3.1 Physical Properties - Fresh
Water Ice
3.2 Seawater Ice
Ice has long been used to cool, and thereby preserve fish both at sea and on-shore. It has a large cooling capacity for a given weight, is relatively cheap, prevents drying by keeping fish moist, is easily portable and maintains a temperature slightly above the freezing point of fish without the need for sophisticated temperature control. It can be made from fresh water or sea water which should in both cases meet micro-biological standards of potable water and be free of objectionable substances. Ice made from polluted water can contaminate fish and reduce its keeping time.
The physical properties of fresh water ice are given in Table 3.
Table 3 Physical properties of fresh water ice
| Property |
|
| Melting point |
|
| Density at 0�C a/ | 0.92 t/m� |
| Specific heat at 0�C | 0.49 Kcal/Kg �C |
| Specific heat at -20�C | 0.46 Kcal/Kg �C |
| Latent heat | 80 Kcal/Kg |
| Thermal conductivity at 0� | 1.91 Kcal/mh �C |
| Thermal conductivity at -10�C | 1.99 Kcal/mh �C |
| Thermal conductivity at -20�C | 2.08 Kcal/mh �C |
a/ The figure quoted for the density of ice should not be confused with the stowage density of different forms of ice which are given in Table 4
Table 4 Stowage densities of ice types
| Type | Stowage (m�/t) a/ |
| Crushed block | 1.4-1.5 |
| Tube | 1.6-2.0 |
| Plate | 1.7-1.8 |
| Flake | 2.3-2.3 |
a/ Note that these stowage densities In Table 4 do not necessarily relate to the size of store required for a given weight of ice without allowance being made for handling. See also Section 5, Ice Storage
The cooling capacity of the different types of ice will be practically the same for equal weights but not for equal volumes due to the difference in stowage density. Subcooled ice will have marginally more cooling capacity due to being sub-cooled and dry. Wet ice caused by hot gas or water defrost systems will have slightly reduced cooling capacity due to the free water contained in the ice, as shown in Figure 7, but for practical purposes the cooling capacity of equal weights of ice types can be considered the same.
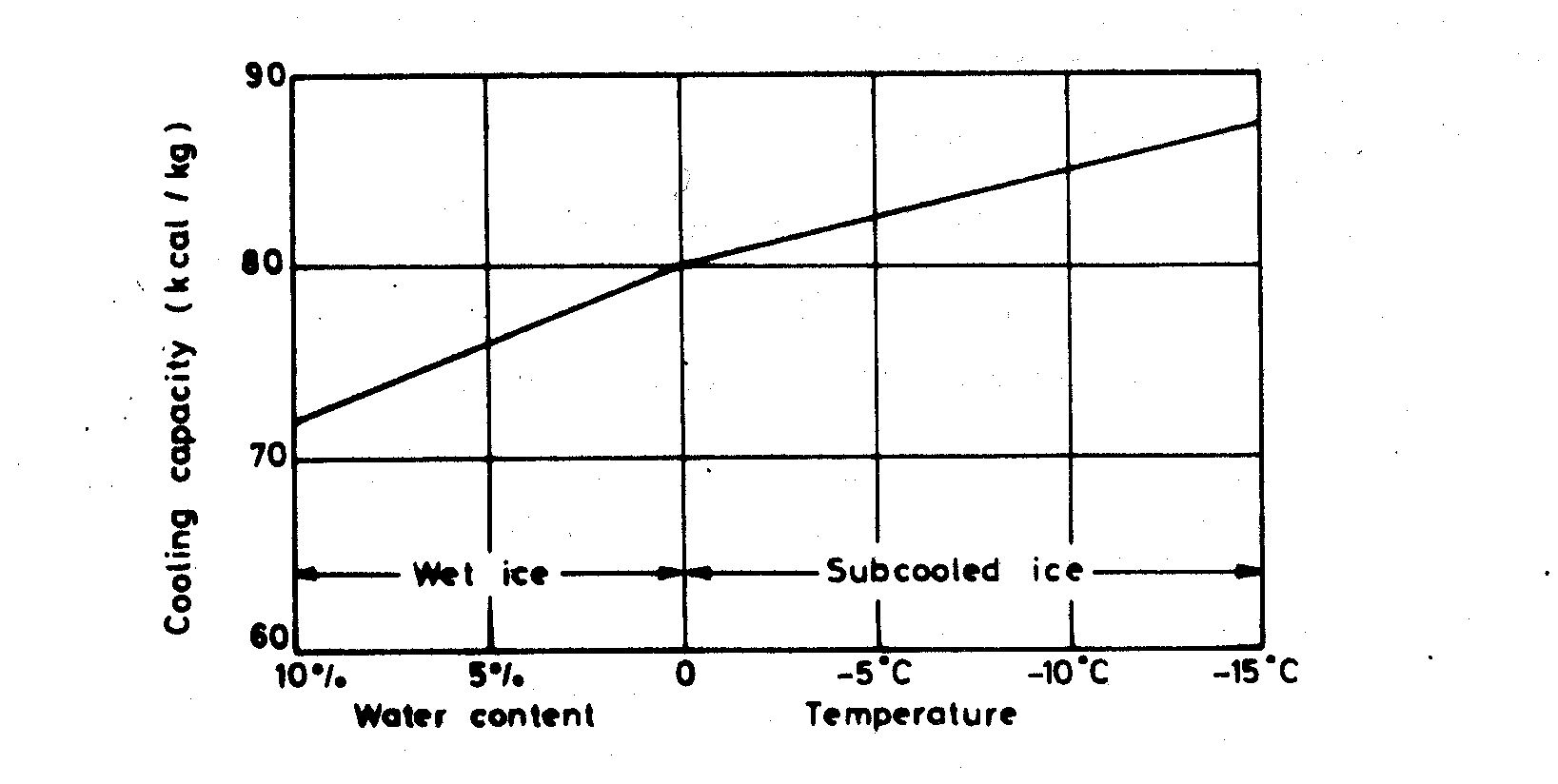
Figure 7. Cooling capacity of ice
The cooling rate or rate of meltage of ice will not be the same for different types of ice and will depend on the surface area of the ice. For example, flake ice has a surface area of over four times that of tube, weight for weight which can be an advantage or a disadvantage depending on its intended application. A poor stowage rate is a disadvantage for applications where space is limited and will give a slightly reduced capacity of fish in a box. Flake ice also has a tendency to bridge over the fish leaving an air space between the fish and the ice, thereby reducing effective cooling but is easier to handle as it is dry and produces the least damage to fish of all the forms of ice.
