by
M. M. KUNJU
Central Marine Fisheries Research Institute 1
Mandapam Camp, India
Abstract
The biology of Solenocera indica Nataraj, commercially exploited around Bombay, was studied based on samples from fishermen's catches. The species is the same as S. subnuda Kubo and its geographic distribution extends from India to Borneo and Hong Kong. It feeds mostly on crustaceans and fish. Breeding behaviour was studied from the periodical occurrence of various maturity stages. The breeding period is protracted, with two peaks in December and April. Main spawning grounds are outside, and probably contiguous with, the fishing area. Males do not leave the fishing grounds for purposes of breeding. The entire population migrates offshore when salinity decreases. Average growth rates of adult females and males, as estimated from length frequency studies, are 6.96 mm and 6.49 mm per month respectively. Juveniles grow faster. Estimated life spans of female and male are 14 to 15 months and 9 to 10 months, respectively. Females always dominate the population.
1 Present address: Central Marine Fisheries Research Sub-station, Bombay
QUELQUES ASPECTS DE LA BIOLOGIE DE Solenocera indica Nataraj
Résumé
L'étude de la biologie de Solenocera indica Nataraj, espèce exploitée commercialement aux environs de Bombay, a été effectuée sur des échantillons provenant des prises des pêcheurs. L'espèce, identique à S. subnuda Kubo, est distribuée géographiquement de l'Inde à Bornéo et Hongkong. Sa nourriture consiste essentiellement en crustacés et en poissons. Le comportement en matière de reproduction a été étudié d'après l'apparition périodique des divers stades de maturité. La période de reproduction est très longue, avec deux pointes en décembre et en avril. Les principales frayères sont situées hors de la zone de pêche, et y sont probablement contigües. Durant la reproduction, les mâles ne quittent pas les fonds de pêche. Lorsque le taux de salinité décroît, toute la population effectue une migration vers le large. Le taux moyen de croissance des femelles et des mâles adultes, estimé sur la base d'études des fréquences de longueurs, est de 6,96 et 6,49 mm par mois, respectivement. Les formes juvéniles croissent plus rapidement. La durée de vie estimée des femelles et des mâles est respectivement de 14 – 15 mois et de 9 – 10 mois. Le nombre des femelles est toujours plus grand que celui des mâles.
ALGUNOS ASPECTOS DE LA BIOLOGIA DE Solenocera indica Nataraj
Extracto
Se ha estudiado la biología de Solenocera indica Nataraj explotado comercialmente en torno a Bombay, sobre la base de muestras tomadas de las capturas de los pescadores. Esta especie es igual a S. subnuda Kubo y su distribución geográfica se extiende desde la India a Borneo y Hong Kong. Se alimenta principalmente de crustáceos y peces. Se ha estudiado su desarrollo por la aparición periódica de las diversas fases de su madurez. El período de cría es largo alcanzando dos máximos en diciembre y abril. Los lugares principales de desove se hallan fuera de la zona pesquera y probablemente contiguos a ella. Los machos no abandonan las áreas pesqueras a fines reproductores. Toda la población emigra hacia alta mar cuando disminuye la salinidad. El promedio de crecimiento de las hembras y machos adultos, calculado mediante estudios de frecuencia de tallas es de 6,96 mm y 6,49 mm mensuales, respectivamente. Las formas juveniles crecen más deprisa. El ciclo vital de hembras y machos se calcula en un período de 14 a 15 meses y de 9 a 10 meses, respectivamente. El número de hembras, en la población, es siempre dominante respecto al de machos.
Our knowledge of the biology of penaeid prawns is mainly based on the various accounts available on the species of the sub-family Penaeinae; and very little information is available on Solenocerinae. Except for the recent accounts on the natural history of Solenocera subnuda (Cheung, 1963; Hall, 1962), there has been only scattered information on the larval and developmental history of other species of Solenocerinae Commercial exploitation of this group is only to a very limited extent, the vast majority of these prawns being abyssal forms inhabiting waters beyond the continental shelf (Alcock and Anderson, 1894; Alcock, 1901; Burkenroad, 1934, Kubo, 1949; Bullis 1956). However, Hymenopenaeus robustus and H. muelleri in the Atlantic coast of America (Bullis, 1956; Eldred and Hutton, 1960), S. membranacea in the Mediterranean coast of Israel (Holthuis and Gottlieb, 1958), S. subnuda, S. alticarinata, S. depressa and S. bedokensis from the sea around Hong Kong (Cheung, 1963) and S. distincta in the Seto inland sea of Japan (Yasuda, 1956) have been reported to occur in the prawn catches of these countries. Of these S. subnuda and S. distincta are of some minor fishery value. S. indica supports a considerable fishery around Bombay and it has been estimated to contribute 9.61 percent of the average annual prawn landings of 53,682 tons in Maharashtra (Kunju, 1967)
Random samples from the commercial catches were collected from Sassoon Dock, Versova and Arnala. The distance from Versova to Arnala is about 32 km to the north and that from Versova to Sassoon Dock is about 28 km to the south. Versova and Sassoon Dock lie within the city of greater Bombay. Care was taken to ensure maximum randomness in the samples, by taking them before the catch had been sorted out and by combining the total sample from different fishing units. The gear used in catching these prawns is a fixed bag net, locally known as ‘Dol’, which is widely used all along the coast of Maharashtra. A smaller version of the net, locally known as ‘Bokshi’ is operated very close to the shore at Arnala. ‘Dol’ is used in all the three centres. The fishing grounds lie within the 30 to 40 m depth range at Versova and Arnala, whereas they are within 20 m depth at Sassoon Dock. The ‘Bokshi’ of Arnala is operated in waters of 6 to 7 m depth. Setna (1949) has given a fairly detailed account of the operation and construction of the ‘Dol’ of the Bombay coast.
