Manfred E. Mielke
(Youqing Luo)
Michael E. Ostry
The Korqin desert region of China is characterized by sand dunes, sand belts, swamps, and lakes. The climate is continental, having an annual mean temperature of 6°C. and ranges from a low of about -30°C in January to + 10°C in July-August. Annual precipitation ranges from 350 to 550 mm. 70 percent of which occurs during July – September. Evaporation from winds is very high, particularly in spring and fall. when velocities reach between 18~35 m sec. There are an average of 8 severe sand storms per year. All of these factors combine to create an environment that is very hostile for the cultivation of trees.
About 6 million hm2 are estimated to be suitable for forestry development if appropriate tree species are planted. and if insects and pathogens do not adversely affect management. Many native and hybrid species of poplar (Populus) have been planted successfully throughout the region. Many pathogens of poplar are indigenous to the region, but presently none appear to threaten its cultivation.
The environmental conditions that make cultivation of trees difficult also are not conducive to the development of most tree leaf diseases. High winds and evaporation in spring in particular, create conditions of low humidity that prevent the formation of free moisture on leaf surfaces. Moisture is necessary for the initiation of infection by foliar pathogens and the development of disease.
Stress caused by drought and frost can predispose poplar to attack by secondary pathogens that cause canker diseases. Branch dieback and subsequent infection by various pathogens will occur in early spring if plants set terminal buds too late the previous fall. Therefore it is vital to select poplar provenances that will set bud early in the fall to prevent frost damage and reduce canker diseases.
Cultural practices common to the region reduce foliar disease development in poplars. Planting trees at low density promotes the movement of air through the plantation, drying leaf surfaces, and reducing chances for development of leaf disease. Raking and disposing of fallen leaves in the dormant season reduces pathogen inoculum and prevents or delays infection the following growing season. In plantations where raking does not occur, plowing under of fallen leaves and cultivation of crops between rows of poplar also reduces the ability of pathogens to infect leaves during the growing season.
Many people harvest the lower branches of poplar in the fall for fuel and fodder. If done correctly, pruning can be beneficial by promoting good tree form, improving wood quality, and reducing disease in branches and leaves in the lower crown area. However, often pruning is done improperly, causing severe injury to tree stems, or creating branch stubs that become infected by canker pathogens. Pathogens that infect the branch stubs are a source of inoculum for the spread of disease throughout the plantation. These stubs also cause the tree to seal the branch wound very slowly. causing a reduction in wood quality and providing sites for invasion by decay organisms. Pruning wounds to the main stem are even more damaging. The wound is a site for introduction of decay and canker organisms. Once infected wood quality and tree survival are threatened. Pathogens such as Agrobacterium sp. can be spread throughout a plantation on contaminated pruning tools.
Proper pruning involves integrating knowledge of the anatomy of tree branches, the use of good pruning tools, and proper timing. Pruning cuts should be made as close to the branch collar as possible, without cutting into the collar area. This collar can be recognized as a small ridge extending from the main stem out onto the branch. The pruning cut should be made using a sharp (and preferably sanitized) cutting tool, and should be done in the dormant season. The cut should be smooth, without ragged edges or torn bark. The younger the branch is when it is pruned, the more quickly the tree will seal the pruning wound.
The major pathogens of poplar planted in the region are described in this field guide. The guide is intended to aid in the early identification of any potential threat in order to maximize management options. Recommended management strategies and other sources of information on poplars are included.
Preventative measures, or indirect controls, are preferred. To apply a direct control measure the following three criteria must be satisfied. The proposed controls must be: 1. biologically effective. 2. economically justified. and 3. environmentally friendly. It must be understood that very few tools exist for the direct control of tree diseases. Most diseases cannot be treated therapeutically. The best control is the prevention of disease. This involves the factors summarized earlier: planting a suitable clone with disease resistance in the appropriate environment. and then applying the best cultural practices to maintain tree vigor.
More than one pathogen may often be present and diagnosis may require microscopic examination. Care must be taken to find the primary cause of a disease since stressed trees often are invaded by several microorganisms.
There may be other pathogen species present in plantations not covered in this guide that could potentially cause serious disease under some conditions. In addition, more pathogenic strains of existing pathogens may develop, Pathogens from outside the region may become established if infected plant material is introduced into an area. Implementing vigilant quarantine measures and good cultural practices will reduce the chances of this happening.