Seawater is suitable for ice making provided it is not contaminated and produces an ice soft and wet in appearance. It is not stable and during storage the brine tends to leak out leaving fresh water ice. The freezing point is dependent on the salt content as shown in Figure 8. When used to cool fish partial freezing of the fish can occur which is not desirable and salt uptake in the fish flesh is noticeable with prolonged storage. Depending on salinity and the amount of entrapped air it has a density in the range of 0.86 to 0.92 t/m3. It has application where fresh water is expensive or in short supply and can be used for production at sea.
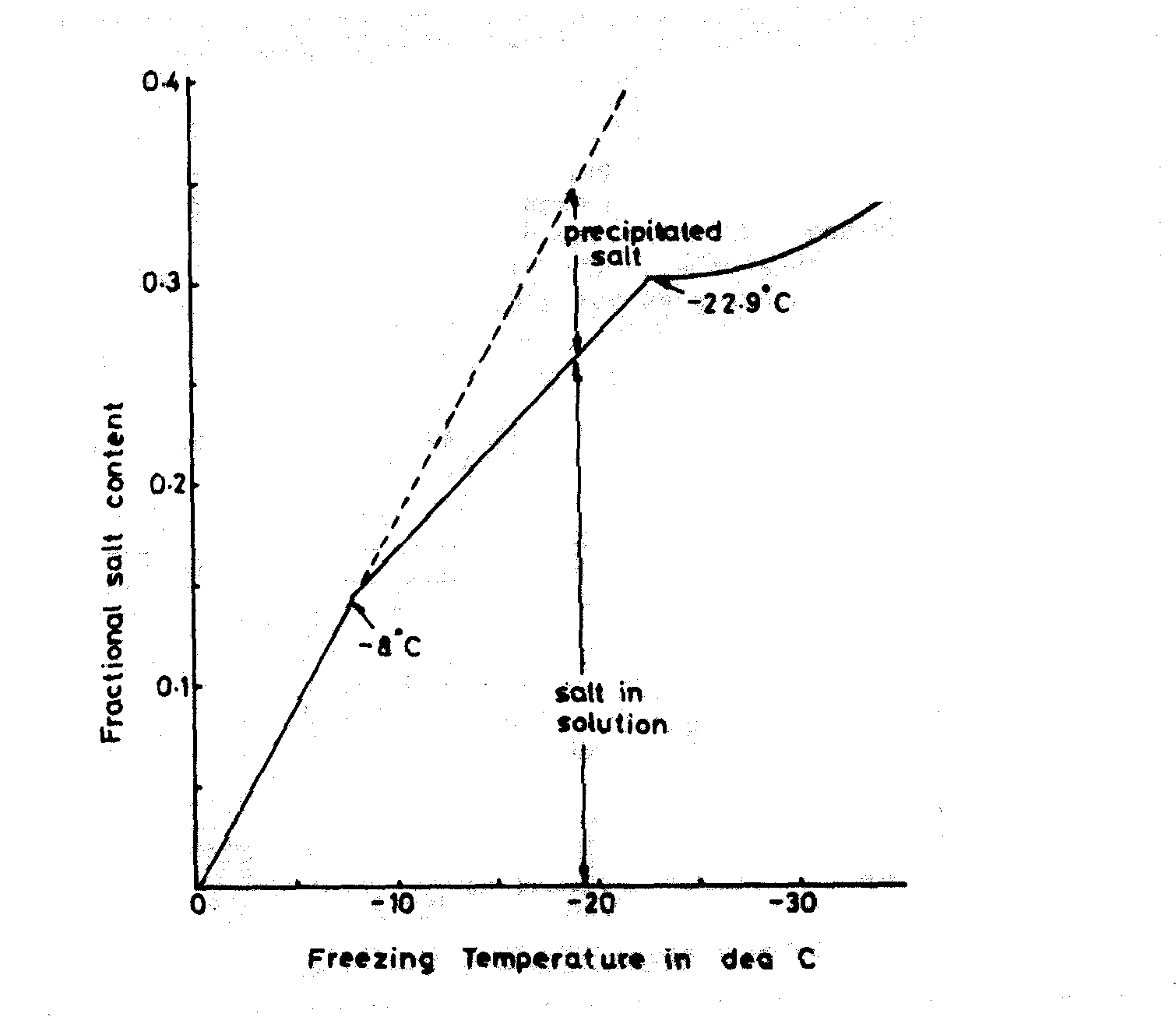
Figure 8. The freezing point of brine as a function of its salt content