Although the Centre's hatchery facility is comparatively well equipped, and the staff have been well trained, further improvements are necessary for full-scale function and operation.
The present temporary seawater intake close by the shore is recommended to be restored to the original point, at the end of the reef. The intake of turbid seawater from the shore causes not only problems with flagellate and ciliate blooms in the larval culture pools, but also it has been attributed as the cause of the epibionts that settle on the larval body surface (Section 2.6).
The capacity of the present seawater reservoir (20 m3 for storage and 20 m3 for filtration) is no longer sufficient for the expanded rearing activities. An additional large capacity roofed reservoir (100 m3) is needed with an automatic pumping switch for the continuous larval rearing operation during the rainy season when the salinity of seawater will be low, and a running seawater system is necessary for holding, rearing, especially for the full functioning of the induction of gonad maturation work. An additional blower (10 m3/min capacity) is needed to meet the hatchery aeration demand. Each of the seawater pumps and blowers is recommended to be powered by a diesel engine for emergency use.
The present plastic-lined pools used for the culture are not permanent installations, and there have been problems with leakages and repairs. There has been a shortage of pools due to the increased postlarval production, food organism culture, and especially with the recent induced maturation and spawning work. Therefore, it is recommended that a series of 20 permanent concrete tanks (5 m long × 3 m wide × 1 m deep) be constructed for both larval rearing and nursery work. These tanks should have sloped bottoms with facilities for outside control stand-pipes with inside screens. Catch basins on both inside and outside the tanks should be provided for easy harvesting. By providing a nearly square dam board near the outlet pipe, soil can be used to fill the entire tank bottom outside this catch basin to serve as an improvised soil bottom for nursery work (Figure 5).
Thermostat controlled submersible electric heaters are needed to maintain culture water temperature during nights in the dry season when the ambient temperature is low. It is essential to dry completely and also to sterilize culture tanks each time after using the chlorine method.
In June 1978, the Centre began to distribute the excess stock of postlarvae to farmers. In order to meet the requirements of forthcoming extension programmes, a large number of postlarvae production is one of the urgent tasks. An accelerated exploratory fishing by the K.M.WINDU is recommended to have a clear understanding of the distribution and seasonal abundance of both gravid females and broodstock for the induction of gonad maturation in this area. This information is also useful for the future programming of induced gonad maturation work to obtain large numbers of spawners at a time. Also an extensive search for broodstock of pond-grown P. monodon is necessary. A trial experiment of the air-transport of broodstock of P. monodon from South Sulawesi by packing with the chilled and dried sawdust should be conducted. This method was used for the transportation of P. japonicus gravid females from Taiwan to Japan. The mortality was only 15 percent and the spawning rate was 32 percent (Imamura, 1974).
For the growth and survival of larvae, natural food is far better than formulated feed, and the role of such a natural diet will be clearly reflected in the size and weight of resulting postlarvae. The mass-culture system of phytoplankton stock culture of Chlorella, and the rotifer, Brachionus has now been established in the Centre. It is essential to prevent possible contamination from other organisms in the early steps of the stock or intermediate culture.
Artemia eggs have recently been available from many parts of the world and the price has been maintained at a more or less constant level. However, because of its importance in the production of P. monodon postlarvae, it is recommended that the feasibility of domestic production of Artemia cysts during the dry season be explored by using the Centre's swimming pool and small nursery ponds following up the work of Mr Marc Talloen, the Associate Expert.
For the larval feed, the concentration of the mass-cultured phytoplankton by a cream separator and the excess production of cultured rotifers are recommended to be collected and stored in frozen form in small containers with a known population.
Holding postlarvae in the hatchery beyond PL-5 or 6 should not be practised to avoid undue mortality. However, due to the culture water problem along with the artificial feed, one of the methods to reduce the mortality is by harvesting the postlarvae at a very early stage (PL-2) and transferring them into a large cement tank which has been fertilized until they attain stocking size.
For the best survival and growth to 0.10–0.15 g for the stocking size, the optimal stocking rate for the well-prepared nursery pond is 150 (PL-5) per m2 for 20 days, and the high density rearing in the aerated nursery tank on formula diet is recommended at a stocking rate not to exceed 700 (PL-5) per m2 with maximum 40 days rearing.