Agrobacterium tumefaciens
Symptoms and Signs:
Crown gall infections appear as round swellings with rough, wrinkled bark usually confined to the root collar and lower stem (Fig. 1). The galls are irregularly shaped and have deep fissures. Galls are hard when young and later become spongy. During moist weather, the galls may ooze a slimy substance, consisting of the infectious bacteria.
Ecology:
Crown gall is caused by a bacterium that exists in the soil and infects trees through wounds. The bacterium can be spread by ground—inhabiting insects, rainsplash, irrigation, cultivation, pruning, and movement of infested soil particles. The bacterium is also spread from infected to healthy trees when infected trees are pruned or otherwise cut (Fig. 2). and the contaminated tools are used to prune healthy trees. The bacteria infect trees and disrupt the cell DNA. This causes cells to proliferate creating galls. When the infected tree parts are sloughed into the soil, the natural cycle begins anew.
Economic Importance:
The galls themselves may be considered unsightly. More important is that galls disrupt nutrient and water transport in the vascular tissues which results in poor growth of young trees. Affected stems are weakened at the points of infection and can be invaded by organisms that cause discoloration and decay resulting in stem breakage.
Management:
Do not plant in soil that is known to be infested by the bacterium. This is especially important when locating nurseries.
Do not propagate infected plant material.
Destroy all infected plant material.
Do not move soil from areas known to be infected with the bacterium, either on plant material or equipment.
If infected trees are pruned or cut. disinfect contaminated equipment before pruning healthy trees.
A possible biocontrol is to inoculate cuttings with a non — gall forming competitor. A. radiobacter. prior to planting in contaminated soils.
Cytospora chrysosperma


Symptoms and Signs:
Cytospora canker (and other similar appearing cankers) affect branches and the main stem. Infected tissue appears sunken, surrounded by a raised margin of healthy tissue. The color of infected tissue varies greatly from yellow, orange, brown to black. The surface of the canker is dotted with small erumpent fruit bodies (pycnidia) of similarly variable color (Fig. 3). During wet weather white, yellow, or orange spore tendrils are extruded from openings in the pycnidia (Fig. 4). These tendrils can reach lengths of up to 2 cm. When dry the spore tendrils appear as fine hairs.
Conidia are very small (1.5μm×4μm). 1-celled, hyaline, elongate and slightly curved.
Ecology:
C. chrysosperma is primarily opportunistic, infecting dead and dying tissue, or trees that are of low vigor or severely stressed. Stress caused by premature defoliation, competition, nutrient imbalance, drought, sunscald, frost, or poor cultural practices predispose trees to attack. Conidia produced on canker surfaces are splashed by rain to wounds, or bud scales and leaf scars of stressed trees. Bark with low moisture content is vulnerable. Trees that set bud late in the fall are subject to frost injury and are particularly vulnerable to infection. The principle cause of infection of poplars by C. chrysosperma in the region is through wounds created by poor pruning practices.
Economic Importance:
As mainly a secondary agent, C. chrysosperma infection is symptomatic of other biotic, environmental, or cultural disorders. The fungus often infects cuttings of poor vigor which do not survive when planted.
Management:
Maintain the health and vigor of trees.
Plant clones that are resistant to pathogens that cause premature defoliation.
Apply cultural practices that reduce infection by foliar pathogens.
Plant clones that are best suited for the environment in which they are grown.
Control weeds and competition, irrigate and fertilize when necessary.
Maintain optimum stand density to prevent leaf disease from developing.
Plant only vigorous. disease-free plant material.
Prune trees properly. Use clean, sharp cutting tools, never leave a branch stub, and especially, do not wound the tree past the branch collar. Prune only during the dormant season from October to April.
Prune infected branches. Excising small stem cankers may eliminate isolated stem infections.
Dothiorella gregaria

