.
For tank rearing of broodfish valuable feed containing high level of animal protein and other essential materials (fatty acids, vitamins) are necessary. Most suitable feed ingredients for preparation of such type of broodfish feed are fresh (ground) trashfish, mustard oil cake powder, cattle blood, wheat bran, rice polish and vitamin premix used in poultry farming. 2.0–2.5 percent food is sufficient for maintenance of broodfish population in sexually active condition.
Selection process of females available for breeding is similar to that of other fish. It is based on evaluation of the belly size and shape and on condition of genital opening. Only fish with empty belly are suitable for selection. Good broodfish have comparatively big swollen soft belly and swollen reddish genital opening.
For accurate selection of female for breeding, a small sample of eggs should be taken out from ovary by catheter or sucking with a special narrow tube (canule) fitted with a syringe (Figure 5). Thin empty ink-tubes of ball-point pens can be used for preparation of the canula. Majority of the eggs (more than 90%) should have diameter bigger than 1 mm in the ripe ovary. This method is suggested for selection of broodfish in off-season period or to select fish available for reproduction from young populations (Viveen et al., 1986).
Due to high environmental stress resistance and other favorable features of the species, artificial reproduction of African magur is easier than that of other fish. Handling of female and male fish is easy because of comparatively small size. The fish are not sensitive to inaccurate administration of hormone doses. The stress during latency period does not affect the process of ovulation. Lack of sufficient oxygen in water has no adverse effect on ovulation. No special treatment is necessary for elimination of stickiness of eggs. It stops during incubation. Normally there is no harmful fungal infection during incubation, if the water temperature is higher than 24–25°C. It is also easy to separate bad eggs, egg shell and deformed fry from healthy larvae.
Though the carp hatcheries can be used for the purpose after some modification, for large scale production of African magur, the best thing is to establish a special magur hatchery.
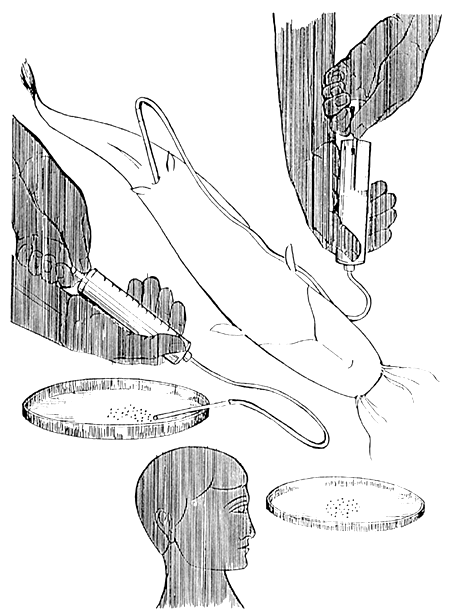 | 1. Inserting a canule in the genital opening for sucking eggs by creating low pressure. |
| 2. Releasing eggs in the petri dish containing water. | |
| 3. Observation of size and shape of the eggs and the position of nucleus. | |
Figure 5.
Use of canule for sampling eggs
All kinds of surface or well waters suitable for rearing of adult population of African catfish or other air-breathing fish are suitable for broodfish manipulation tanks in magur hatcheries. Oxygen level in the tanks should exceed 3 mg/l. Naturally, it is easier to work with broodfish in clean well water free from turbidity, because the good visibility facilitates the determination of proper time of stripping. If the broodfish tanks are supplied with turbid surface water or the water rich in plankton organisms it is advisable to filter it with a pebble filter (with approximately 4 m2 of basic area), and to aerate the tanks at night (Figure 6).
The broodfish tanks, should carry an average number of 5–6 broodfish injected at the same time, because the time required for stripping more fish is too long. Long stripping time may cause overripening of eggs. For maintenance of such a number of fish, a tank area of 1.0–1.5m2, at least, is necessary, but 3–4 m2 will be more comfortable. A tank containing such a broodfish group required approximately 5 liter/min water supply. Depth of broodfish tanks should be 0.5–0.6 m. The most comfortable water depth is 0.3 m. The UNICEF carp hatcheries in FSMFs are suitable for manipulation of broodfish.
Naturally, all types of tanks with sufficient water supply or aeration facilities are suitable for broodfish manipulation. It is suggested to cover the fish manipulation tanks, because broodfish sometimes try to escape by jumping causing serious selfinjuries. Most active jumping is detectable at night.
Egg production of one group (5–6 individuals) of injected female is approximately 0.6–0.7 kg. For incubation of this quantity of eggs 4–5 hatching troughs are necessary. The surface area of troughs is 1 m2, dimensions being 2m×0.5m×0.25m, with little sloping of sidewalls. The most suitable material for preparing trough in Bangladesh is the galvanized iron sheet. For avoiding the direct contact between metal and fish it is better to cover the inner surface of trough with polyethylene sheet. A special overflow has to be arranged in trough, covered with brass-net of mesh size 0.4–0.5 mm (Figure 7). Brass net of this mesh size is used for filtering of shallow tube well water. A short movable PVC pipe should be installed in the water outlet for maintenance of necessary water level. Water volume in a trough should be 50– 100 liter. Required water supply is 3–6 liter/min. Water should be sprayed in the tank from a perforated PVC tube. Water quality requirement of the hatchery unit is similar to that of other catfish hatcheries (See Table 2).
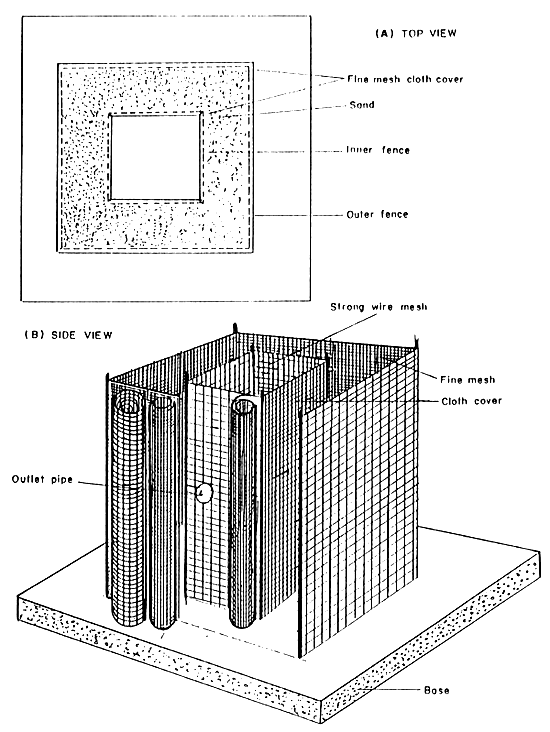
Gravel filter constructed on a concrete platform. Water outlet pipe is installed before filling up the filter bed. Space between inner and outer walls must be filled up with pebble of 3–7 mm particle size.
Water level should be kept lower than the filter height. Central hole must be covered with wooden platform to avoid the light penetration.
Figure 6.