Material from Arnala was obtained once a fortnight from November 1959 to June 1962, and from Versova and Sassoon Dock once a week from October 1959 to April 1965. Samples of different weeks of the month were combined to represent the month's sample. In all, about 8,000 prawns were examined. Total length was measured from the tip of rostrum to the tip of telson after having stretched the prawn dorsoventrally on a graduated scale. Carapace length was measured from the mid-dorsal line, opposite the posterior orbital margin, to the posterior end of the mid-dorsal line of the carapace, by using a vernier caliper calibrated to 1/10 mm. Measurements were recorded mostly from specimens preserved in 5 percent formaldehyde for about a month; only a few samples could be measured in the fresh condition.
Other details of techniques employed have been given in the appropriate sections.
After the species was first described by Nataraj in 1945 several authors have dealt with the systematics of Solenocerinae, but none of them has referred to S. indica. This omission is particularly evident in the monograph of Kubo (1949) wherein six new species of Solenocera have been proposed, of which five are closely related to the Indian form. Among them S. subnuda, a species erected on material obtained from the south coast of Borneo appears to be the same as S. indica. The characters of the two species as described by the original authors were compared by the present author and slight variations were noticed in the post-rostral carina, antennular flagellum, second and third abdominal somites, thelycum and the pereiopods. On examination of a large number of specimens of S. indica these variations were found to occur within the species and hence were not considered of specific significance.
TABLE I
Percentage composition of the stomach contents of Solenocera indica
| Sex | Size in mm | No. of prawns examined | Crustacea | Fish | Polychaetes | Molluscs | Sand | Debris | Miscellaneous | ||
| Decapod | Copepod | Unidentified Crustacea | |||||||||
| Female | 20–50 | 6 | - | - | 40.00 | 16.67 | - | - | 6.67 | 36.67 | - |
| 51–70 | 24 | 47.50 | 1.67 | 7.50 | 8.75 | 6.25 | 2.09 | 0.83 | 24.59 | 0.83 | |
| 71–90 | 84 | 27.98 | 0.36 | 10.60 | 24.88 | 4.05 | 3.57 | 1.43 | 27.02 | 0.12 | |
| 91–110 | 32 | 54.69 | 2.19 | - | 27.19 | - | - | - | 15.94 | - | |
| All sizes | 146 | 35.89 | 0.96 | 8.97 | 22.40 | 3.36 | 2.40 | 1.23 | 24.59 | 0.21 | |
| Male | 20–46 | 4 | 12.50 | 7.50 | - | 10.00 | - | - | 30.00 | 35.00 | 5.00 |
| 47–70 | 76 | 27.63 | 1.05 | 15.13 | 22.24 | 2.36 | 1.05 | 1.84 | 27.76 | 0.92 | |
| All sizes | 80 | 26.88 | 1.38 | 14.38 | 21.63 | 2.25 | 1.00 | 3.25 | 28.13 | 1.13 | |
| Both sex | 226 | 32.70 | 1.11 | 10.88 | 22.12 | 2.96 | 1.90 | 1.95 | 25.84 | 0.53 | |
Hall (1956) erected the species S. kuboi on material collected from Malaysian waters and compared it with S. subnuda. The differences noticed by Hall were mostly the same as mentioned above and these were subsequently reconciled (Hall, 1962) and S. kuboi and S. subnuda considered synonymous. His description and illustration of the antero-ventral region of the carapace of S. subnuda show perfect agreement with S. indica. However, the transitory spines along the lateral margins of the telson of juveniles are not found in S. indica.
Cheung (1960) pointed out the similarity between S. indica and S. subnuda, and his opinion that “in the absence of type specimens the name subnuda is to be preferred to indica for geographical reasons” is not considered acceptable for obvious reasons. Dr. A.J. Bruce of the Fisheries Research Station, Hong Kong, believes (personal communication) that the two forms are the same, but both are likely to be synonymous with S. chinensis Yu. Kubo and Hall seemed to be unaware of the work of Nataraj.
S. indica is found all along the coast of India. Nataraj (1945) recorded the species from Gulf of Cambay, Bombay, Madras, Visathapatnam, Orissa and Ganjam coasts, and from the sandheads and the mouth of River Hooghly. His material also came from Mergui Archipelago and Singapore. Ahmad (1957) recorded the species from Khulna in East Pakistan. As S. subnuda is to be considered a synonym of S. indica the range of distribution of the latter may be said to extend up to the coasts of Borneo and Hong Kong. All these records were from within 40 m depth.
Along the coast of Maharashtra the species is abundant, particularly around Bombay. In the course of the present study it was collected from Dahanu, Satpati, Arnala, Versova, Worli, Sassoon Dock, Alibag, Murud and Paj. Its abundance is conspicuous in Versova from where the largest catches are recorded.
The stomach contents of 146 females, ranging from 20 to 110 mm in length, and 80 males ranging from 20 to 70 mm in length, selected from samples of different months from all the three centres were examined in detail in order to determine the nature of feeding. The stomachs were dissected and the contents examined under microscope. The percentage composition of the constituents was determined by eye estimation, and analyzed separately for the various length groups of prawns. The stomach contents were found mostly in an advanced stage of digestion and hence identification of the constituents was extremely difficult.
The percentage composition of the stomach contents is given in Table I. The food items were classified as crustaceans, fish, polychaetes, molluscs, sand, debris and miscellaneous.
