Although numerous papers have been published on the hydrobiological characteristics of fish ponds and experience gained on certain practical aspects, no uniformity could be found in the data on different parameters relevant to fish culture. A comparative assessment of the basic architectural characteristics of such undrainable rural fish ponds is thus problematic. Therefore, to make a uniform quantification of the main characteristics of the ponds, a routine environmental monitoring system has been developed and monitoring of about 30 ponds in the Cuttack and Puri Districts has been completed.
The bottom of the perennial ponds is never exposed to sunlight and therefore their ecosystem is quite different from those of shallow and seasonal ponds. Although these ponds serve multipurpose uses ranging from supplying drinking water for human consumption, for agriculture and livestock, the recent trend is to utilize them for fish culture. In this context also, it is essential to develop a reasonably rapid environmental monitoring system to survey the basic architecture and production processes in this particular type of fish pond ecosystem to ensure successful management for optimization of fish production. The system needs to be simple with regard to the instrumentation, time-schedule and labour, while offering sufficient information on the nutrient and productive status of the pond sediment and water. On the basis of detailed environmental analysis of several types of shallow water ecosystems, it was decided to include 31 parameters in this monitoring system. Most of the parameters can easily be measured on the pond site except for the six chemical components of the monitoring system. Quantification of some of the above parameters may at times be made on visual site estimation. Figure 1 shows the design of the environmental monitoring system and Table 1 shows the evaluation sheet. A brief description follows on the relevance, importance and methodology of all the parameters selected for this environmental monitoring system.
1. Coding pond number and name. Presently there exists no system of coding the name of the ponds. It is essential to give name and code number of the ponds which may be based either on postal district/unit or other uniform standard.
2. Water area. Measurement of the water area is essential in order to know the size of the pond for proper fish stocking and quantifying the production processes. This can be done easily with the help of a bamboo rod.
3. Age. Age is one of the most important parameters since it has direct relevance with the productivity of the pond; and can vary from one to several hundred years.
4. Management. Management status should include any existing management technique; and its level (intensive or extensive.) The species of fish present, details of culture activities, stocking structure and density, fertilization, feeding, harvesting, marketing, etc., need to be known. To obtain quantified data on the organic carbon and biogenic nutrient load it is necessary to know the number of livestock and human population associated with the particular pond.
5. The visual colour. The visual colour is a simple but important reflection of the basic production processes.
6. Water transparency. Water transparency measured with Secchi disc is intended to quantify the result of those processes which determine and modify the visual colour. However, a low transparency alone may result either from high turbidity or by dense algal population and thus cannot reflect the correct trophic or production level of the water. However, at the same time, the quantitative nature of the Secchi transparency together with the visual colour has a high practical value in fish pond management.
7. Water depth. The primary water source is the heavy rainfall during the monsoon. After the rainy season the water level gradually decreases which results in a very shallow water column by the end of the dry season. The water depth can be measured with a 6–8 m long bamboo rod equipped with a wooden disc of 25 cm diameter.
8. Soft sediment depth. Depending on the texture and chemistry of the basic soil and on the quantity of the sedimenting organic and inorganic particles, a soft sediment layer may be present. The depth of this layer can be measured with a 6–8 m long bamboo rod equipped with a wooden disc of 10 cm diameter.
9. Solid sediment layer. In older ponds, in addition to the soft sediment layer, a solid sediment layer with a low water content is also present, as a result of diagenesis and fossilization of the nutrients contained in this layer. The thickness of the layer can be measured with a 6–8 m long bamboo rod with a sharp end. The total thickness of the soft plus solid sediment layers has a direct relation to the age of the fish ponds, and at times the sediment layer measures more than 2 m. Such thick sediment, having rich nutrients and anaerobic conditions with a very slow bacterial decomposition and mineral cycling, should be properly utilized for fish culture.