It is desirable to have some source of freshwater on the nursery ponds site to dilute the high salinity pond water during the dry season.
As indicated earlier, there is no tradition of local fish farmers using P. merguiensis for deliberate stocking of ponds because the unexplained disappearance of this species in culture ponds is a common occurrence.
A series of experiments has been carried out to develop the possibility of culture of this species: (a) Under controlled conditions in cement tanks; the survival rate was quite high mainly due to the absence of predators and cannibalism was low (Section 2.5.3); (b) On the contrary, in the shallower earthern ponds with seepage, higher temperature fluctuation and the presence of predators, the growth of surviving specimens was remarkable although the survival rate was low (Secion 2.5.4); (c) Based on the above results, experiments were conducted initiating interim fish eradication and maintaining water level by taking in fresh tidal water or by pumping as frequently as possible (Section 2.5.6).
The last experiment proved that annual production of shrimp can be projected as high as 600 kg per ha without direct feeding; only the increment of natural productivity by fertilization of the pond was utilized. Culture of this species is to be encouraged.
For the development of further production increase, the following activities are recommended.
Because of the narrow tidal amplitude and the shallowness of the ponds, it is desirable to have a wide and deep peripheral furrow in culture ponds and the central plateau must be dried by pumping out water from the pond. The stocking rate under Jepara conditions should not exceed two shrimps per m2, and it was recommended that large postlarvae over 0.1 g size take advantage of their exponential growth period. The shrimp must be harvested at nearly 10 g, which takes about two months growing period for attaining this size, in order to prevent further mortality. Intensive trapping is necessary for nearly complete harvesting and later harvesting by drying the pond through pumping.
The initial fish extermination is essential and must be effective. Derris plus tobacco dust is recommended for those ponds heavily infested by polychaetes. An interim fish extermination after 20 days of stocking is an essential practice to increase shrimp production. Derris root is used and the strength varied from time to time because of the differences in root size and prolonged storage in water after pounding (Section 2.5.8). In order to obtain constant results and effective operation, the dried roots have to be collected in large quantities from South Sumatra and dried, and finely powdered by the hammer mill and stored in airtight packages after determining the strength.
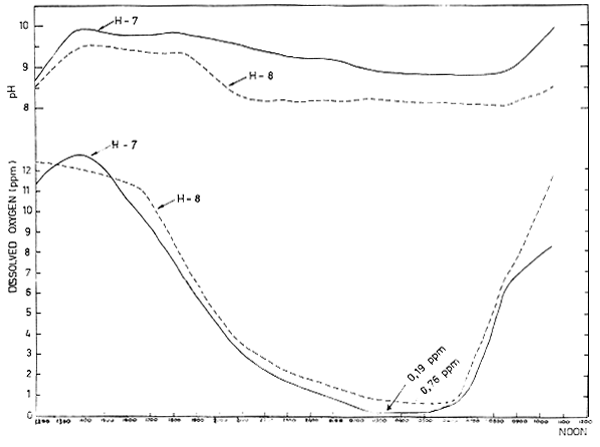
As previously stated, in shallow ponds resulting from limited tidal amplitude, the dissolved oxygen in the water often reaches lethal levels for shimp, particularly when continuing inputs of fertilizers are provided for increasing production. An example of the dissolved oxygen and pH fluctuations in a pond with heavy fertilization prior to stocking is shown in Figure 6. This often leads to difficult management problems when intake of water for the application of fertilizers cannot be carried out because of limited tidal range. It is therefore essential to have a well timed fertilizer application schedule that takes into account the period when high tidal range is available, so that drastic depletion of oxygen can be avoided. It is therfore advisable to continually check the oxygen level by the oxygen meter in the stocked ponds and when it reaches below 3.0 ppm level action should be taken to allow sufficient new seawater into the ponds. If this does not become feasible because of insufficient tidal difference, a positive measure such as by pumping in water should be arranged especially during early morning hours.
The behaviour of shrimp should be checked even at daytime, including the examination of phytoplankton population present, to forestall the appearance of Peridinium sp. bloom in the ponds.
It is strongly recommended to experiment the progression farming system in the five ha “F” series ponds, originally designed for this system of culture, and used by the Research Group of the Ministry of Agriculture. The location of series pond requires additional excavation of the canal to connect the existing canals on both sides to improve water circulation.