Scheme of gravel filter for hatchery supply
(After Woynarovich and Horvath, 1980)

1. Perspective view of trough containing water outlet covered with fine mesh size brass net.
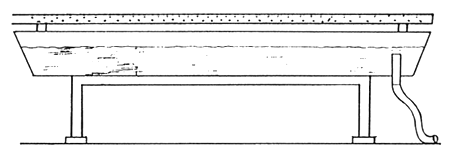
2. Cross section of a trough located on wooden stand. A water supply tube above the tray.
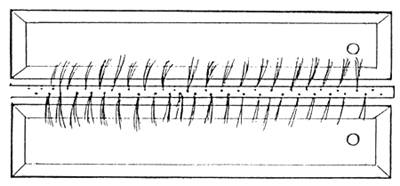
3. View from above of two parallely located trough with central water supply tube.
Figure 7.
Galvanized iron trough for incubation and larval rearing
An inner hatching frame made of iron frame and covered with mosquito net installed temporarily into troughs, is used for incubation of the eggs. The inner frames should have small supports (legs) keeping them at some distance from the bottom of trough, facilitating oxygen supply of eggs stuck to the surface of inner hatching frames (Figure 8). The fertilized eggs should be spread uniformly on the surface of inner hatching frames. Soon after hatching the embryos can penetrate through the mosquito net and they gather at the corners of troughs. Only bad eggs, egg shell and deformed embryos remain on the surface of stretched net. The whole inner frame should be removed carefully from trough a few hours after hatching.
A separate area should be made available for injection and stripping of fish and a store unit should be installed in the hatchery building. Moreover, a tank for temporary holding of fish before packing and an area for packing should also be arranged in hatcheries.
For one such unit of hatchery with 5 troughs supply of approximately 30–40 liter water/min is necessary. Supply of good quality aerated well water is the most appropriate. If surface water is used for hatchery supply, a big pebble filter should be installed. Pond water loaded heavily with organic materials is not suitable for supply to incubators. Ponds used for water supply of hatcheries should be stocked with grass carp, silver carp and catla at the rate of 300–500 kg/bigha for controlling plankton and macrovegetation blooming. Continuous or frequent aeration of such type of pond waters is essential in the hatcheries.
There is no significant difference between the works carried out in carp and African magur hatcheries. So, no special equipment is required for magur reproduction except incubators. A list of materials necessary for magur hatcheries are detailed in Appendix 1.
For transport of selected fish from ponds to hatchery, hundies covered with small pieces of net are suitable. No dry transport of fish is allowed. Adequate water should be poured into the hundies before transport. Janssen (1987) suggested using tranquilizers for longer transport of fish. According to Janssen 10g MS 222, 2.5 ml Quinaldine or 30 ml Phenoxy ethanol diluted in 100 liter of water are the proper concentrations of different tranquilizers. Moreover, he suggested disinfecting the fish with formaldehyde at the rate of 0.15ml/10 liter of water for 6 hours before stocking them into broodfish tanks. As broodfish are reared nearby to hatcheries and no recirculation of water used for broodfish tanks and incubators, it appears that the above mentioned treatments are not necessary in Bangladesh hatcheries.
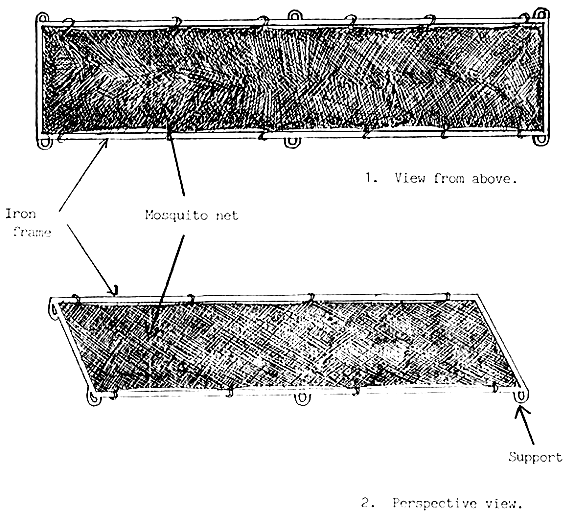
The short supports keeping the frame 2–3 cm distance above the bottom facilitating the water flow aroung the eggs.
Figure 8.
Inner (incubator) frame of trough for spreading eggs
For hormonal treatment of broodfish, morning period is suggested instead of evening administration of hormone. There are several advantages of this schedule of broodfish treatment: The temperature of water and air is lower in the morning and it facilitates handling of broodfish. The latency time is 8–10 hours in the warm well water of Bangladesh fish hatcheries. Consequently after evening injection the time of ovulation would be too early, making it difficult to observe the proper stripping time. Moreover, as the fish are more aggressive in the dark, fighting would occur in broodfish tank and the jumping of broodfish would be frequent.
Carp PG or HCG is recommended for injection of broodfish, dose being the same for both the sexes. Both female and male fish have to be injected at same time. The proper dose of PG is 4–6 mg/kg, the HCG dose being 2–4 IU/g. Janssen (1987) suggested changing the doses based on ripeness of females. Experience gathered in Bangladesh shows that the changing of doses depending on condition of broodfish is not necessary. The optimum dose is 5–6 mg PG/kg fish round the year.
This dose is approximately equal to 1.5–2.0 PG, available in the market. The fish are not sensitive to overdosing. However, it is very difficult to define the proper dose of PG, because same size glands have different individual weight depending on the method of preservation and storage (Table 8). The suggested volume of injection is not more than 1.0 cm3/fish. The place of injection is the dorsal muscle.
Table 8 Weight of pituitary gland preserved in alcohol and acetone after drying
| Preservative | M E A S U R I N G T I M E | ||||
| 2 minutes* | 10 minutes* | 30 minutes** | 4 hours** | 12 hours** | |
| Alcohol | 7 mg | -- | 5 mg | -- | 1.5 mg |
| Acetone | 13 mg | 8 mg | -- | 4 mg | 3.7 mg |
*: Kept in air;
**: Kept in desiccator
The most appropriate temperature for artificial reproduction of African magur is 25°C. Survival of developing embryos is highest and ratio of deformed embryos is lowest at this temperature.
The best method is to keep female and male fish in separate tanks after injection. If evening administration of hormone is done, it is advisable to close (to sew) the mouths of male fish for avoiding serious biting. It is not necessary for morning injection (Figure 9).
Data of Janssen (1987) show that the latency time (time between injection and ovulation) is different at different water temperatures (Table 9). The latency time after HCG administration is longer by 30 %.
Table 9 Time between pituitary gland administration and stripping (After Janssen 1987)
| Water temperature | C | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 |
| Latency time | h | 21 | 18 | 15.5 | 13.5 | 12 | 11 | 10 | 9 | 8 | 7.5 | 7 |
In contrast to other fish species it is possible to strip magur females about 1 hour before ovulation. Ovulation, sometimes remain hidden in abscene of male fish in the tank. Eggs stripped earlier or later than the proper time is not of good quality. Percentage of unfertilized eggs and ratio of deformed larvae increase with increasing temperature and with increasing differences between ideal and actual time of stripping (Table 10). Moreover, early stripping is more harmful than late stripping. The easiest way for exact determination of latency period is the use of a “signaller male” in female group. This male should be introduced to female group a few (1–2) hours before estimated time of ovulation. It starts to spawn by a special choreography with ovulating female in the most suitable time (Figure 10).