4.1 Crustaceans
It could be seen from the table that the most important item in the diet was of crustacean origin, constituting 44.69 percent of the stomach contents. The 51 to 70 mm and 91 to 110 mm groups among females and the 47 to 70 mm group among males were found to consume more of this item than the other size groups. Decapods such as Acetes indicus and Palaemon tenuipes, both of which were abundant in the inshore waters of Bombay, formed the dominant group among crustaceans. In several cases palaemonid zoeae were found in large numbers. Since Palaemon tenuipes is the only palaemonid found occuring in vast numbers in the area of investigation these zoeae were assigned to it. Most of them were found entire without having been mutilated by the masticatory or digestive processes. Calanoid copepods and fragments of various parts of other crustaceans constituted the rest of the crustacean item. These fragments were broken appendages, pieces of exoskeleton, setae, etc.
4.2 Fish remains
As stated earlier, it was extremely difficult to differentiate the identity due to the fish being found only in fragments consisting of various bones, eye balls, scales, otoliths and sometimes portions of the muscle, of very small fishes, probably larval or post-larval forms. This item constituted 22.12 percent of the stomach contents. Larger prawns were found to feed on fish more than the others.
4.3 Polychaetes
Setae and fragments of polychaetes constituted 2.96 percent of the stomach contents. Medium sized prawns of both sex were found to feed on this item more than the others.
4.4 Moluscs
Forming 1.90 percent of the stomach contents, these could be recognized only by the presence of broken pieces of shell, probably those of small gastropods.
4.5 Sand
Sand grains constituted 1.95 percent of the stomach contents and they were present in all the size groups, though more in the smaller prawns. Sand particles have, obviously, got in along with food ingested from the bottom of the sea.
4.6 Debris
In almost all the prawns examined a sizeable portion of the stomach contents (25.84 percent) consisted of unidentifiable finely ground up matter. The word ‘debris’ is used instead of the more common ‘detritus’ (Panikkar and Menon, 1956) since it was not known whether it was of organic or inorganic origin. It was not possible to decide whether the debris had been ingested as such or ground up in the stomach.
4.7 Miscellaneous items
This included various items, like foraminiferans (Pulvinulina sp.) and algal filaments which occurred in isolated cases.
Prawns are believed to be omnivorous in habit, feeding mostly over mud flats. Forster (1951) stated that since prawns can catch small amphipods and other crustaceans it may be that their habits are not so much scavenging as is commonly supposed. The preponderance of crustaceans and fishes in the diet of Solenocera indica may not be the result of scavenging activities alone. Kunju (1956) has observed seperate habits in aquarium reared Leptocarpus fluminicola. In the study of the feeding habits of S. subnuda, Hall (1962) found that polychaetes formed the dominant food item which were ingested by a definite process of selection.
Maturity stages in the female penaeids have been determined by different methods by various authors. King (1948) and Lindner and Anderson (1956) designated the various ovarian stages of Penaeus setiferus as “undeveloped”, “developing”, “yellow”, “ripe” and “spent”. Eldred et al. (1961) and Cummings (1961) classified the ovary of Penaeus duorarum into “undeveloped”, “developed”, “nearly ripe”, and “ripe”. Shaikhmahmud and Tembe (1961) used the terms “immature”, “early maturing”, “late maturing”, “mature”, and “spent”, while differentiating the maturity stages of the ovary of Parapenaeopsis stylifera. The method adopted by Cheung (1963) was similar to that of Shaikhmahmud and Tembe, but he combined the “early” and “late maturing” stages into a single “maturing” stage. These stages were recognized by the various authors by taking into account both macroscopic and microscopic characters of the ovary and ovum.
In the present study the following stages were recognized.
Immature: Ovary small, thin and colourless, not visible through the exoskeleton. Ovum transluscent and nucleus clearly visible; yolk granules sparsely distributed.
Maturing-early: Ovary opaque and from pale to bright yellow in colour; still not visible through the exoskeleton. Nucleus only faintly visible through the yolk granules in the ovum.
Maturing-late: Ovary orange in colour, which is darker in advanced stages; visible through the abdominal tergites and the membraneous connectives between the first abdominal somite and the carapace. Ovum opaque, packed with yolk granules and nucleus not visible.
Mature: Ovary occupies all the available space in the abdomen and cephalothorax, dark brown in colour and clearly visible through the exoskeleton all along the abdomen. Surface of the ovum appears corrugated due to the presence of rod shaped peripheral bodies.
Spent: Ovary greatly reduced in size, loose and flaccid in appearance, cream or dirty yellow in colour. As the spent stage advances the ovary is found further reduced in size, opaque and pale yellow in colour, rendering it difficult to be distinguished from the “maturing-early” stage. Under the microscope several disintegrating ova and myriads of follicle cells are visible.
Colour of the ovary in the various stages described pertained to preserved material as it was not possible to record it before preservation. Structure of the ovum was examined when gross macroscopic characters of the ovary failed to yield dependable clues to the precise maturity stage.
Since the smallest maturing female recorded was 51 mm (total length), sizes below this were excluded from the study. Incidence of the various maturation stages in the different months from October 1959 to July 1963 at each centre of observation was recorded. The material from Arnala was meagre and that from Sassoon Dock had only very few individuals with the ovary in different maturation stages. The Versova samples had a fair representation of all these stages (Table II). One or the other of the maturity stages was present in all the months and “mature” and “spent” conditions were found occurring together. The distribution of the various maturity stages among prawns collected from Versova in the different months is graphically shown in Fig. 1. Generally, there is a gradual decline in the incidence of the various stages from October onwards. Among the “maturing-early” stage there were two peaks, in October and February. These peaks were found progressing towards the spawning stage in December and April. The December to January peak of “mature” prawns was probably derived from the “maturing-early” peak of October, though the corresponding “maturing-late” stage was not clear. On the other hand, the “maturing-early” peak of February was found to pass through a well defined peak of “maturing-late” stage in March and attain the “mature” stage in April. The peaks formed by the “mature” and “spent” prawns were found in the same period. Among the “mature” prawns a good many may have been in such an advanced stage as to spawn immediately. It has been observed in other penaeids also that once the ovary reaches the “mature” stage spawning follows soon after (King, 1948; Cheung, 1963, and 1964).