It is necessary to feed shrimp during the rainy season since the provision of natural food and salinity is low in ponds, as mentioned earlier. A supplementary feeding experiment is recommended by collecting pond-grown fish or various formulated feed. Cheap trash fish available in the market and shell fish not being utilized in the locality should also be examined.
As mentioned in a previous section, the tidal range of this area is narrow, and the problems of ponds are insufficient water depth and the difficulties in water addition to the shrimp pond. It is therefore desirable to have comparative P. merguiensis farming experiments with selected fish farmers in East Java area, where the tidal amplitude is wide, to provide guidance for proper shrimp pond management in these different areas.
Sufficient facilities and expertise are now available to organize a training course on induced maturation and hatchery production of postlarvae of penaeid shrimp. It would be organized by the staff of the hatcheries, and operated by provincial fisheries administration. This can aim at providing individual participants with first-hand experience in rearing at least four batches of postlarvae.
A training of selected fish farmers in industry as well as fisheries extension personnel can provide field training in the preparation, stocking and management of nurseries and ponds. The postlarvae required for stocking their ponds could be given free. Technical guidance of culture operation by the farmers should be continued by the Centre, preferably through a regular extension unit attached to the Centre. After a reasonable period of trial, postlarvae could be sold to bona fide farmers at cost.
Acuacop., 1977 Reproduction in captivity and growth of P. monodon Fabricius in Polynesia. Centre oceanologique du Pacifique CNEXD-COP B.P. 7004 Taravao Tahiti. 20 pp.
Alikunhi, K.H., A. Poernomo, S. Adisukresno, M. Budiono and S. Busman, 1975 Preliminary observations on induction of maturity and spawning in Penaeus monodon Fabricius and Penaeus merguiensis de Man by eyestalk extirpation. Bull. Shrimp Cult. Res. Cent., 1(1):1–11
Cook, H.L., 1976 Problems in shrimp culture in the South China Sea Region. SCS/76/WP/40, 29 pp.
Djajadiredja, D. and A. Purnomo, 1974 Review of coastal water resources in relation to coastal aquaculture. IPFC Procs., 15(III):159–172
Hirata, H., Y. Mori and M. Watanabe, 1975 Rearing of prawn larvae, Penaeus japonicus, fed soycake particles and diatom. Marine Biol., (29):9–13
Hudinaga, M. and J. Kittaka, 1966 Studies on food and growth of larval stage of a prawn, Penaeus japonicus, with reference to the application to practical mass culture. Inform. Bull. Planktol.Jap., 13:83–94
Imamura, S., 1974 Taiwan san kuruma-ebi no sairanyo oya-ebi to shi te no riyo kachi ni tsuite (On the utilization value of Formosan Penaeus japonicus as the spawning gravid females). Saibai Kiken 3(1):77–85 (in Japanese)
Schuster, W.H., 1952 Fish culture in brackishwater pond of Java. IPFC Spec.Publ.No.1, 143 pp.
Tuma, D.J. 1967 A description of the development of primary and secondary sexual characters in the banana prawn, Penaeus merguiensis de Man (Crustacea Decapods:Penaeinae). Aust.J.Mar.Freshwat.Res., 18:73–88
Wongwinyutrakarn, S., 1971 A preliminary study on the maturity stages of Kung Chaebuoy, Penaeus merguiensis de Man and Kung Kula-Lai, Penaeus semisulcatus de Haan. Second Sympo.Mar.Fish., Mar.Fish.Lab., Bangkok., 49 pp. (Mimeographed)
Yang, W.T., 1975 A manual for large-tank culture of penaeid shrimp to the postlarval stages. Miami, Florida, Sea Gant Technical Bulletin No. 31, University of Miami, 94 pp.