As a result of spawning, some eggs are detectable at the bottom of tank containing female fish. The proper time of stripping is after second “wild spawning”. Since ovulation of females having similar background (females, with similar age, body weight and receiving same hormone) happens in the same time, after stripping of first female spawned with “signaller male” usually the whole group of females is ready for stripping, regardless whether the individuals spawned or not with the male.
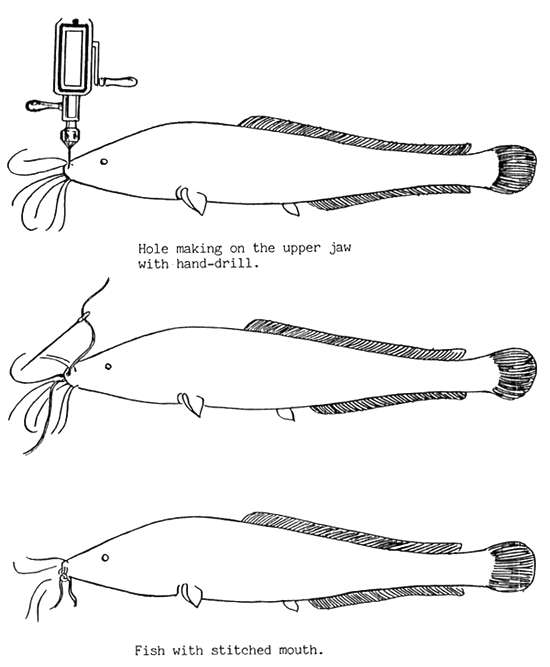
Figure 9.
Mouth stiching of male magur
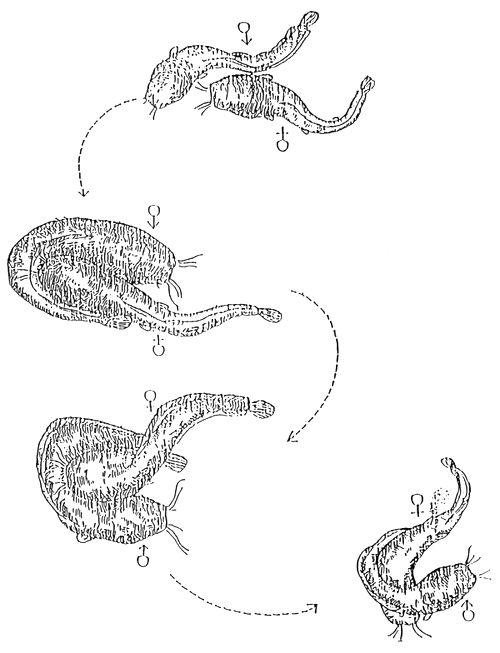
Figure 10.
Significant phases of mating and spawning
(After Janssen, 1987)
Table 10 Effect of stripping time on the percentage of hatched and deformed fry in different temperature (After Hogendoorn and Vismans, 1980)
| Water temperature | 20°C | 25°C | 30°C | ||||||
| Time after injection (hours) | 17 | 20 | 23 | 9 | 10 | 13 | 6 | 7 | 8 |
| Hatched eggs % | 45 | 62 | 51 | 40 | 85 | 73 | 45 | 75 | 40 |
| Deformed eggs % | 10 | 20 | 23 | 15 | 5 | 12 | 15 | 20 | 20 |
It is advisable not to use two signaller males, because the fighting of two males will interrupt the process of egg releasing. However, when the breeding is attempted in lean period, regardless of the difficulties mentioned, two or more males are necessary for determination of proper time of stripping, because most of the male are inactive at such time.
The easiest method of catching fish for stripping from broodfish tank is by using a rectangular scoopnet or a piece of net hold by two fishermen. The process of catching does not disturb the ovulation. It is preferable to start stripping of both sexes at the same time. If sufficient workers are not available, at first the testis has to be removed after slaughtering of fish. The testis should be wrapped in dry cloth and can be stored at room temperature or in refrigerator for 1–3 hours. Janssen (1987) suggested squeezing the sperm from testis and keeping it in physiological solution (0.65% salt solution) in refrigerator. Viveen et al. (1986) revealed that no deterioration of sperm quality in such conditions for two days. Neither the sperm nor the testes should contact water, because if such contact takes place the sperm may loose its fertility within one minute.
If there is scarcity of male fish, it is better to remove the testis by operation, instead of killing the individuals. Only 3/4 of the testis should be removed during this process. 6–7 months after operation new fertile test is will develop in body cavity of processed males. Operation procedure is shown in the Figure 11. Special antibiotic treatment is necessary following operation. Fish should be injected 2–3 times with Terramycin or other wide-spectrum antibiotics during a 8–10 days period after operation. It should be released into pond after partial recovery of wound and removal of thread from body. During regeneration fish should be kept in the hatchery. (Injection antibiotics diluted in oil to body cavity is forbidden though allowed in the muscle of fish.)
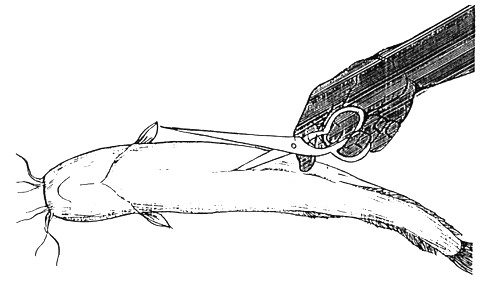
1. Incision of belly. Shallow incision advised to avoid the intestinal injuries.
2. 3/4 of testis should be removed from body cavity.
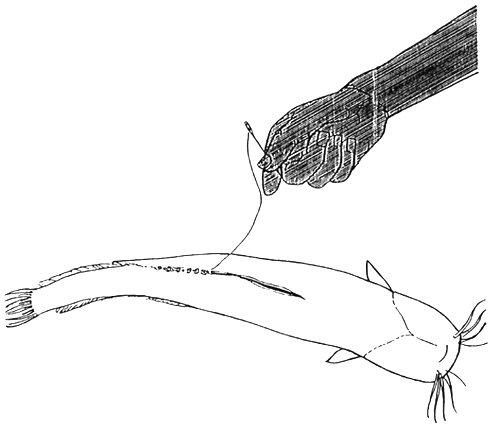
3. Two sides of grash being pulled together and stitched tightly. Thread being tied in a knot after each stitch.
Figure 11.
Operation of male fish for removing testis
Process of stripping is similar to that of other fish species. Two workers are necessary for satisfactory work: one holds the head of the fish and performs stripping, the other holds the tail and the pot for egg collection. Fish should be wrapped in dry cloth before the process starts, and the parts of the body not wrapped will have to be dried. Process of stripping should be easy, no strong pressure is necessary for getting eggs. Volume of eggs stripped in one pot should not exceed 150–200 g.
Some forecast on egg quality can be made while stripping. This may be done on the basis of color and density of stripped eggs. Eggs of immature females are green. Color of good eggs is brownish or brownish pink. Eggs stripped earlier than the proper time is dense, small batch of eggs remain together and does not disperse uniformly on bottom of the pot used for egg collection. Eggs stripped at proper time are not so dry and are similar to eggs of mirror carp stripped immediately following ovulation.