It is evident that the prawn spawns throughout the period of the fishery, and has two peak spawning periods, in December and April (Fig. 1). In S. subnuda two peak spawning periods were observed, but in certain months there was a total absence of maturing stages (Cheung, 1963).
TABLE II
Occurrence of various maturity stages of S. indica at Versova
| Month | Total No. | Spent | Mature | Maturing-Late | Maturing-Early | Immature |
| October 1959 | 34 | 1 | 3 | 14 | 10 | 6 |
| November | 58 | 5 | 11 | 21 | 13 | 8 |
| December | 65 | 5 | 8 | 15 | 16 | 21 |
| January 1960 | 42 | 1 | - | 5 | 9 | 27 |
| February | 120 | - | - | 1 | 42 | 77 |
| March | 92 | 1 | - | 10 | 13 | 68 |
| April | 95 | 8 | 1 | 5 | 18 | 63 |
| May | 48 | - | - | - | 4 | 44 |
| June | 12 | - | - | - | - | 12 |
| October | 21 | - | - | 3 | 7 | 11 |
| November | 24 | 1 | 1 | 4 | 3 | 15 |
| December | 81 | 6 | 31 | 15 | 10 | 19 |
| January 1961 | 92 | 5 | 21 | 9 | 11 | 46 |
| February | 98 | 6 | 5 | 21 | 15 | 51 |
| March | 68 | 2 | - | 10 | 13 | 43 |
| April | 65 | 2 | 5 | 12 | 9 | 37 |
| May | 50 | - | 1 | 2 | 8 | 39 |
| October | 5 | - | - | - | 2 | 3 |
| November | 91 | 1 | 10 | 12 | 17 | 51 |
| December | 60 | 2 | 4 | 11 | 10 | 33 |
| January 1962 | 50 | - | 8 | 7 | 16 | 19 |
| February | 33 | - | - | 4 | 6 | 23 |
| March | 56 | - | 1 | 15 | 21 | 19 |
| April | 37 | - | - | - | 1 | 36 |
| May | 58 | - | - | - | 3 | 55 |
| June | 14 | - | - | - | - | 14 |
| December | 47 | 2 | - | 5 | 16 | 24 |
| February 1963 | 69 | - | - | 3 | 23 | 43 |
| March | 82 | 1 | 4 | 12 | 12 | 53 |
| April | 63 | 2 | 2 | 4 | 12 | 43 |
| May | 79 | 2 | - | - | 10 | 67 |
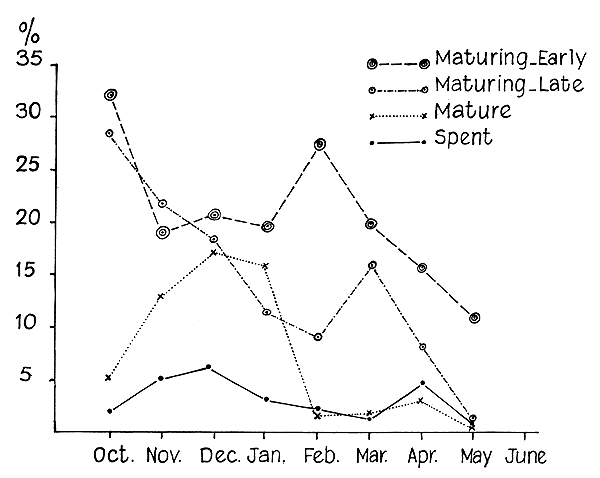
Fig. 1. Incidence of maturity stages of female S. indica in different months at Versova.
From the progression of modes described above (Fig. 1) it is seen that the “immature” prawns took about three months to attain the “mature” condition and eventually spawn.
There was a gradual reduction in the relative abundance of prawns in progressive conditions of maturity, from the “immature” to the “spent”, the number of “spent” prawns being conspicuously low, compared to the other stages. In instances where spawning takes place within the fishing grounds the number of “spent” prawns encountered was far greater than observed in the present species (Cheung, 1963 and 1964; Lindner and Anderson, 1956; Eldred et al., 1961), especially in the peak spawning periods. Therefore, it is possible that the main spawning ground of S. indica is not within the fishing area from where the material was collected. Nevertheless, the occurrence of “spent” prawns in the catches, although not in large numbers, may indicate that the fishing area is probably contiguous with the main spawning ground of the prawn. This assumption is further supported by the fact that “mature” and “spent” prawns were rarely encountered at Sassoon Dock where fishing is carried out within 20 m depth, whereas the ‘dol’ fishery at Versova and Arnala, where the fishing grounds lie within 30 to 40 m depth yielded larger proportions of these stages.
Two types of migratory movements were discernible in S. indica, one in connection with spawning and the other in relation to salinity. As stated earlier the presence of such a small number of “spent” prawns would indicate that the females move out of the fishing grounds for spawning. Males probably do not participate in this migration, vide infra. Besides, the gradual decrease in the number of individuals in advanced stages of maturity could also be due to such a movement. How far out the prawn migrates and its further destiny is not known. As explained in connection with the incidence of “spent” stages, the spawning migratory movement was towards an area closely adjacent to the fishing grounds thus indicating a limited spawning migration, similar to that of S. subnuda (Cheung, 1963). These migrations were found to take place throughout the year, with two peak periods in December and April. In the length frequency studies (see below) the larger size groups were found to disappear from the exploited area in regular progression and these groups constituted the breeding females which were migrating away from the fishing grounds.