NUMBER OF OVIGEROUS PENAEID SHRIMPS COLLECTED BY K.M. WINDU
(During the period from May 1976 to June 1978)
Year Month | No. of days in operation | No. of hauls | Penaeus merguiensis | Penaeus monodon | Penaeus semisulcatus |
| 1976 | |||||
May1 | 9 | 20 | 16 | 1 | 0 |
June1 | 6 | 36 | 11 | 3 | 1 |
July1 | 2 | 11 | 3 | 3 | 3 |
August | 6 | 29 | 24 | 1 | 1 |
September2 | No operation | ||||
October | 12 | 40 | 1 | 0 | 2 |
November | 7 | 29 | 15 | 0 | 1 |
December | 9 | 44 | 14 | 2 | 7 |
| 1977 | |||||
January | 7 | 39 | 15 | 2 | 1 |
February | 7 | 28 | 8 | 0 | 2 |
March | 9 | 43 | 18 | 0 | 0 |
April2 | 8 | 36 | 17 | 3 | 0 |
May | 14 | 70 | 48 | 6 | 3 |
June | 7 | 43 | 35 | 2 | 1 |
July | 6 | 34 | 9 | 0 | 0 |
August | 15 | 73 | 30 | 0 | 2 |
September | 9 | 44 | 31 | 0 | 0 |
October | 15 | 81 | 19 | 2 | 0 |
November | 12 | 68 | 11 | 0 | 0 |
December | 14 | 74 | 7 | 2 | 20 |
| 1978 | |||||
January | 10 | 50 | 8 | 4 | 7 |
February2 | 1 | 7 | 2 | 1 | 0 |
March | 13 | 71 | 17 | 7 | 0 |
April | 7 | 29 | 18 | 2 | 0 |
May | 16 | 94 | 9 | 1 | 6 |
June3 | 4 | 21 | 1 | 7 | 2 |
Total: | 225 | 1,114 | 387 | 49 | 59 |
Remarks: The ovigerous females include those with ovaries in stages III and IV
1 Boat engaged on the other research activities during part of month
2 Boat was under repair service during part of this month
3 Data up to 10 June 1978
OBSERVED FECUNDITY (AS NAUPLIUS 1–2) OF P. MERGUIENSIS AND P. MONODON
| Date obtained | Weight (g) | Total length (mm) | Number of early nauplii produced |
| P. merguiensis | |||
| 3/12/75 | 32 | 156 | 210 000 |
| 1/7/77 | 46 | 164 | 345 000 |
| 15/5/75 | 54 | 187 | 446 000 |
| 13/8/75 | 54 | 185 | 375 000 |
| 31/8/75 | 80 | 200 | 186 000 |
| 28/7/77 | 105 | 225 | 494 000 |
| 10/7/75 | 110 | 224 | 342 000 |
| 29/7/75 | 125 | 235 | 44 300 |
| P. monodon | |||
| 31/10/77 | NW1 | 218 | 515 000 |
| 9/1/78 | 200 | 275 | 1 285 000 |
| 24/4/76 | 240 | 290 | 1 216 000 |
HATCHERY PRODUCTION OF PENAEID POSTLARVAE IN 1975 TO 1978
| Species | 1975 | 1976 | 1977 | 19781 |
| Penaeus merguiensis | 583 800 | 1 032 000 | 2 341 000 | 1 222 000 |
| Penaeus monodon | - | 167 900 | 591 000 | 1 045 000 |