Dry method of fertilization is suggested for African catfish. The sperm should be squeezed on eggs after incision of the surface of testes. (White drops of sperm should appear following the incision of test is containing ripe sperm). For pressing out sperm the test is should be covered with plankton net or other fine mesh size plastic net and squeezed with strong pressure of fingers. Approximately 7–10 drops of sperm (0.5 ml) have to be pressed on 150 g eggs. Clean water is used for fertilization. Volume of this water should not exceed over 1/3 of the volume of eggs. The fertilization is accomplished by gently shaking the plate containing eggs, sperm and water. Stirring of eggs with spoon is not suggested. A little more water should be poured on the fertilized eggs in 15–20 seconds and after further shaking and dilution the eggs should be spread on the surface of hatching frame using a teaspoon. Only one layer of eggs should be spread. Eggs should be distributed within 1.0–1.5 minute after fertilization and before developing stickiness, because survival of embryos in eggs which are distributed after starting of stickiness is usually low. Percentage of developing eggs should be counted 12–15 hours after fertilization. It is usually 50–80% at reproduction carried out in the peak season.
Developing eggs are sensitive to mechanical treatment in the first 8–10 hours. Accordingly, no treatment of egg is allowed for elimination of stickiness. If Zoug type jars are used for incubation, the fertilized eggs should be poured in the jars one minute after fertilization and should be dispersed in ten hours with smooth stirring. No strong water current or agitation is allowed earlier.
Dead eggs are usually infected by fungi and bacteria during incubation. No treatment is necessary for controlling this infection above 25°C, because the speed of development of egg is high and they are not endangered by mass infection of the slowly developing fungi. If the incubation is performed at lower temperature, malachite green (5 mg/liter) or methylene blue (0.1–0.2 mg/liter) or formalin treatment (1ml/5 liter of water) is necessary following the fertilization, and subsequent in 12 hours. To avoid extreme hardening of egg shell later treatment is discouraged.
Approximate time of hatching (duration of embryonic development) has been shown in an earlier table (See Table 5). After hatching, majority of viable embryos escape from inner hatching frame and gather at corners of trough. At that time the mass development of fungi on inner hatching frame holds together the dead eggs, empty egg shells and deformed embryos. Then removal of inner hatching frame is easy without contamination of the trough with falling pieces of eggs. The frames should be cleaned with high pressure of water and sundried for disinfection. If the newly hatched fry are accidentally mixed with dead eggs and egg shell, it is easy to clean the population after 24 hours because by that time the fry are strong enough to escape from the trap of fungal mesh.
No special treatment is necessary during the first day after hatching, except for rubbing bacteria off from surface of polyethylene sheet. The rubbing is to be carried out with open hand pressing strongly against the wall of trough. Use of foam or cloth is not advised, because becomes a source of contamination from one trough to another. Moreover, by doing so some fry may be killed. The tiny larvae gather near the water outlet on the first day. If the filter of water outlet is not fixed properly a significant number of fry may escape from the tank. To avoid such type of loss, frequent checking is necessary by installing a bucket or basin to collect escaping fry from outlet water. Two days after hatching color of fry becomes dark. Approximately 50 hours after hatching they are capable of taking external food.
For production of 1–2 inch size fingerlings, three different methods were developed (Janssen, 1987). Each of them has different labour requirement. Production costs of the methods vary and their hazards differ.
Pond production of fingerlings is simplest of the technologies. 3 days old larvae are stocked in well prepared ponds for 2–3 weeks nursing. The main hazard of this technology is the predation by aquatic insects and copepods. With appropriate chemical treatment of pond water this problem should be eliminated or decreased. Survival rate of fry in this type of nursing is 30– 60%.
The second methodology is the combination of hatchery and pond rearing. In this method the fry are reared in hatchery for approximately ten days and then stocked in ponds when they are 20–40 mg in body weight. Main advantage of this method is that fry are not sensitive to Copepods predation at this age and it is easier to protect them from water insects. The disadvantage of the method is the labour-intensive hatchery nursing. Survival rate of fry is 50–80% here. Duration of nursing period is 3–4 weeks.
The most laborious and expensive method of fingerling production is the hatchery nursing. Moreover it is the most hazardous way of fingerling production, as there is risk of frequent infection caused by Myxobacteria. Duration of fry production by this method is 3–5 weeks, depending on the final size of produced fry or fingerling. Survival can exceed 85–90 percent.
Shallow ponds with any type of arrangements for draining are suitable for pond nursing of African magur fry. A pond not more than one bigha size is the most appropriate one. Newly constructed ponds with clay soil are not suitable for magur nursing, as the African catfish fry are sensitive to turbidity of water created by clay-colloids. (It is not clear yet whether the turbidity is primary or secondary reason of mass death of fry detectable in the ponds containing clay- colloids. Naturally the older ponds of clayey areas where the pond bottom is covered with accumulated organic materials are suitable for magur fry production.)
For satisfactory survival in ponds, special pre-and post-stocking treatments are necessary. Following steps are to be taken in pre-stocking management:
Population of unwanted fish must be eradicated from pond either by dewatering or using fish poisons;
Process of decomposition in pond bottom should be promoted with aeration of upper layer of pond soil. This process should be accelerated with repeated racking of inundated soil or by keeping the pond in dry condition for a short period;
Liming of pond;
Partial refilling of pond and management of plankton population;
Eradication of water insects;
Installation sufficient number of shades for fry.
Important steps of post-stocking management:
Maintenance of productivity of pond ecosystem;
Controlling predators;
Regular feeding of fish.
The fish that remains in the pond after harvesting (regardless of whether they are cultured or wild species) are harmful for new stock. They may be the sources of fish diseases. They consume newly hatched fry reducing survival of young population. If the ponds are kept in dry condition for a few days, these weed fish can not survive. Moreover, the decomposition process of the partially degradated organic materials is facilitated when the pond bottom has direct contact with air and the gas accumulated in the upper layer of pond bottom can escape into air.
If there is no way of dewatering the pond fully, before new stocking, the unwanted fish and other organisms (for example the crustaceans) are to be eliminated by applying poison. Three different materials are used for elimination of fish in Bangladesh: Rotenone, Phostoxin and Thiodin.
The most effective fish poison without risk of secondary or side-effect is Rotenone. Effective dose of Rotenone is 2–3 mg/liter (2.7–4.0 kg/bigha at 1 m water depth). Residual effect of Rotenone lasts for 6–7 days. Fish killed with Rotenone are fit for human consumption.
The other fish poison widely used in Bangladesh is Phostoxin. It is harmful not only to fish, but to other organisms including humans. The gas developed from tablets is harmful on lungs. So in using Phostoxin the wind direction is to be considered. Suggested concentration of this material for fish poisoning is 0.25 mg/liter (0.25 g/m3, 340 g/ bigha) at 1 m water depth. (Considering that one tablet is 3 g, the requirement of 1 bigha pond with 1 m water depth is 114 tablets.) Regular use of “horra” is suggested following application of tablets for promotion of proper mixing of developing gas with the water. Netting in this period is not suggested, as the net can concentrate the distributed pills in one part of pond, decreasing effect of poison on other places. Residual effect after Phostoxin treatment lasts for one week, which may be decreased to 4–5 days by liming. Fish killed by this material are also edible.