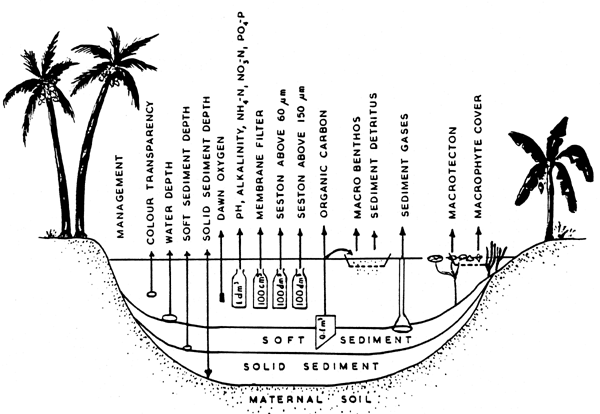
FIG. 1. DESIGN OF THE ENVIRONMENTAL MONITORING SYSTEM FOR PERENNIAL POND
10. Sediment gases. The thick, organic rich and anaerobic sediment contains a large amount of several gases, mainly methane, nitrogen, hydrogen, carbondioxide, nitrous oxide. Quantification of this parameter can be made by a standard sediment stirrer grid super-imposed on a funnel mounting connected to a gas collector through a rubber tube. This small device can be attached for gas collection to the bamboo rod used for depth measurements.
11. Sediment organic carbon. The sediment layer is the result of accumulation and depositing of the silt and the energy-rich organic matter. The extreme oxygen deficiency and the electron oversupply in these thick sediments create an environment which retards the mineralization and keeps the sediment organic pool out of the rapid nutrient cycling in the overlaying, oxygen-saturated water column. The anaerobic nature of the organic and reduced substance-rich sediment is also the site for the early development of the Microcystis species blooming in their final developmental stage on the water surface and causing adverse effects on the fish pond. The Hargrave type sampler is the most suitable tool for collecting sediment samples and the simple ignition procedure is sufficient to measure the sediment organic carbon.
12. Sediment detritus. It consists mainly of decomposing aquatic plant and animal organisms. In addition to the autochthonous organic matter, the detritus in ponds also contains a significant amount of allochthonous organic particles of external origin as the ponds are used for bathing and washing of human and livestock population.
To measure the quantity of this important nutrient resource a simple sieving procedure is adopted with a mesh size of around 400 μm. The wet weight of the residue may simply be taken to quantify the sediment detritus, but special care needs to be taken to remove all the inorganic particles of higher specific weight, and remaining in the corners of the washing tray during decantation. Selection of the mesh size for detritus should be dependent on its utilization by the benthos feeder fish, taking into consideration the mouth morphology. Detailed study using different mesh sized sieves would be required to quantify in more detail the whole size spectrum of the sediment detritus.
13–17. Chemical environment in the water column. The water is chemically characterized by pH, alkalinity, NH4-N, NO3-N and PO4-P measurements with standard methods. Normally, the pH and alkalinity do not change from pond to pond on the same types of maternal soil. The measurement of NH4-N, NO3-N, and PO4-P indicate the basic inorganic nutrient status of the pond.
18. Dawn oxygen. Generally, fish ponds exhibit wide fluctuations in the oxygen content from day to night. This diurnal oxygen fluctuation is normally measured to calculate the community metabolism of the whole pond while quantifying the production and respiration processes in the ecosystem. For a routine monitoring system, however, the measurement of the whole diurnal oxygen curve is not essential since the basic production processes may be estimated, although less accurately from other parameters of the monitoring system. Although many environmental parameters influence the concentration of the oxygen both in time and space, a single measurement at the end of the dark period would be an important indicator of the risk of fish kill. Oxygen measurement can be made by oxygen electrode in situ.