| Penaeus semisulcatus | - | - | 53 000 | - |
| Metapenaeus monoceros | - | - | 1 078 000 | - |
| Total | 583 800 | 1 199 900 | 4 063 000 | 2 267 000 |
1 The production figures at June 1978, excluding the culture in progress
EXAMPLE OF RESULTS OF POSTLARVAL PRODUCTION
Species: Penaeus monodon
(BP pool: bottom area 10 m2)
| Pool | Original spawning | Divided | Total |
| No. of spawner and size (mm) | 1 (12.8) | - | |
| Nauplii population | 515 000 | - | 515 000 |
| Population division at mysis-II | 208 0001 | 137 000 | |
| Harvested population | 257 000 | 118 000 | 375 000 |
| Average survival rate (%) | 72.8 | 72.8 | |
| Individual weight at PL-5 (mg) | 1.2 | 1.2 | |
| Duration: | |||
| N-1 to PL-1 (days) | 10 | 10 | |
| Total Artemia given (g) | 700.9 | 279.3 | 980.2 |
| No. of rotifer (× 106) | 29.4 | 3.8 | 33.2 |
Species: Penaeus merguiensis
| Culture number | 1 | 2 | |
| Pool | Spawned | Original spawning | Divided |
| No. of spawners and size (g) | 3(36, 38, 59) | 5(40, 44, 45, 80, 86) | |
| Nauplii population | 580 000 (N-1) 145 000/m3 | 740 000 148 000/m3 | 610 000 122 000/m3 |
| Harvested population | 302 000 (PL-6) 43 100/m3 | 324 000 (PL-5) 46 300/m3 | 201 000 (PL-5) 28 700/m3 |
| Survival rate (%) | 52 | 44 | 33 |
| Individual weight at PL-5 (mg) | 1.5 | 1.5 | 1.7 |
| Duration: N-1 to PL-1 (days) | 9 | 10 | 10 |
| Total Artemia given (g) | 1 490 | 926 | 789 |
| No. of Copepoda given (× 1,000) | 1 354 | 796 | |
1 Population counting underestimated
FEEDING OF LATE PENAEUS MERGUIENSIS LARVAE ON BRACHIONUS DIET
| Larval stages | Population (estimated) | Total Rotifer supplied | Rotifer/ml of culture water | Rotifer/larvae |
| (× 103) | ||||
| Z-3 | 301 000 | 3 790 | 2.5 | 10.1 |
| Z-3 (19 %) M-1 (81 %) | 303 000 | 17 960 | 11.7 | 71.8 |
| M-1 | 175 000 | 19 680 | 12.9 | 112.5 |
| M-2 | NE1 | 8 528 | 5.6 | NC* |
| M-3 | 146 000 | 5 365 | 3.6 | 36.6 |
| M-3 (81 %) PL-1 (19 %) | 122 400 | 4 000 | 2.6 | 32.7 |
| M-3 (14 %) PL-1 (86 %) | 103 900 | 5 062 | 3.3 | 48.7 |
| Average | 6.0 | 52.1 | ||
MORTALITY OF P. MERGUIENSIS POSTLARVAE UNDER DIFFERENT DENSITIES OF PACKING