The Thiodin is the third fish poison used in Bangladesh. Effective concentration is 0.10–0.15 mg/liter. The Thiodin is very dangerous for human beings. Fish killed with Thiodin are not fit for consumption. Waiting time after Thiodin treatment is more than one month.
Considering the fact that the residual or lethal effect of different fish poisons is determined by several factors, a small group of fish stocked in a hapa should be exposed for 24 hours to observe the effect of pond water before stocking.
Lime is used for killing different living organisms remaining in muddy pools after dewatering of ponds. Moreover, with sharp changing of soil pH, bacteria prevailing in upper layer of mud are controlled by lime scattered on the bottom surface and the new chemical environment facilitates blooming of more productive aerobic bacteria population. Some minerals become more accessible for living organisms after application of lime (Boyd, 1982).
Two different types of lime are available in Bangladesh for pond treatment:
Efficiency of the two materials differ. In effectiveness 100 kg of hydrated lime is equal to 70 kg quicklime. Considering the fact that soils in Bangladesh have usually significant quantity of colloid fractions, depending on the soil pH, 40–100 kg quicklime or 60–140 kg hydrated lime are suggested for liming 1 bigha of fish pond. While applying lime it should be taken into account that the efficiency of lime depends on the particle size. So, powdered quicklime or well diluted hydrated lines are the most effective.
If the ponds are not dried before preparation for new stocking, frequent application of small lime doses is suggested. 10–20 kg hydrated lime scattered on one bigha pond surface daily has a favorable effect on pond ecosystem. When combined with organic manuring it eliminates the turbidity of pond water. Frequent recking of pond bottom is necessary before new stocking.
Nursery ponds are to be partially replenished 3–4 days before the time of planned stocking. Desirable depth of pond water is not more than 2.0–2.5 feet. As time of stocking is imminent, no big dose of manure is suggested. Poultry manure 100–150 kg or 200–250 kg cattle dung per bigha should be scattered uniformly on commencing day of pond preparation. Manure should be distributed simultaneously with refilling of pond. No additional manuring is suggested on the day after preparatory dose of manure, but from the following day 10–15 kg poultry manure or 20–25 kg cattle manure/bigha should be spread daily on pond water surface for a 5–7 day period. If refilling of pond is carried out with well water, it is better to add in addition to organic manure 3–5 kg of urea and 2–3 kg superphosphate/bigha for promotion of algal development. If the pond water loses its green color after organic manuring, it is advisable to mix daily dose of organic manure with 400–450 g urea and 50–100 g superphosphate per one bigha for promotion of algal blooming for a few days. In absence of organic manure a mixture of soaked mustard oil cake and inorganic fertilizers can be used for pond preparation and plankton maintenance. Suggested doses for preparation of one bigha pond are: 10 kg mustard oil cake, 3 kg urea and 1 kg TSP, soaked for 24 hours.
Preparation of an undrainable pond should commence about one week before stocking. Frequent application of small doses of manure is suggested. The doses are similar to manure doses mentioned before for drainable ponds.
If the ponds are filled with well water and are manured as suggested above, usually mass blooming of pure rotifer population will occur. In undrainable ponds or in ponds filled with pond water, the plankton organisms that prevail from before (usually mixed population of cladoceras and copepods) start blooming after application of preparatory doses of manure. If the pond water is treated with Dipterex at the rate of 0.5–1.0 mg/l, the copepods (Cyclops) and cladoceras are killed, and the most favorable organisms, the rotifers, start to develop. The best time for chemical eradication of unwanted plankton organisms is 3 days before fish stocking. It is worthwhile to mention that after repeated Dipterex treatments of a water body organophosphate resistance of Cyclops population may develop, and it is not possible to eliminate such resistant populations. The pond containing resistant organisms are not suitable for stocking of newly hatched magur fry.
Newly hatched larvae of African magur prefers the shades instead of remaining in open and well illuminated areas. So, they try to hide at pond bottom. The environment at the pond bottom is not favorable for newly hatched fry, because the level of oxygen is sometimes lower as a result of higher rate of bacterial decomposition of organic materials. To avoid concentration of fry in this environment, sufficient number of shades need to be installed in nursery ponds. The shades are prepared with rice straw fixed on bamboo frame. Surface area of one shade is 9–10 square-feet. For a 1 bigha pond 30 shades are suggested, installing 20–30 cm under the water surface (Figure 12). The shades serve as a hiding place for fry, and Paramecium developing on the surface of straw is excellent first food for the fry.
In pond waters rich with organic materials, as a consequence of organic manuring, dense population of water insects can develop within 1–3 days after refilling. Chemical treatment (Sumithion or Dipterex) of water can control this booming of insects within 1–3 days period. The most common aquatic insects are the backswimmers (Figure 13). Majority of backswimmers are harmful for newly hatched magur fry. So, frequent control of insects is essential. Sumithion treatment 10–15 hours before stocking, applied at the rate of 0.25 mg/liter can temporarily eliminate backswimmer population. Considering the suggested 2.5 feet water depth of an one bigha pond, 200–250 ml of Sumithion is necessary for treatment. Dipterex, Sumithion and Thiodin (mentioned earlier in section 3.3.1.1.1) are strong poisons, and can enter through the skin. For handling and sprinkling of these materials wearing of rubber gloves is a must. If such gloves are not available, a polyethylene bag can be put on hand before working these chemicals.
Shades being provide on water surface or few cm under the surface in an area where the water depth is at least two feet.
Figure 12.
A shade installed in a nursery pond.
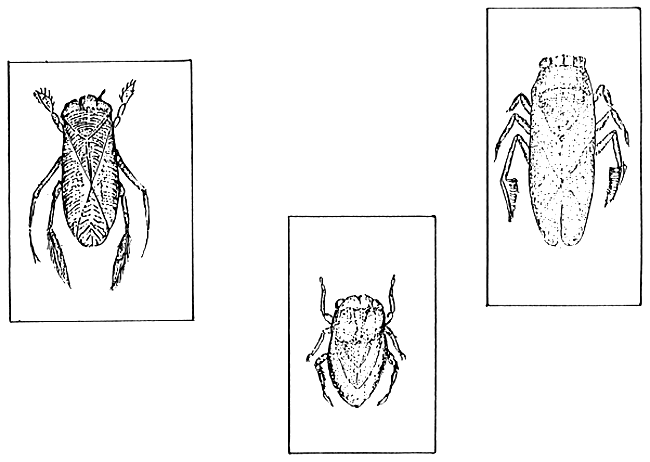
Figure 13.
Backswimmers
The best time for stocking of magur fry are the morning hours in cloudy days and the late afternoon period in sunny days. Most reasonable stocking density of fry is 1–2 lakh/bigha (80–150 fry/m2). They should be released after continuous equalization of water temperature and quality, near the straw-shades. Considering the suggested stocking density and the number of shades for one bigha pond, 3000–6000 fry have to be released at one shade.
Sequence and short description of activities necessary for pond preparation are summarized in Table 11. Synchronization of hatchery activities and the works of nursery pond preparation is summarized in Table 12.