Symptoms and Signs:
The first evidence of infection appears as approximately 10 mm diameter dark rings on branches and stems of trees with rough bark. On trees with smooth bark, infections appear as raised blisters 5~20 mm in diameter (Fig. 5). These blisters ooze a brown fluid when cut. Trees in young nurseries and recently - established plantations are most susceptible due to transplant stress. Dieback of branches is often associated with blister canker.
Conidia are produced in black pycnidia. They are hyaline. 1-celled, and range from 19~29μm×5~7μm.
Ecology:
D. gregaria is primarily opportunistic, infecting dead and dying tissue, or trees that are of low vigor or severely stressed. Stress caused by premature defoliation, competition, nutrient imbalance, drought, sunscald, or poor cultural practices predispose trees to attack. Conidia produced on canker surfaces are splashed by rain to wounds, or bud scales and leaf scars of stressed trees. Bark with low moisture content is vulnerable.
Asymptomatic trees often are infected in the nursery and only show symptoms of the disease following outplanting due to transplant stress. The incidence of infection depends on the vigor of nursery material and the presence of inoculum.
Economic Importance:
As mainly a secondary agent, infection by D. gregaria is symptomatic of other biotic, environmental, or cultural disorders. Nursery plants of low vigor may be killed. Newly planted trees or cuttings that are infected in the nursery may sustain dieback, or may be killed if subjected to other severe stresses. Once trees establish healthy growth this disease becomes insignificant.
Management:
Maintain the health and vigor of trees.
Plant clones that are resistant to pathogens that cause premature defoliation.
Apply cultural practices that reduce infection by foliar pathogens.
Plant clones that are best suited for the environment in which they are grown.
Control weeds and competition, irrigate and fertilize when necessary.
Maintain optimum stand density to prevent leaf disease from developing.
Plant only vigorous, disease-free plant material.
Prune trees properly. Use clean, sharp cutting tools, never leave a branch stub. and especially. do not wound the tree past the branch collar. Proper pruning will prevent most canker disease.
Prune infected branches.

Dothichiza populea
Symptoms and Signs:
D. populea infects young branches of stressed trees. The infection begins as a tiny, brown spot in the bark. The canker expands beneath the bark into the cambium causing a perennial canker (Fig. 6). The bark splits open over these infection sites forming a vertical slit that later closes. Older trees rarely are damaged by this canker.
Spores are, 10~13μm×7.5~10μm, avoid and hyaline.
Ecology:
D. populea infects those trees stressed by other factors. Spores are able to infect buds and new growth.
Economic Importance:
As mainly a secondary agent. Dothichiza bark canker is symptomatic of other, biotic. environmental, or cultural disorders. Nursery plants of low vigor may be killed. Newly planted trees that are infected in the nursery may sustain dieback, or may be killed if subjected to other severe stresses. Once trees establish healthy growth, this disease becomes insignificant.
Management:
Maintain the health and vigor of trees.
Plant clones that are resistant to pathogens that cause premature defoliation.
Apply cultural practices that reduce infection by foliar pathogens.
Plant clones that are best suited for the environment in which they are grown.
Control weeds and competition, irrigate and fertilize when necessary.
Maintain optimum stand density to prevent leaf disease from developing.
Plant only vigorous. disease-free plant material.
Prune trees properly. Use clean, sharp cutting tools, never leave a branch stub. and especially. do not wound the tree past the branch collar.
Prune infected branches.
Coryneum populinum