| No. 1 | No. 2 | No. 3 | No. 4 | |
| Number of larvae packed | 5 000 | 10 000 | 15 000 | 20 000 |
| Number died | 266 | 1 564 | 1 978 | 4 443 |
| Mortality rate (%) | 5 | 16 | 13 | 22 |
GROWTH AND FEEDING OF P. MERGUIENSIS POSTLARVAE ON A MIXED DIET OF
KLEKAP, CHIRONOMID LARVAE AND GROUND FISH
(Bottom area 40 m2)
| Postlarval designation | Rearing days in tank | Average weight (mg) | Food material (wet weight) | ||
| Weight of klekap (kg) | Chironomid larvae (g) | Ground fish (g) | |||
| PL - 6 | 1 | 1.2 | 22.5 | - | 900 |
| PL - 11 | 6 | 9.8 | 101.0 | - | 500 |
| PL - 16 | 11 | 25.0 | 55.0 | 200 | 500 |
| PL - 21 | 16 | 34.0 | 110.0 | 1 000 | 3 250 |
| PL - 36 | 31 | 45.0 | - | - | 2 250 |
| PL - 42 | 37 | 47.0 | |||
| Total (kg) | 288.5 | 1.2 | 7.4 | ||
STOCKING, GROWTH AND APPROXIMATE SURVIVAL RATES OF P. MERGUIENSIS IN NURSERY PONDS
| Pond | 1 | 2 | 3 | |
| Area (m2) | 480 | 410 | 420 | |
| Stocking rate/m2 | 60 | 150 | 320 | |
| Pond rearing (days) | Postlarvae | Weight (mg) | Weight (mg) | Weight (mg) |
| 1 | PL - 6 | 1.1 | 1.1 | 1.1 |
| 6 | PL - 11 | 6.6 | 9.1 | 11.4 |
| 11 | PL - 16 | 31.0 | 39.0 | 34.0 |
| 16 | PL - 21 | 168.0 | 118.0 | 79.0 |
| 31 | PL - 36 | 452.0 | 139.0 | 64.0 |
| 35 | PL - 40 | 470.0 | 173.0 | - |
| 41 | PL - 46 | 540.0 | 345.0 | 100.0 |
| No. harvested | 19 100 | 25 800 | 17 400 | |
| Approximate survival rate (%) | 66 | 41 | 12 | |
| Inputs (kg) | ||||
Cowdung | 210.60 | 196.30 | 186.20 | |
Coconut oil cake | 1.86 | 2.03 | 1.82 | |
Urea | 3.87 | 3.84 | 3.62 | |
Superphosphate | 0.77 | 1.01 | 0.92 | |
| Derris roots | 0.75 | 0.75 | 0.75 | |
GROWTH, SURVIVAL AND CONVERSION RATES OF P. MERGUIENSIS POSTLARVAE
ON FORMULA DIET AND COBALT CHLORIDE TREATMENT
(Stocking rate: 1 000/m2, duration 15 days)
| Pool no. | 1 | 2 | 3 | 4 |
| Cobalt chloride | Treated | Not treated | ||
| Stocking | ||||
No. stocked | 2 500 | 2 500 | 2 500 | 2 500 |
Ave. weight (mg) | 1.2 | 1.2 | 1.2 | 1.2 |
Total weight (g) | 5.0 | 5.0 | 5.0 | 5.0 |
| Harvesting | ||||
| No. harvested | 2 373 | 2 390 | 2 442 | 2 378 |
| Survival rate (%) | 94.9 | 95.6 | 97.7 | 95.1 |
| Ave. weight (mg) | 40.0 | 34.0 | 31.5 | 32.0 |