19. Bacterioplankton. Bacteria, converting a large number of important processes in the energy flow and mineral cycling, also serve as food for the aquatic animals including fish species such as silver carp, tilapia and many benthos feeder species. Therefore, it is important to measure the actual number of bacteria and select this parameter for environmental monitoring. Normally, the classical practice of counting the bacterial colonies appearing on the agar surface or in the agar column after a given incubation period is not recommended since the number of bacteria growing on any kind of nutrient media represents only a very small portion of the total bacterial community present in natural waters. Instead, 100 cm-3 pond water may be collected and preserved immediately in 2% formalin. The preserved water may be filtered through a millipore membrane filter with a pore size of 0.2 μm, and residue on the membrane filter is stained with erythrosin dissolved in freshly distilled phenolic acid. After drying the membrane filter the bacteria may be counted under a microscope with a magnification higher than 1 200.
As far as quantitative assessment of the bacterial community is concerned, it is essential to make an estimate of the main groups taking part in the mineralization process as shown in Table 2. Presently, there is no information available on the bacterial community in such ponds either in the water or in the sediment. However, it is not necessary to assess this parameter pond by pond since in similar agroclimatic conditions this will not vary greatly unless different management techniques are applied in different ponds.
20–21. Phytoplankton and seston detritus. Collecting and measuring the volume and wet weight of two seston size fractions (as suggested in items nos. 22–25) can quantify the planktonic fish food resources. The membrane filter prepared for bacterioplankton counting (as in item 19) offers simple, but reliable means to quantify the phytoplankton structure with the help of a microscope with a lower magnification of about 400. The amount of freely-suspended detritus consisting of very fine particles can also be quantified from the above sample.
22–25. Seston size fractions. Avoiding the detailed plankton analysis, two seston samples using, first a mesh size of around 60 μm to collect food particles including phytoplankton, zooplankton and even detritus, filtrable easily by fine filter feeders like silver carp and tilapia: and second, a mesh size of around 150 μm to collect organisms large enough for the rough filter feeders or even for the common carp, would be adequate for quantification of natural food resources available in the water column. After centrifugation the volume of seston may be read and the wet weight may be measured. However, to establish the real nutritive value of the planktonic organisms in both size fractions of seston may at times be difficult since many undesirable and indigestable algae may constitute major items of such fractions. However, formalin preserved samples can be analysed to obtain some idea of dominant species of both phyto- and zooplankton.
26–29. Zoobenthos and Zootecton. The growth of some important fish species, e.g., mrigal and common carp, depends primarily on the abundance of the larger animals. In most of the ponds with a thick anaerobic sediment layer, the bottom environment is not suited to maintain any significant animal populations. Even certain chironomids and oligochaete species well adapted to the sediment environment have difficulty in establishing themselves due to the total absence of oxygen and the high concentration of different reduced compounds. The complete lack of these important food items in ponds thus indicates highly adverse conditions for pond productivity. However, if the ponds have a significant macrophyte cover, many large animals, mainly crustaceans, insects and molluscs can establish a flourishing population of fish food organisms. However, at times, the large animal feeder fish populations sometimes overgraze on them and gradually destroy their populations. Moreover, considerable amounts of macrophytes are not desirable since these compete seriously for food with the phytoplankton.
The presence and the number of benthic animals in the sediment and of the zoobiotecton living among the macrophytes can easily be detected and counted after rapid sieving with a mesh size of around 400 μm. The samples for zoobiotecton counting may be taken simply by a sieving tray, placing it and retrieving it carefully beneath the macrophyte cover. After sieving and washing both the sediment and macrophyte samples with 400 μm pore size, all the washing remains may be poured into a large white tray and the animals analysed quantitatively and qualitatively.
30–31. The percentage cover of macrophytes. In addition to factors of nutrient stabilization and competition for food, macrophytes also compete with the phytoplankton for the light. Fish ponds which have a significant macrophyte cover contain practically no phytoplankton, and as a consequence the natural food organisms have no primary food to synthetize their protein for the fish community. Only the zoobiotecton with a long generation period prevail in these ponds. A simple visual examination and estimation of the percentage cover of the macrophytes are sufficient for quantitative and qualitative estimations.