Symptoms and Signs:
Initial infections by C. populinum cause small, water soaked, round, ovate or irregular spots on leaves. Petioles and tender new growth also are infected. Older leaf spots are larger, often several millimeters across, and irregular, with a very definite margin. The inner part of the spot is a shiny gray color, hence its name gray spot. Frequently the center of the spot falls away forming a shothole (Fig. 7). Often many shotholes occur on each leaf.
Clusters of black acervuli form in the middle of the spot late in the growing season. The conidia are oblong, dark, several celled, and 32~54μm×7~10μmin size.
Ecology:
C. populinum overwinters in lesions on the previous year's growth and in fallen infected leaves. During periods of moist weather the following growing season, ascospores are rain-splashed to the current year's leaves. Conidia produced from lesions in the previous year's growth also infect new leaves when moisture is present on leaf surfaces. Trees infected early in the growing season sustain premature defoliation and saplings may sustain terminal dieback.
Economic Importance:
Severe infections, especially those initiated early in the growing season, cause premature defoliation, loss of tree vigor, and predisposition to secondary disease fungi such as Cytospora canker and root disease. These secondary fungi often kill the tree.
Management:
Monitor plantations for symptom development early in the growing season to enable timely control if disease exceeds acceptable levels.
Maintain low density plantations to promote air flow and to dry free moisture on leaves necessary for infections.
Plant only clones that have demonstrated resistance to the pathogen.
Rake, burn, plow under or otherwise dispose of fallen leaves before the onset of the following growing season.
Marssonina brunnea
Symptoms and Signs:
M. brunnea causes numerous small leaf spots less than 1 mm across. Each spot often is surrounded by a chlorotic ring, especially visible when held up to the light (Fig. 8). Later in the season leaf spots often merge, forming larger spots or leaf blotches. These blotches often appear rust brown to black. Infections on the leaf petiole and new growth are lens-shaped.
The conidia are 2-celled, ovoid to elongate, ranging in size from 13~18μm×4.5~5.7μm. and are formed in pale acervuli.
Ecology:
M. brunnea overwinters in lesions on the previous year's twig and stem growth, and in fallen infected leaves. During periods of moist weather the following growing season, ascospores are rainsplashed to the current year's leaves.
Economic Importance
Severe infections, especially those initiated early in the growing season, cause premature defoliation, loss of tree vigor, and predisposition to secondary disease fungi such as Cytospora eanker and root disease. These secondary fungi often kill the tree.
Management:
Monitor plantations for symptom development early in the growing season to enable timely control if infections exceed acceptable levels.
Maintain low density plantations to promote air flow and to dry free moisture on leaves necessary for infections.
Plant only clones that have demonstrated resistance to the pathogen.
Rake, burn, plow under or otherwise dispose of fallen leaves before the onset of the following growing season.

Melampsora larici-populina
Symptoms and Signs:
Bright orange to yellow pustules develop on the underside of leaves in the later part of the growing season (Fig. 9). These pustules are clusters of uredospores, and may appear early in the growing season if alternate hosts are present nearby (Fig. 10). The topside of the leaf consists of discolored spots.
The uredospores are clavate and covered with small spines. Size ranges from 30~49μm×13~16μm.
Ecology:
Species of poplar rust fungi have a very complex ecology. They require two hosts to complete their life cycle. The alternate host required to complete the life cycle depends on the species of rust. The alternate host of M. larici-populina is larch.
During wet weather in the spring spores produced on overwintered infected poplar leaves are blown by wind to emerging larch needles. These needles are infected and by early summer aeciospores produced on the needles are blown by wind to leaves of poplar where the uredospores are produced. These uredospores then reinfect leaves on susceptible poplars throughout the remaining growing season. The infected leaves fall and the cycle repeats itself. The closer the poplar trees are to the alternate host, the earlier in the season they can become infected.
Economic Importance:
Severe infections, especially those initiated early in the growing season, cause premature defoliation, loss of tree vigor, and predisposition to secondary disease fungi. Infections early in the season have been shown to reduce growth potential up to 20 percent and predispose trees to winter injury.
Management:
Monitor plantations for symptom development early in the growing season to enable timely control if disease exceeds acceptable levels.
Maintain low density plantations to promote air flow and to dry free moisture on leaves necessary for infections.
Plant only clones that have demonstrated resistance to the pathogen.
Rake, burn, plow under or otherwise dispose of fallen leaves before the onset of the following growing season.
Do not establish plantations in close proximity to alternate hosts in order to reduce or delay infection.