| Net production (g) | 89.9 | 76.2 | 71.2 | 71.1 |
| Total feeding (g) | 162 | 162 | 162 | 162 |
| Conversion ratio | 1.8 | 2.0 | 2.3 | 2.3 |
GROWTH, SURVIVAL AND FOOD CONVERSION RATES OF P. MONODON POSTLARVAE
IN POOLS UNDER DIFFERENT DIETS AND STOCKING RATES
| Pool No. | 1 | 2 | 3 | 4 | 5 | 6 |
| Duration (Days) | 15 | 15 | 15 | 15 | 15 | 15 |
| Bottom soil | Fresh | Old | Fresh | Old | Fresh | Fresh |
| Stocking /m2 | 500 | 500 | 750 | 750 | 1,000 | 1,000 |
| Stocking | ||||||
| No. stocked | 5 000 | 5 000 | 7 500 | 7 500 | 10 000 | 10 000 |
| Ave. weight (PL-5) (mg) | 1.83 | 1.83 | 1.83 | 1.83 | 1.83 | 1.83 |
| Total weight (mg) | 9.15 | 9.15 | 13.73 | 13.73 | 18.30 | 18.30 |
| Sampling | ||||||
| Ave. weight (mg) | ||||||
| 5th day (PL-10) | 4.5 | 4.1 | 4.3 | 4.3 | 4.2 | 5.3 |
| 10th day (PL-15) | 16.0 | 18.3 | 16.8 | 11.9 | 12.8 | 12.5 |
| 15th day (PL-20) | 46.0 | 51.0 | 46.0 | 21.0 | 33.0 | 28.0 |
| Ave. length (mm) | 19.9 | 20.1 | 19.6 | 16.1 | 18.2 | 18.0 |
| Harvesting | ||||||
| No. harvested | 3 456 | 990 | 3 705 | 3 633 | 6 711 | 7 459 |
| Survival rate (%) | 69 | 20 | 49 | 48 | 67 | 75 |
| Net production (g) | 149 | 50 | 170 | 76 | 203 | 191 |
| Total feeding (g) | 223 | 223 | 315 | 315 | 440 | 440 |
| Conversion ratio | 1.5 | 4.5 | 1.9 | 4.1 | 2.2 | 2.3 |
| DO ppm at 04.00 hrs | ||||||
| 19/5/76 | 2.04 | 2.19 | 2.67 | 2.04 | 2.98 | 2.82 |
| 20/5/76 | 3.14 | 3.14 | 2.67 | 2.04 | 2.51 | 2.20 |
RESULTS OF MIXED CULTURE OF PENAEID SHRIMPS
Duration: P. merguiensis 2 months
P. monodon 5 months
Stocking rate: 2 shrimp/m2 Pond area: 5 000 m2
| Pond No. | 1 | 2 | 3 | 4 | 5 | 6 |
| Stocking | ||||||
| P. monodon | ||||||
| No. stocked | 5 400 | 5 000 | 2 000 | 1 500 | 1 750 | 2 000 |
| Ave. weight (g) | 0.8 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| P. semisulcatus | ||||||
| No. stocked | 5 000 | - | - | - | - | - |
| Ave. weight (g) | 0.3 | - | - | - | - | - |
| P. merguiensis | ||||||
| No. stocked | - | 5 000 | 8 000 | 8 500 | 8 250 | 8 000 |
| Weight range (g) | - | (0.1) | (0.1) | (0.03–0.1) | (0.3) | (0.03–0.1) |
| Harvest | ||||||
| P. merguiensis | ||||||