Table 11 Pre-stocking pond management schedule
| Actions | P r o c e d u r e s |
| Dewatering | 5–7 days before stocking. The most effective method of eradication of un-wanted fish and aeration of pond soil. |
| Poisoning | 7–10 days (or one month) before stocking. Rotenon 2–3 mg/l, Phostoxin 0.25 mg/l, or Thiodin 0.5–0.7 mg/l (waiting time one month) are suitable. |
| Liming | 5–6 days before stocking. 40–140 kg lime/bigha after dewatering, or 40 kg/bigha followed by daily 10–15 kg for 1 week. |
| Bottom treatment | Recking the bottom of undrainable ponds for few days for aeration of upper soil layer. |
| Partial refilling | 3–4 days before the planned stocking. The required water depth is 2.0–2.5 feet. |
| Manuring | 3–4 days before the stocking (on the day of refilling), 100–150 kg poultry or 200–250 kg cattle manure/bigha as a preparatory dose followed by 10–15 kg or 20–25 kg daily dose. |
| Fertilization | If well water is used for refilling (or the algal blooming is not sufficient in undrainable ponds), in addition to manure 5–7 kg/bigha Urea and 3–4 kg/bigha TSP followed by daily dose of 0.5 kg Urea and 0.2 kg TSP. |
| Eradication of copepods | Dipterex treatment (0.5–1.0 mg/l) 30–50 hours before stocking if Cyclops are present in the water. (This treatment is not necessary while stocking 7–10 days old fry.) |
| Eradication of water insects | 10–15 hours before stocking by Sumithion treatment (0.2–0.3 mg/liter). |
| Installation of shades | 2–3 hours before stocking, 30 shades (surface area about 9–10 square feet) made of rice straw should be installed for 1 bigha pond. |
| Stocking | 100 000–200 000 fry per 1 bigha of pond. |
Table 12 Synchronizing works in hatchery and pond
| Time | Works in hatchery | Pond preparation |
| 7 days before stocking | - | Dewatering |
| 6 days " " | - | Liming |
| 4 days " " | Injection and stripping | Refilling, manuring |
| 3 days " " | Hatching, removing hatching frames | Preparation of shades |
| 2 days " " | Cleaning of troughs | Dipterex treatment |
| 1 day " " 12 hours before stocking | Cleaning, starting Artemia incubation | Sumithion treatment |
| 0 day | First feeding of fry 3– 4 hours before stocking | Stocking |
Important task of fish culturists is the maintenance of plankton population following stocking. Most efficient way of plankton maintenance is frequent manuring with small doses of poultry or cattle manure. In addition to plankton maintenance, frequent manuring maintains an active and effective bacterial population in pond water. It facilitates decomposition of metabolites hampering fish growth. Poultry (5–15 kg/bigha) or cattle (10–20 kg/bigha) manure is sufficient for frequent treatment of pond. If no animal manure is available, soaked mustard oil cake is also good for pond maintenance. If the alga blooming is not sufficient, some chemical fertilizers should be applied for daily treatment. The manure, soaked mustard oil cake and chemical fertilizers should be scattered equally on pond surface. As a consequence of organic material treatment, backswimmers can bloom soon in treated ponds. Sumithion treatment should be repeated frequently during first ten days of nursing. In abscene of Sumithion the Diesel oil treatment is suitable also for elimination of backswimmers, at the rate of 4 liter/bigha. Application of oil film for insect killing is harmful for fish populations older than 7–8 days, because later clogging of accessory breathing organ may occur.
Feeding of fish is not necessary in first 7–8 days period of nursing. Later feeding is required in ponds where stocking density is higher than 1 lakh/ bigha. No artificial feeding is required in ponds with low stocking density. Use of feeding tray is suggested for feeding of fish. Five to six feeding trays, each of 6–8 square feet, are necessary for feeding in a 1 bigha fish pond (Figure 14). Fifty percent of daily feed should be fed from trays split in two (morning an afternoon) halfes, and 50% should be scattered on pond surface. Quality of feed for developing fry should be same as that suggested for broodfish. The ration is 50–70% of the body weight on second week of nursing, followed by 30–40% and 20–25% on consecutive weeks. Important works of post stocking pond management are summarized in Table 13.
When fish are 12–14 days old, their accessory air breathing organ starts functioning. Fish frequently swim up to breath air. Average individual weight (and size) of fish at this stage is 0.1–0.15 g (1.5 cm). The developing fry reach 0.4–0.5g (2.5 cm) at 20 days of age and exceeds 1.5–2.0g (3–5 cm) after one month rearing. Severe cannibalism may develop in fish population older than 20 days.
Table 13 Summary of post stocking pond management activities
| Activities | D e t a i l s |
| Increasing the water level in the pond | 5–6 cm increase in water level daily for 5–10 days, for maintenance of good water quality. |
| Treatment of bottom | Harrowing the pond bottom during rearing period. |
| Maintenance of plankton | 5–10 kg poultry or 10–20 kg cattle manure per bigha daily. If the algal production (the water color) is not satisfactory, in addition to manure use of 0.5 kg urea and 0.2 kg TSP is suggested. |
| Insect control | Two-three Sumithion treatment (0.2 mg/l) on every second day in ponds where newly hatched fry were stocked. One-two treatment after stocking 7–10 days old fry. |
| Feeding | No feeding is necessary in first week. 50–70% of the body weight in second week, 30–40% in third and 20–25% in fourth week. |
If proper management of plankton population in fish pond is not possible and cyclops are present in water, the combined method of nursing is to be followed for fingerling production of magur. Fry should be reared in fish hatcheries for about 10 days in troughs, and fry of about 0.04–0.1 g body weight should be stocked in ponds.
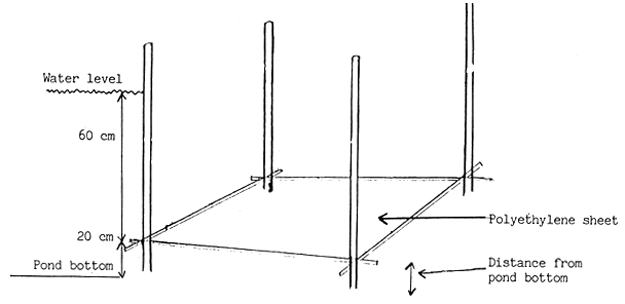
1 Feeding tray prepared from polyethylene sheet stretched on bamboo frame. The distance of sheet being kept at least 10–15 cm from pond bottom.

2. Feeding tray under the water.
Figure 14.
Feeding tray and its placement in nursery pond
As mentioned earlier quality of water required for African catfish fry is similar to that of other catfish species in initial period of rearing. Consequently stocking in trough (with surface area of 1 m2 and water volume 70–100 liter) should not exceed 40–60,000 newly hatched fry in the first 4–5 days. For maintenance of good water quality in trough, 3–5 liter clean well water supply is necessary per minute. Later during intensive feeding, the stocking has to be decreased to 20–25,000 and stocking rate of 20–30 mg fish should not be more than 10,000.
For maintenance of good health and growth of fry, frequent cleaning of troughs and feeding of fish is required. Further frequent prophylactic treatment of troughs is necessary. Accordingly, wall of troughs should be rubbed daily twice ones in morning and evening, for removing bacterial layer accumulated on inner surface of rearing tanks. Faeces, waste particles of food and dead bodies of fish should be syphoned every third hour. Two formalin treatment at the rate of 10 ml/100 liter at beginning of rearing period (morning and late afternoon) and later three treatment per day is necessary. The treatment is to be carried out without decreasing water exchange rate of troughs. This concentration of formalin is harmless on fry but effective for controlling of bacteria development. The formaldehyde should not be poured on fry directly, but a two-three liter solution should be prepared and sprayed uniformly on whole surface of trough to avoid local overdosing.