Septoria populi
Symptoms and Signs:
Disease symptoms caused by S. populi are highly variable. Leaf spots often appear circular, over 1 cm in diameter with dark brown or yellow margins. Symptoms may also consist of irregular large blotches with dark margins and tan centers with small black fruiting bodies at the center (Figs. 11. 12). Leaf spots may also be small. 1~2 mm in diameter, silvery and angular, often merging to form larger spots.
Fruit bodies are pycnidia which often form in the center of some spots. Conidia are slender, straight to often curved. 1 septate, hyaline. 32~48μm×3.0~3.5μm.
Ecology:
S. populi overwinters in fallen infected leaves. During periods of moist weather the following growing season, ascospores are rainsplashed to the current year's leaves. Conidia produced during the growing season infect leaves when moisture is present on the leaf surfaces. Trees infected early in the growing season sustain premature defoliation and terminal dieback.
Economic Importance:
Severe infections, especially those initiated early in the growing season, cause premature defoliation, loss of tree vigor, and pre-disposition to secondary disease fungi.
Management:
Monitor plantations for symptom development early in the growing season to enable timely control if infections exceed acceptable levels.
Maintain low density plantations to promote air flow and to dry free moisture on leaves necessary for infections to occur.
Plant only clones that have demonstrated resistance to the pathogen.
Rake, burn, plow under or otherwise dispose of fallen leaves before the onset of the following growing season.


Venturia populina
Symptoms and Signs:
Infected leaves have very large, irregular brown to black blotches. The fungus also infects current year shoots which become blackened and brittle, forming a distinctive curl, or “Shepherd's crook” (Fig. 13). Recently-infected tissue has a greenish appearance caused by the production of conidia.
Conidia are smooth. 2 (rarely 0-1-3) septate, straight or curved. 23~39μm× 9–14μm.
Ecology:
Only young leaves and shoots are susceptible. Older leaves become resistant. Infections usually occur from conidia produced on shoots and leaves killed the previous year. Conidia are spread by rainsplash.
Economic Importance:
This disease is rarely economically important.
Management:
Control is not necessary, because tree tissues develop resistance during the course of the growing season. Some clones are more resistant and should be favored.

Phyllactinia populi

Symptoms and Signs:
A white powdery patch with small, embedded, scattered, black fruit bodies, grow on infected leaves late in the growing season. Fig. 14
Fruit bodies are cleistothecia with distinctive appendages.
Ecology:
Powdery mildews are weak, late season parasites that primarily attack shaded leaves.
Economic Importance:
This disease is not economically important.
Management:
No control is necessary.