| No. harvested | 124 | 3 364 | 7 455 | 8 032 | 7 048 | 6 998 |
| Survival (%) | - | 67.3 | 93.2 | 94.5 | 85.4 | 87.5 |
| Ave. weight (g) | 9.8 | 6.3 | 9.1 | 4.3 | 5.7 | 5.4 |
| Net production kg/ha crop | 2.4 | 41.4 | 134.2 | 68.0 | 80.0 | 74.6 |
| P. monodon | ||||||
| No. harvested | 517 | 580 | 604 | 863 | 161 | 152 |
| Survival (%) | 9.6 | 11.6 | 30.2 | 57.5 | 9.2 | 7.6 |
| Ave. weight (g) | 22.2 | 22.5 | 24 | 18.1 | 17.6 | 12.8 |
| Net production kg/ha/crop | 14.4 | 24.0 | 28.2 | 30.6 | 4.8 | 3.0 |
| M. monoceros (Natural stock) | ||||||
| No. harvested | 1 143.0 | 1 646.0 | 1 656.0 | 233.0 | 780.0 | 1 558.0 |
| Ave. weight (g) | 6.1 | 3.4 | 4.5 | 3.1 | 3.2 | 2.9 |
| Production kg/ha/crop | 14.0 | 11.2 | 15.0 | 1.4 | 5.0 | 9.0 |
| Projection for shrimp production kg/year1 | 65.62 | 210 | 597 | 278 | 340 | 334 |
| Edible fish (Tilapia and other) | ||||||
| Production kg/ha/crop | 116.0 | 158.0 | 28.8 | 122.2 | 159.4 | 345.8 |
| Total gross production kg/ha/crop | 146.8 | 234.6 | 206.2 | 222.2 | 249.2 | 432.4 |
2 Production based only for wild stock brought in with the water supply.
INTAKE RATE OF BURROWED CHIRONOMID LARVAE BY JUVENILE P. MERGUIENSIS FOR 24-HOUR PERIOD
| Number of shrimp | Average shrimp weight (g) | Depth of mud (cm) | Depth of water | Total number of chironomid larvae taken | Average number of chironomid larvae taken per shrimp |
| 4 | 0.05 | 2 | 9 | 28 | 7.0 |
| 2 | 0.05 | 3 | 8 | 11 | 5.5 |
| 4 | 0.06 | 4 | 7 | 87 | 23.2 |
Fig. 1 View of semi automatic water level adjusting siphon
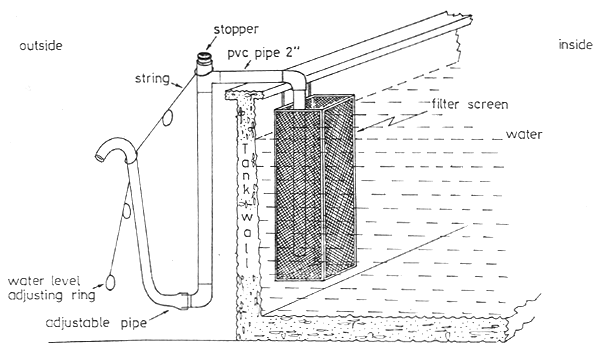
 Fig. 2 LAYOUT Experiment and demonstration ponds BRACKISH WATER SHRIMP AND MILFISH APPLIED RESEARCH AND TRAINING PROJECT Jepara, Indonesia scale. 1 : 7000  | 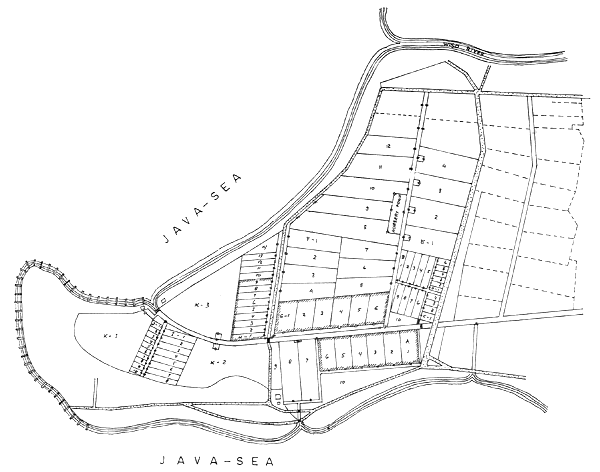 |
| SHRIMP CULTURE PONDS : | |
| A1 - 6 H1 - 9 G1 - 6 |
Fig. 3 View of sluice gate with filter and dam board
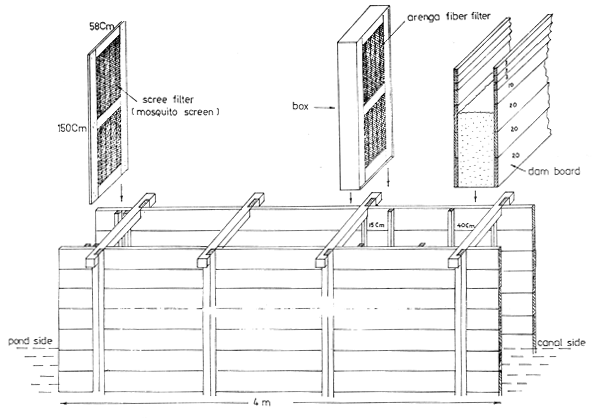
Fig. 4 LARGE SHRIMP SAMPLING TRAP
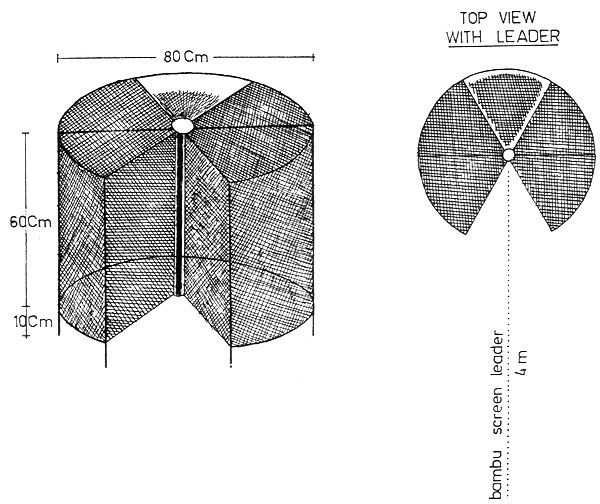
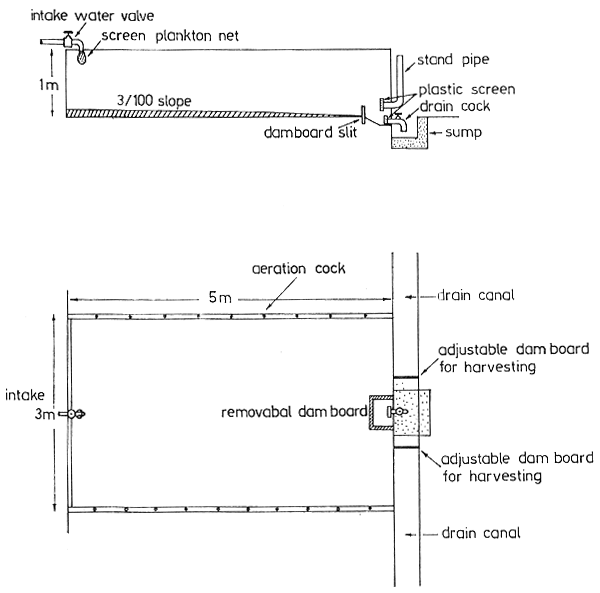
Fig. 5 Cement tanks for larval rearing and nursery
Fig. 6 DIURNAL DO AND pH CHANGES AFTER HEAVY FERTILIZATION