From the 5th or 6th day of rearing antibiotic treatment of fry is necessary one or two times daily. The most efficient antibiotic is the Furazolidone, at the rate of 1 teaspoon/trough/treatment. It is suggested to dissolve the antibiotic in water and spray the solution on surface of trough. Terramycin is suitable for prophylactic treatment also. Four-six tablets of terramycin (2–3 g) produced for veterinary treatment can control the outbreak of bacterial disease, if additional treatment with formalin and continuous cleaning of troughs are carried out properly. For applying antibiotic, the water exchange rate of troughs need not be changed. Treatment should be carried out in running water.
Artemia (brine shrimp) is the best starter food for African magur fry. For feeding a fry population of 100–150,000 individuals, daily four spoons of Artemia egg is sufficient during first two days of feeding. (Incubation methods of Artemia and method of preparation of hatched nauplii for feeding are shown in Appendix 2.) Feeding unhatched Artemia eggs and egg shells should be avoided, because consumption of this undigestible materials can cause significant mortality. It is recommended to feed the fry frequently in first period of rearing. The daily ration should be fed in 5–7 portion. If the quantity of Artemia used for feeding is sufficient, not less or not more than requirement of fish, it should be consumed within 10–15 minutes. Though Artemia is an import item in Bangladesh, its use for first feeding is highly recommended. (It is available in aquarium shops in Dhaka.) Though the unit price of the Artemia is high (Tk 1500–2000/liter), considering the fact that 1 liter of brine shrimp egg is sufficient for starter feeding of about 1 million magur fry, the feeding cost of fry is practically low.
African catfish fry can consume finely chopped Tubifex from second day of rearing. Daily 50 g Tubifex is necessary for feeding one through at the beginning of rearing period. Tubifex consumption can be approximately 150–200 g/day/trough at the end of hatchery rearing.
The collected Tubifex should be transported to fish hatcheries in dry condition. The most efficient and safe way of transport is carrying it in carton or wooden box padded with a few layers of newspaper. Transportation of Tubifex in wet condition or in plastic bags will cause mass mortality of worms due to high temperature and lack of sufficient oxygen.
The mass of Tubifex collected for feeding should be split into small batches (each of 0.5–1.0 kg) and kept in bucket or basins under flowing water in hatcheries. It is not advised to keep Tubifex under water outlet of fish tanks, because the worms would be contaminated with materials washed away from the troughs. The accumulated faeces, mud and dead individuals are excreted by worms and concentrated under the colonies. It should be removed from basins daily. Method of Tubifex collection, transport and treatment are summarized in Appendix 3.
Small batch (not more than 30 g) of Tubifex should be chopped on a small wooden platform for 5–7 minutes for feeding. Blood should be washed out from chopped material through a fine mesh size plankton net before feeding. After this procedure approximately 60 g fish food will remain from 100 g fresh Tubifex (Figure 15).
As cyclops can attack young fry of catfish and troughs would be contaminated by organic materials collected from pond with plankton, it is suggested not to feed the fry with collected zooplankton. If there is no way to avoid zooplankton feeding, cyclops should be removed by filtering. If proper mesh sized filter is not available, the collected material should be kept in deep freeze and thawed before feeding. This procedure kills the plankton without significant deterioration of its quality.
Portion of Tubifex being finely chopped with a small light knife on a wooden platform for 5–7 minutes.
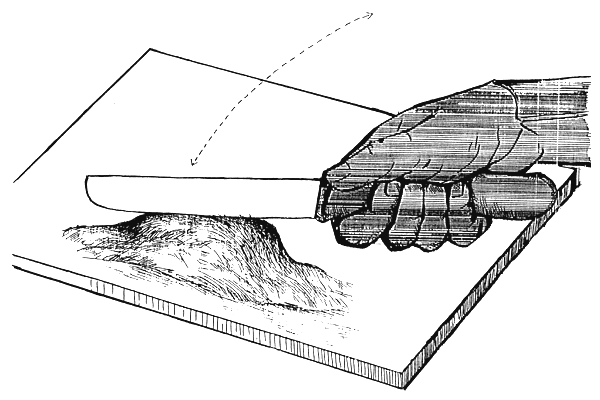
Figure 15.
Chopping of Tubifex before feeding fry
If Artemia is not available for starter feeding, yolk of boiled chicken eggs can be used for feeding on first day, followed by finely chopped Tubifex feeding from the second. Overfeeding with egg yolk can cause mass mortality of fry, especially in high water temperature.
The survival of fry in first ten days, if cleaning and prophylactic treatments are satisfactory, may exceed 90%. Fish are suitable for pond stocking at 20– 40 mg individual weight. Cyclops can not hurt the skin of such size fish.
Schedule of daily treatment of fry rearing in troughs is summarized in Table 14.
Table 14 Daily schedule of works in trough rearing of magur fry
| Time | Treatment |
| 6 a.m. | Rubbing the bottom and sides of trays by hand and syphoning
of feces. Feeding. |
| 8 a.m. | Syphoning the feces and scattering a handful of kitchen salt on surface of water. Antibiotic treatment, if necessary. |
| 9 a.m. | Feeding. |
| 11 a.m. | Formalin treatment. Concentration should be 1:10 000. |
| 12. a.m. | Feeding. |
| 1 p.m. | Antibiotic treatment if necessary. |
| 4 p.m. | Syphoning, feeding. |
| 7 p.m. | Syphoning, rubbing the surface of tray, feeding. |
| 10 p.m. | Formalin treatment. Antibiotic treatment, if necessary. |
| 12 p.m. | Feeding. |
| 2 a.m. | Syphoning. |
Process of pond preparation for this phase is the same as that of one phase nursing when newly hatched fry are stocked. The only difference is that for second phase of nursing Dipterex or Sumithion treatment is not necessary in case of stocking of fish bigger than 40 mg. Diesel oil treatment of ponds is strictly forbidden in this type of rearing technic.
Post stocking pond management and feeding of fish are also similar to the method recommended for one phase nursing. Harvesting commences 8–10 days after stocking.
For intensive culture of African magur fingerling, optimum stocking in a trough (surface area 1 m2, water volume 70–100 1) is 6–7000 fry. Duration necessary for 1 inch size fingerling production is approximately one month. Survival rate of rearing is 70–90%.
Though there are fish feeds available for intensive production of catfish fingerling as mentioned earlier, only the live food organisms (mainly Tubifex) are suitable for nursing in trough of African magur in Bangladesh. After one - three days feeding of fry with Artemia, feeding of fish can be changed to collected Tubifex.
Stocking and feeding of fish during the first week of trough-nursing are similar to stocking and feeding of fish reared in troughs during first phase of combined nursing. At the stocking density of 7–8000 fish/trough the rations from the second week of rearing are:
| Second week | : 150 g chopped Tubifex daily |
| Third week | : 150–350 g " " " |
| Fourth week | : 400–800 g " " " |
There is no significant difference in the treatment of troughs during first phase of combined nursing (when the fry are kept for 8–10 days in hatcheries before releasing them into ponds) and tank rearing of fish. Same schedule of cleaning, feeding and prophylaxis is required. Naturally, with the increase of fish biomass during rearing period, hazards of bacterial diseases are higher. So three formalin and at least one antibiotic treatments are suggested daily from 7–8th day of rearing.