The second migratory movement was found to take place when the population en masse moved offshore when the salinity of the coastal waters decreased from June to early October as a result of the southwest monsoon rains. Jayaraman et al. (1961) recorded the lowest salinity conditions in Bombay in these months. During this period fishing activities were entirely suspended at Versova, but the ‘dol’ fishery at Sassoon Dook and the ‘bokshi’ fishery at Arnala were continued uninterrupted (Kunju, 1967). In the latter two S. indica was completely absent in these months. As in the spawning migration it is likely that the prawn moves out only to adjacent areas of higher salinity. Trawl catches taken from within 40 m depth usually contained a small number of large sized S. indica which were altogether absent in the monsoon months. A sciaenid fish (Otolithoides brunneus) caught off Bombay, in August 1960, in a depth of about 60 m was found to have fed on S. indica.
In certain penaeid prawns, Cummings (1961) and Eldred et al. (1961) found a direct relation between salinity and temperature on one hand and spawning and migration on the other. The period of low salinity in the surface waters of Bombay was recorded as from June to October, the salinity ranging from 23 to 27‰, whereas in the other months it was between 35 and 37‰ (Jayaraman et al., 1961). It is when the salinity begins to fall on the onset of the southwest monsoon rains that S. indica migrates en masse, to return to the fishing grounds in October when the pre-June salinity is restored. Though there is a slight decline in the temperature also in these months, it does not appear significant.
In the peak spawning months of December and April the temperature is relatively low (25 to 26°C) in the former and high (29 to 31°C) in the latter month (Jayaraman et al., 1961) indicating that spawning does not probably depend on temperature conditions. But both these peak periods coincide with the period of highest salinity of 35 to 37 ‰ in the surface waters.
Several early workers considered total length, from the tip of rostrum to the tip of telson, as the most reliable measure of size in prawns, while Burkenroad (1934; 1951) contended that length did not reveal certain important increases in size, and that volume or weight should be used. Kubo (1949; 1956), Dall (1958) and Cheung (1963; 1964) have been using carapace length in assessing growth and other characteristics of the prawn. In the present investigation total lengths alone were recorded, from which length frequency studies were made, and carapace lengths of 255 prawns, collected in 1965, were recorded in order to determine the relationship between these two measurements.
Carapace measurements were grouped in 1.0 mm classes and their averages were plotted against average total lengths. A straight line fitted by the method of least squares gave a good fit (Fig. 2). The relationship was found to be
L = 6.91 + 3.20 C
where L is the total length (mm) and C the carapace length (mm)
Size frequency studies were based on samples collected from Versova from October 1959 to April 1965. The frequency groups for males were represented by sharp peaks owing to their small size range, while those for females were more flattened. The frequencies for the period October 1964 to April 1965 are shown in Fig. 3. The modes, exhibited by both the sexes, were in many places rather obscure and hence drawing conclusions on these was rendered difficult. However, certain definite trends in the progression of several series of modes were found significant (Table III).
The rates of growth of penaeid prawns vary in different species. In Metapenaeus dobsoni it was 4 to 14 mm (Menon, 1952), in M. bennettae (as M. mastersii) 10 mm (Dall, 1958) and in M. monoceros 5 to 10 mm (George, 1959). The averages derived from modal progressions of prawns of 33 mm and above in length (Table III) showed that the monthly growth of S. indica was 6.96 mm in females and 6.49 mm in males, which is about the same reported for S. subnuda (Cheung, 1963).
Recruitment of juveniles of both the sexes measuring 33 mm and less was evident in the months of January to March every year, and probably they were the progeny of the spawning group of November to December. Therefore, they may be 2 to 3 mo old having grown at a faster rate than those of over 33 mm length. The generation derived from the second spawning peak in April was not represented in the length frequency graphs, since by June the fishery at Versova was suspended.
The largest female and male encountered during this study measured 114 mm and 80 mm respectively, and taking into consideration the monthly average growth and the approximate age of the juvenile recruits discussed above, these prawns may be 14 to 15 and 9 to 10 mo old respectively.
As the smallest female found with “maturing-early” ovary was 51 mm it may probably attain this stage when it is 5 to 6 mo old. Since the immature ovary takes about 3 mo to attain the spawning stage, the prawn may spawn for the first time when it is 8 to 9 mo old. This inference is further supported by the fact that the majority of spent females were within 81 to 85 mm length (Table IV) and by the method of estimate shown above they are approximately 9 mo old. As the life span of the female was estimated to be approximately 14 to 15 mo it is possible for the prawn to spawn at least once again; but, in the absence of other evidence this inference can only be tentative.
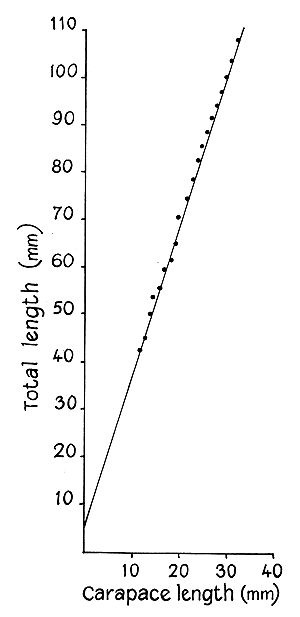
Fig. 2. Relationship between total length and carapace length of S. indica.