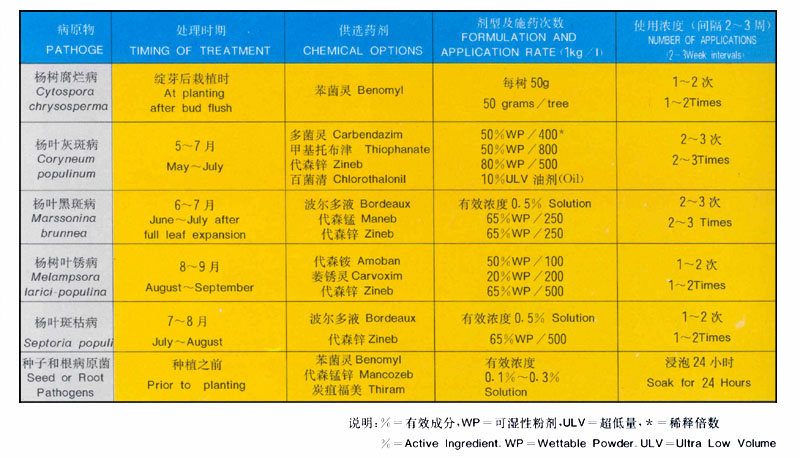
In most cases chemical control of poplar diseases cannot be justified. When diseases do become a problem the underlying cause is often stress because of poor cultural practices. planting highly susceptible clones. or not planting species that are best suited to the site. Prevention is the best strategy for management of poplar diseases.
There are. however. some circumstances when chemical control may be appropriate. Treating valuable seed to prevent damping—off disease caused by seed and soil—borne fungi. treating cuttings prior to planting. or treating newly established nurseries or plantations for foliar diseases if the disease outbreak occurred because of unusual weather conditions. Regular treatment of damaging levels of foliar diseases cannot be justified economically, biologically or environmentally. Plant resistant clones instead. Chemical treatment of canker diseases is usually not practical. and most often is treating the symptom. not the cause.
If chemical control is justified. tab. 2 provides the necessary information for treatment. Apply chemicals to foliage of individual seedlings or saplings uniformly. taking care not to apply more than will adhere to leaves.
Abiotic: nonliving disease agent, such as weather, mechanical, nutrient, or chemicals
Acervuli: saucer—shaped fruit bodies containing asexual conidia
Aeciospores: spores of rusts produced on the alternate host
Canker: a localized dead portion of the cambium and bark of branches and stem of a tree
Chlorosis: absence of sufficient chlorophyll in foliage resulting in yellow leaves
Conidia: asexual fungus spores
Fruit body: a general term for spore—bearing organs in both micro and macrofungi
Gall: swelling of plant tissues caused by certain fungi, bacteria, viruses, insects, mites, or nematodes
Inoculum: spores, mycelium, or other propagules of a pathogen that can infect a plant
Lesion: an injury or wound on a plant
Pycnidium: a flask—shaped fruit body containing asexual conidia
Septum: a cell wall or partition
Sign: visible evidence of a pathogen or insect
Symptom: visible response of a plant to a pathogen or an insect
Uredospores: repeating vegetative spores of rusts produced on the primary host
Bin—Cheng Zhang and Yi—Chun Huang. 1990. A list of important plant diseases in China. Review of Plant Pathology. Vol.69 No. 3. pp. 97~118.
Dali Tao. P. H. Li. J. V. Carter. M. E. Ostry. 1984. Relationship of environmental stress and Cytospora chrysoperma infection to spring dieback of poplar shoots. For. Sci. Vol. 30. No. 3. pp. 645~651.
FAO. 1979. Poplars and willows in wood production and land use. International Poplar Commission. FAO UN. Rome. FAO Forestry Series No. 10. 330 p.
Gremmen. J. 1978. Research on Dothichiza—bark necrosis (Cryptodiaporthe populea) in poplar. Eur. J. For. Path. 8 pp. 362~368.
McNabb Jr. Harold S. R. B. Hall. M. E. Ostry. 1982. Biological and physical modifications of the environment and the resulting effect upon the host—parasite inter-actions in short — rotation tree crops. IN: Resistance to diseases and pests in forest trees. Proc. Workshop 1980. Pudoc Wageningen. pp. 60~71.
Ostry. Michael E. L. F. Wilson. H. S. McNabb. Jr. L. M. Moore. 1989. A guide to insect, disease, and animal pests of poplars. USDA Forest Service. Agricultural Handbook 677. 117 p.
Ostry. Michael E.. H.S. McNabb. Jr. 1990. Minimizing disease injury to hybrid poplars. J. Environ. Hort. 8(2): 96–98. pp. 96~98.
Pinon. J. 1984. Management of diseases of poplar. Eur. J. For. Path. 14. pp. 415~425.
Pinon. J. 1982. Variability in the genus Populus in sensitivity to Melampsora rusts. Silvae Genetica 41. 1. pp. 25~34.
Spiers. A. G. 1984. Comparative studies of host specificity and symptoms exhibited by poplars infected with Marssonina brunnea. Marssonina castagnei and Marssonina populi. Eur. J. For. Path. 14 (1984) 202~218.