Cement tanks with 2–4 m3 water volume are suitable for nursing magur fry. Tanks used for intensive culture of fry should have well or filtered pond water supply at the rate of 10–20 liter/minute. Water outlet of tanks should be covered with proper mesh size net. The mesh size should be changed (increased) during fish rearing to facilitate washing out the feces and waste food. The proper mesh size is 1.0–1.5 mm at beginning of tank rearing. Water depth of tanks used for intensive nursing should not exceed 40 cm. UNICEF hatchery tanks of Fish Seed Multiplication Farms are suitable for tank rearing of African magur fry.
Magur fry at least 6–8 days old (pre-nursed in trough) are suitable for
stocking at the range of 5–10 000/m3. Chopped Tubifex is the best food for
tank nursing. Growth of fish depends highly on food quantity and on frequency
of feeding. The best schedule of feeding is similar to that of trough rearing.
Cleaning and prophylactic treatment of tanks are similar also. The only
difference is that such frequent antibiotic treatment is not necessary because
of lower stocking density. Here also the survival rate is similar.
Cement tanks without continuous water supply may be used for rearing of fry
also. The best ones are the long rectangular tanks with 3–5m3 water volume.
The ideal water depth of tanks is 25–35 cm. Such type of tank culture should
be based on zooplankton, produced in tank water. Methodology of water
treatment in such tank is similar to that of nursery ponds. The tank should
be filled with well or filtered pond water approximately one week before
stocking. Jute bags filled with 10–20 kg poultry manure are necessary for
enrichment of water. To help releasing nutritients the bags should be shaken
frequently during preparation. Application of some artificial manure (4–5 g
urea/m3 and 1–2 g TSP/m3) also is advised. Zooplankton organisms, mainly
rotifers and Paramecium, usually start to develop 2–3 days after manuring. The
bags containing manure should be removed 1–2 days before stocking.
Stocking density of tanks should not exceed 100 larvae/m2. The larvae should be stocked after 2–3 days feeding in troughs. Before stocking, installation of two shades prepared from rice straw is necessary for 10 m2 tank area. The shades should not be laid at the bottom of tank, but 8–10 cm above the bottom level using bricks as support. Feeding with chopped Tubifex, is advised for first 10 days of rearing. Later, in addition to Tubifex, some artificial starter feed (available at “SABINCO”) is allowed. For maintenance of plankton blooming, after 6th-7th day of rearing daily application of 100–150 g soaked rice polish or wheat bran spread uniformly on water surface is necessary.
Daily brushing of wall and bottom of the tanks is essential. Moreover, when there is deterioration in water quality, 10–20 % water replacement is suggested. Chemical treatment of fish is not necessary.
Survival and growth of fish primarily depends on management, mainly on frequency of feeding and on maintenance of good water quality. It may range from few percent to 70–80%.
Usually no disease breaks out during pond nursing of African magur, if the pond preparation for nursing is carried out according to above suggestions. As a result of high temperature and abundance of natural food organisms, resistance of growing fish is excellent.
Bacterial infection of fish population reared in hatcheries is quite common. The most frequent pathogen is Flexibacter columnaris (Janssen, 1987). Fish reared in overstocked condition or in tanks with insufficient cleaning and also the starving fish populations are susceptible to such type of bacterial infection. Color of infected fish is whitish. Bacterial colonies can develop mainly on epithelium of fins and barbels and around the mouth of fry. Sometime small pieces of epithelium between the rays of fins become loose. Position of barbels can draw attention to weak health condition of fish. Barbels of healthy fry are pointed forward, while barbels of sick fish are curved backward. Infected fish stay sometimes in vertical position, “hanging” near surface of water. After outbreak of disease same antibiotic and formalin treatments with higher frequency are required than for prophylaxis (Figure 16).
Fungal and parasitic infections may occur among different size of fry and fingerlings. Malachite green, Formalin or Dipterex baths are the best measures to control them. Suggested concentration of malachite green treatment is maximum 0.07 mg/liter. Frequency of therephautic treatment is 4–5/day. Formalin is a good tool for controlling bacterial and protozoan infections at the rate of 5–10 ml/100 liter applied as it was detailed before. Suggested frequency of formalin baths is 4–6 times daily. Organophosphate baths at the rate of 0.1 g/liter for 20–30 minutes are effective against worm-type gill or skin parasites. The allowed frequency of such treatment is not more than two-three days.
Diseases occurring in catfish populations, their pathogens, symptoms and treatments are summarized in Appendix 4.
As pointed out earlier, prior to the development of accessory breathing organs in the fry, the environmental requirement of African magur is same as that of other fish species. Consequently, for transportation of newly hatched fry and nursed fry, fish density suggested, is similar to that of the carp fry. In plastic bags with total volume 16–20 liter, containing 10–13 liter oxygen the transportation of
35–40,000 fry is suggested for 2 hours journey,
13–18,000 " 6–8 ".
For transportation, such a bag should not contain more than 4000 fry of 1.3– 1.5 cm (approximately 60–100 mg individual body weight) for 6–8 hors journey.
Areas where the symptoms are usually detectable
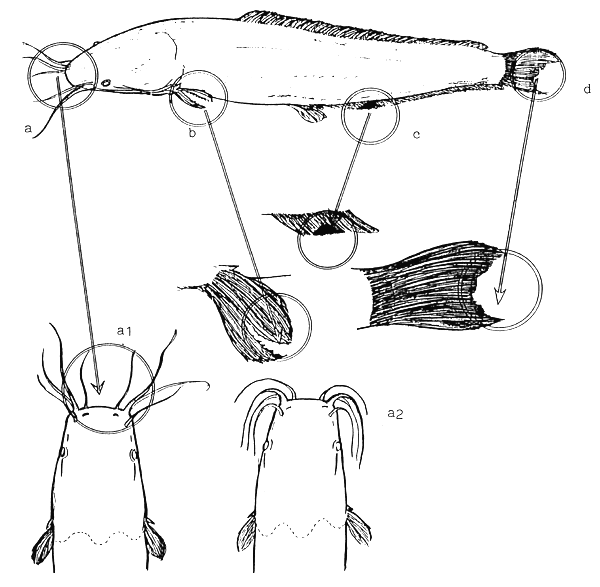
a1. barbels of healthy fish remains pointed forward
a2. barbels of sick fish curved backward
b. epithelium between the fin rays destroyed by bacteria
c. bacteria colony on fin
d. bacterial erosion of fin
Figure 16.
Typical symptoms of Myxobacter infection
African magur fry are stronger than any other species after final development of accessory breathing organ. Packing of 1000–1200 one inch size fish is advised in an average size (20 1 volume) plastic bag for 5–6 hours journey. As in the case of other fish species, intestine should be emptied before packing, but unnecessarily long conditioning should be avoided, because Myxobacteria infection may break out in strongly stressed population. Use of antibiotic in fish transport bags with the concentration of 20–50 mg/liter is highly recommended.