TABLE III
Progression of modes from length frequency polygons of S. indica from Versova
| Female | |||||||
| From | To | ||||||
| Month | Mode (mm) | Month | Mode (mm) | Growth (mm) | Period in months | ||
| October | 1959 | 38 | June | 1960 | 93 | 55 | 8 |
| October | 58 | March | 93 | 35 | 5 | ||
| October | 78 | January | 98 | 20 | 3 | ||
| January | 1960 | 48 | May | 1960 | 78 | 30 | 4 |
| October | 48 | March | 1961 | 88 | 40 | 5 | |
| October | 63 | March | 98 | 35 | 5 | ||
| November | 38 | May | 88 | 50 | 6 | ||
| January | 1961 | 33 | May | 1961 | 63 | 30 | 4 |
| November | 48 | April | 1962 | 88 | 40 | 6 | |
| October | 58 | March | 93 | 35 | 5 | ||
| December | 33 | June | 73 | 40 | 6 | ||
| December | 1962 | 63 | April | 1963 | 88 | 25 | 4 |
| December | 53 | May | 88 | 35 | 5 | ||
| February | 1963 | 48 | May | 1963 | 73 | 25 | 3 |
| February | 33 | May | 58 | 25 | 3 | ||
| October | 53 | April | 1964 | 88 | 35 | 6 | |
| December | 43 | May | 63 | 20 | 4 | ||
| February | 1964 | 33 | May | 1964 | 53 | 20 | 3 |
| October | 68 | April | 1965 | 108 | 40 | 6 | |
| October | 43 | April | 83 | 40 | 6 | ||
| Average growth of female per month = 6.96 mm | |||||||
| Male | |||||||
| From | To | ||||||
| Month | Mode (mm) | Month | Mode (mm) | Growth (mm) | Period in months | ||
| October | 1959 | 43 | December | 1959 | 58 | 15 | 2 |
| December | 43 | March | 1960 | 63 | 20 | 3 | |
| January | 1960 | 38 | May | 1960 | 58 | 20 | 4 |
| November | 38 | February | 1961 | 58 | 20 | 3 | |
| January | 1961 | 33 | May | 1961 | 58 | 25 | 4 |
| October | 43 | December | 58 | 15 | 2 | ||
| February | 1962 | 33 | May | 1962 | 58 | 25 | 3 |
| February | 1963 | 38 | May | 1963 | 58 | 20 | 3 |
| December | 43 | February | 1964 | 58 | 15 | 2 | |
| February | 1964 | 33 | May | 1964 | 53 | 20 | 3 |
| March | 53 | May | 63 | 10 | 2 | ||
| October | 48 | January | 1965 | 63 | 15 | 3 | |
| January | 1965 | 38 | April | 1965 | 58 | 20 | 3 |
| Average growth of male per month = 6.49 mm | |||||||
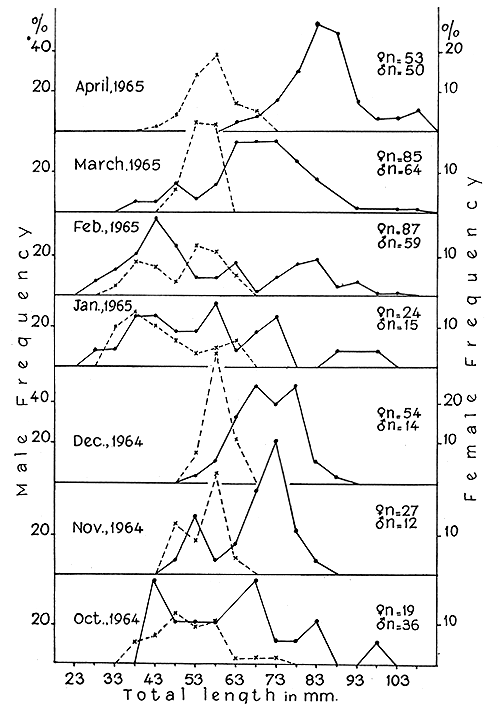
Fig. 3. Length frequency distribution of S. indica from October 1964 to April 1965 at Versova.
TABLE IV
Distribution of the various maturity stages of S. indica among the different size groups from all the centres
| Size group (mm) | Total No. | Spent | Mature | Maturing-Late | Maturing-Early | Immature |
| 51–55 | 330 | - | - | 8 | 3 | 319 |
| 56–60 | 349 | - | - | 9 | 8 | 332 |
| 61–65 | 335 | - | 8 | 15 | 22 | 290 |
| 66–70 | 403 | 3 | 17 | 42 | 63 | 278 |
| 71–75 | 395 | 11 | 22 | 46 | 85 | 231 |
| 76–80 | 395 | 8 | 28 | 48 | 93 | 218 |
| 81–85 | 289 | 20 | 20 | 49 | 74 | 126 |
| 86–90 | 260 | 12 | 28 | 37 | 79 | 104 |
| 91–95 | 104 | 5 | 6 | 30 | 35 | 28 |
| 96–100 | 44 | 2 | 6 | 10 | 18 | 8 |
| 101–105 | 12 | 1 | - | 6 | 1 | 4 |
| 106–110 | 3 | - | 1 | - | 1 | 1 |
| 111–115 | 2 | - | 1 | - | - | 1 |
The female-male ratio in the population varied from month to month (Table V) but with the females always dominating. The disparity between the sexes was least in the month of October and high in the months of January, April and May when more than half the population was constituted by females. A breakdown of the data for the entire period of investigation showed that the number of females was twice that of males (Table V).
The sex-wise size distribution of the prawn for the entire period is shown in Fig. 4. Up to about 48 mm size the disparity between the sexes is not pronounced, whereas from this stage onwards there is a striking preponderence of males in the population, which is probably because the sexually maturing females move out of the fishing grounds. Therefore it is possible that mating and impregnation take place within the fishing grounds only and that the males do not move out of the grounds along with the females. The disparity seen in the higher size groups could be only due to the shorter length attained and the comparatively short life span of the male. Menon (1957) suggested that the disparity in the sexes of older size groups of four penaeid species he studied, viz. Metapenaeus dobsoni, M. monoceros, Penaeus indicus and Parapenaeopsis stylifera was due to their inshore and offshore migrations.
TABLE V
Sex-wise size distribution of S. indica in different months in all the centres
| Size group | October | November | December | January | February | March | April | May | All months | |||||||||
| F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | |
| 21– 25 | - | - | 1 | 1 | - | - | - | 1 | 2 | - | 10 | 5 | 1 | 1 | 2 | - | 16 | 8 |
| 26– 30 | 3 | - | 11 | 2 | 9 | 4 | 3 | 4 | 19 | 6 | 34 | 16 | 6 | 5 | 1 | - | 86 | 37 |
| 31– 35 | 7 | 1 | 13 | 8 | 13 | 9 | 18 | 4 | 36 | 17 | 32 | 13 | 15 | 6 | 3 | 3 | 137 | 61 |
| 36– 40 | 24 | 7 | 39 | 14 | 30 | 17 | 21 | 8 | 44 | 39 | 44 | 23 | 49 | 14 | 9 | 4 | 260 | 126 |
| 41– 45 | 35 | 19 | 19 | 35 | 51 | 41 | 53 | 22 | 73 | 53 | 75 | 26 | 58 | 19 | 17 | 9 | 381 | 224 |
| 46– 50 | 27 | 50 | 35 | 69 | 49 | 74 | 37 | 26 | 96 | 77 | 109 | 85 | 69 | 27 | 32 | 9 | 454 | 417 |
| 51– 55 | 29 | 54 | 37 | 70 | 65 | 91 | 45 | 83 | 76 | 149 | 99 | 183 | 73 | 91 | 36 | 31 | 460 | 752 |
| 56– 60 | 41 | 53 | 50 | 63 | 70 | 89 | 40 | 76 | 64 | 169 | 103 | 106 | 67 | 78 | 52 | 70 | 487 | 704 |
| 61– 65 | 36 | 20 | 60 | 29 | 74 | 40 | 37 | 18 | 63 | 48 | 103 | 42 | 58 | 28 | 57 | 40 | 488 | 265 |
| 66– 70 | 31 | 10 | 75 | 6 | 89 | 6 | 68 | 9 | 75 | 5 | 99 | 9 | 61 | 8 | 64 | 14 | 562 | 67 |
| 71– 75 | 14 | 5 | 61 | 1 | 79 | - | 71 | - | 84 | - | 108 | 2 | 73 | - | 52 | - | 542 | 8 |
| 76– 80 | 16 | 1 | 47 | - | 62 | - | 61 | - | 81 | 1 | 98 | - | 79 | - | 70 | - | 514 | 2 |
| 81– 85 | 19 | - | 46 | - | 43 | - | 34 | - | 71 | - | 70 | - | 59 | - | 40 | - | 382 | - |
| 86– 90 | 11 | - | 33 | - | 31 | - | 22 | - | 46 | - | 58 | - | 65 | - | 57 | - | 323 | - |
| 91– 95 | 3 | - | 12 | - | 12 | - | 5 | - | 24 | - | 33 | - | 14 | - | 20 | - | 133 | - |
| 96–100 | - | - | 12 | - | 9 | - | 3 | - | 5 | - | 9 | - | 9 | - | 12 | - | 59 | - |
| 101–105 | 1 | - | 3 | - | 2 | - | 1 | - | 3 | - | 4 | - | - | - | 2 | - | 16 | - |
| 106–110 | - | - | 2 | - | - | - | - | - | - | - | 2 | - | 2 | - | 1 | - | 7 | - |
| 111–115 | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | 1 | - |
| Total | 297 | 220 | 556 | 298 | 688 | 371 | 520 | 251 | 862 | 564 | 1,090 | 510 | 758 | 277 | 527 | 180 | 5,308 | 2,671 |
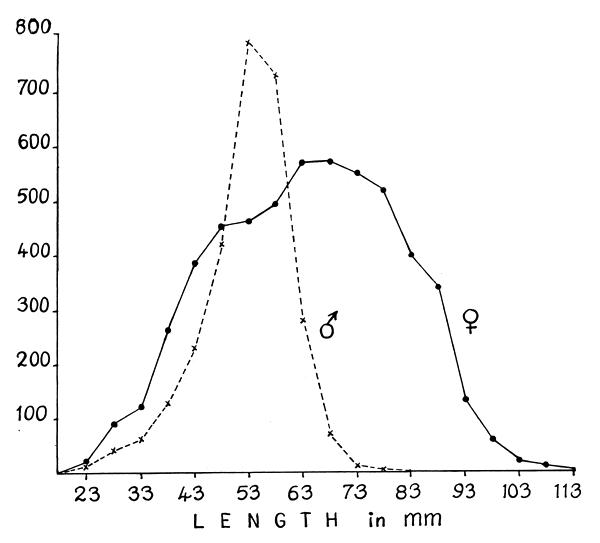
Fig. 4. Size frequency of female and male S. indica for the entire period from October 1959 to April 1965 from all the centres.
Ahmad, N., 1957 Prawn and prawn fishery of East Pakistan. Dacca, East Pakistan Government, Press, 31 p.
Alcock, A., 1901 A descriptive catalogue of the Indian deep-sea Crustacea Decapoda, Macrura and Anomala in the Indian Museum, being a revised account of the deep-sea species collected by the Royal Marine Survey Ship ‘INVESTIGATOR’. Calcutta, 286 p.
Alcock, A. and A.R.S. Anderson, 1894 Natural history notes from H.M. Indian Marine Survey steamer ‘INVESTIGATOR’, Commander C.F. Oldham, R.N., commanding. Series 2. No. 14. An account of a recent collection of deep-sea Crustacea from the Bay of Bengal and Laccadive Sea. J.Asiat.Soc.Beng., 63(2):141–85
Bullis, H.R., 1956 Preliminary results of deep water exploration for shrimp in the Gulf of Mexico by the M.V. OREGON (1950–1956). Comml Fish Rev., 18(12):1–12
Burkenroad, D., 1934 The Penaeidae of Louisiana with a discussion of their world relationships. Bull.Am.Mus.nat.Hist., 68(2):61–143
Burkenroad, D., 1951 Measurement of the natural growth rates of decapod crustaceans. Proc. Gulf Caribb Fish.Inst., 3:25–6
Cheung, T.S., 1960 A key to the identification of Hong Kong Penaeid prawns with comments on points of systematic interest. J.Hongkong Univ.Fish., 3:61–9
Cheung, T.S.vv, 1963 The natural history of the commercial species of Hong Kong Penaeidae (Crustacea, Decapoda). Ann.Mag.nat.Hist., 6(13):401–33
Cheung, T.S., 1964 Contributions to the knowledge of the life history of Metapenaeus ensis and other economic species of penaeid prawns in Hong Kong. J.appl.Ecol., 1:369–86
Cummings, W.C., 1961 Maturation and spawning of the pink shrimp, Penaeus duorarum Burkenroad. Trans.Am.Fish.Soc., 90(4):462–68
Dall, W., 1958 Observations on the biology of the greentail prawn, Metapenaeus mastersii (Haswell) (Crustacea Decapoda: Penaeidae). Aust.J.mar.Freshwat.Res., 9(1):111–34
Eldred, B. and R.F. Hutton, 1960 On the grading and identification of domestic commercial shrimps (Family Penaeidae) with a tentative world list of commercial penaeids. Q.Jl Fla Acad.Sci., 23(2):29–118
Eldred, B., et al., 1961 Biological observations on the commercial shrimp, Penaeus duorarum Burkenroad in Florida waters. Prof.Pap.Ser.mar.Lab.Fla., 3:1–139
Forster, G.R., 1951 Biology of the common prawn, Lender serratus Pennant. J.mar.biol.Ass U.K., 30:333–60
George, M.J., 1959 Notes on the bionomics of the prawn Metapenaeus monoceros Fabricius. Indian J.Fish., 6(2):268–79
Hall, D.N.F., 1956 The Malayan Penaeidae (Crustacea, Decapoda). Part 1. Introductory notes on the species of the genera Solenocera, Penaeus and Metapenaeus. Bull. Raffles Mus., 27:68–90
Hall, D.N.F., 1962 Observations on the taxonomy and biology of some Indo-West Pacific Penaeidae (Crustacea, Decapoda). Fish.Publs colon.Off., 17:229 p.
Holthuis, L.B. and E. Gottlieb, 1958 An annotated list of the Decapod Crustacea of the Mediterranean coast of Israel, with an appendix listing the Decapoda of the the eastern Mediterranean. Bull.Res.Coun.Israel(B), 7(1–2):1–126 also issued Bull.Sea Fish.Res.Stn Israel, (17):1–126
Jayaraman, R., R. Viswanathan and S.S. Gogate, 1961 Characteristics of sea water near the light house, Bombay. J.mar.biol.Ass.India, 3(1–2):1–5
King, E.J., 1948 A study of the reproductive organs of the common marine shrimp, Penaeus setiferus (Linnaeus). Biol.Bull.mar.biol.Lab.,Woods Hole 94(3):244–62
Kubo, I., 1949 Studies on the penaeids of Japan and its adjacent waters. J.Tokyo Coll. Fish., 36(1):1–467
Kubo, I., 1956 A review of the biology and systematics of shrimps and prawns of Japan. Proc.Indo-Pacif.Fish.Coun., 6(3):387–98
Kunju, M.M., 1956 Preliminary studies on the biology of the palaemonid prawn, Leander styliferus Milne Edwards. Proc.Indo-Pacif.Fish.Coun., 6(3):404–16
Kunju, M.M., 1967 Observations on the prawn fishery of Maharashtra coast. Proc.Symp. Crustacea mar.biol.Ass.India, Part 4:1382–97
Lindner, M.J. and W.W. Anderson, 1956 Growth, migrations, spawning and size distribution of shrimp, Penaeus setiferus. Fishery Bull.Fish Wild.Serv.U.S., 56(106):555–645
Menon, M.K., 1952 The life history and bionomics of an Indian penaeid prawn Metapenaeus dobsoni Miers. Proc.Indo-Pacif.Fish.Coun., 3(2):80–93
Menon, M.K., 1957 Contributions to the biology of penaeid prawns of the south-west coast of India. 1. Sex ratio and movements. Indian J.Fish. 4(1):62–74
Nataraj, S., 1945 On two new species of Solenocera (Crustacea Decapoda: Penaeidae) with notes on Solenocera pectinata (Spence Bate). J.Asiat.Soc.Beng., 11:91–8
Panikkar, N.K. and M.K. Menon, 1956 Prawn fisheries of India. Proc.Indo-Pacif.Fish.Coun., 6(3):328–44
Shaikhmahmud, F.S. and V.B. Tembe, 1961 A brief account of the changes in the developing ovary of (Penaeid Prawns) Parapenaeopsis stylifera (Edw.) in relation to maturation and spawning cycle. J.Univ.Bombay, 29(3–5):62–77
Setna, S.B., 1949 Bombay fishermen's ingenuity. J.Bombay nat.Hist.Soc., 48(3):444–9
Yasuda, J., 1956 Shrimps of the Seto Inland sea of Japan. Proc.Indo-Paci.Fish.Coun. 6(3):378–86
Acknowledgments
I am deeply indebted to Dr. S. Jones, Director, Central Marine Fisheries Research Institute, Mandapam Camp, for his constant encouragement during the course of this work. Grateful thanks are due to Shri K.H. Mohamed and Shri K. Virabhadra Rao, Senior Research Officers, who suggested improvements in the manuscript.