Broadhead, G. C.. 1953 Investigations of the black mullet, Mugil cephalus L, in Northwest Florida. State Board of Conservation Technical Series No. 7, Marine Laboratory, University of Miami, Fla., 34 p.
Bhinyoying, S. 1980 Sperm preservation technique, hormone bank, and fish seed production in Thailand. Asean Meeting of Technical Experts on Aquaculture, 3–4 Jan. 1980, National Inland Fisheries Institute Bangkok, Thailand. Asean 80/Aquaculture 1/XI, 5 p.
Chan, W. L. 1982 Management of the nursery of seabass fry In Report of training course on seabass spawning and larval rearing. SCS/GEN/82/39. South China Sea Fisheries Development and Coordinating Programme, Manila, Philippines p. 34-7
Maneewongsa, S. and T. Tattanon. 1982a Nature of eggs, larvae and juveniles of the seabass. In Report of training course on seabass spawning and larval rearing. SCS/GEN/82/39. South China Sea Fisheries Development and Coordinating Programme, Manila, Philippines, p. 22-4
Maneewongsa, S. and T. Tattanon, 1982b Growth of seabass larvae and juveniles. In Report of Training course on seabass spawning and larval rearing. SCS/GEN/82/39. South China Sea Fisheries Development and Coordinating Programme, Manila, Philippines, p. 31-2
Tattanon, T. and S. Maneewongsa. 1982a Larval rearing seabass. In Report of training course on seabass spawning and larval rearing. SCS/GEN/82/39. South China Sea Fisheries Development and Coordinating Programme, Manila, Philippines, p.29–30
Tattanon, T. and S. Maneewongsa. 1982b Distribution and transport of seabass fry. In Report of training course on seabass spawning and larval rearing. SCS/GEN/82/39. South China Sea Fisheries Development and Coordinating Programme, Manila, Philippines, p.33
Withler, F. C. and L. C. Lim. 1982 Preliminary observations of chilled and deep frozen storage of grouper (Epinephelus tauvina) sperm. Aquaculture 27:389-92
Table 1
STAGES OF GONADAL DEVELOPMENT IN SEABASS, LATES CALCARIFER
(adapted from Broadhead, 1953)
| Stage | Gonadal Condition | |
| Female | Male | |
| I Virgin | Glassy, rounded and 1/4 the body cavity in length. | Colourless thin strap lying along the blood vessel. One half body cavity in length. |
| II Maturing virgin and recovering spent | Definite gonadal appearance. The same length as stage I. | Whitish and has assumed a definite gonadal appearance. The same length as stage I. |
| III Developed gonad | Yellowish and easily detectable as female. Ovary about two thirds of the body cavity. | Whitish with gonadal appearance. |
| IV Developing | Fills half the body cavity. The eggs can be distinguished separately. | Fills half the body cavity. Whitish. |
| V Fully ripe | Eggs are separate and fill the entire body cavity. | Milt fills the body cavity and can be expelled without difficulty. White and sticky. |
| VI Spent | Ovary flaccid. May have some eggs remaining. | Testes thin although not as flaccid as the female. Some spawner may have the testes remain and fill to one half of body cavity. |
| VII Resting | Ovaries reddish and small. Easily confused with stage II and a microscope may be necessary for identification. | Testes are small and thin. They are sharp viewed from the edge. |
Table 2
SUITABLE RANGES OF WATER QUALITY OF THE BROODSTOCK TANK
| Parameters | Suitable range of values | ||
| Temperatures | 28°C – 32°C | ||
| Salinity | 29 – 32 ppt | ||
| Hardness (as CaCo3) | 40 – 100 mg/1 | ||
| pH | 6.8 – 8.0 | ||
| Dissolved oxygen | 8 – 10 mg/1 | ||
| Phosphate content | 10 – 100 mg/1 | ||
| Unionized ammonia (NH3) | below | 0.5 mg/1 | |
| Ionized ammonia (NH+4) | below | 1.5 mg/1 | |
| Aluminium sulphate (Alum) | below | 80 mg/1 | |
| Turbidity : Suspended solid size bigger | 1 μm | 2 – 10 mg/1 | |
| Suspended solid size smaller | 1μm | 2 – 3 mg/1 | |
| BOD (5 day) | maximum | 3 mg/1 | |
| Nitrite (NO2) | below | 6 mg/1 | |
| Nitrate (NO3) | below | 150 mg/1 | |
| Chlorine | below | 0.08 mg/1 | |
Table 3
EFFECTS OF SALINITY ON HATCHING RATE OF SEABASS EGGS
| Salinity (ppt) | Rate of hatching (%) |
| 0 | 0 |
| 5 | 2.86 |
| 10 | 58.56 |
| 15 | 75.03 |
| 20 | 82.35 |
| 25 | 83.36 |
| 30 | 80.78 |
| 35 | 46.90 |
Source: Maneewongsa and Tattanon, 1982a
Table 4
EMBRYONIC DEVELOPMENT OF SEABASS EGG AT 27°C
| Stage of development | Hour | Minutes |
| Fertilized egg | 0 | 0 |
| 1-celled | 0 | 35 |
| 2-celled | 0 | 40 |
| 4-celled | 0 | 45 |
| 8-celled | 1 | 00 |
| 32-celled | 2 | 15 |
| 64-celled | 2 | 45 |
| 128-celled | 2 | 55 |
| Multi-celled | 3 | 15 |
| Blastula | 5 | 30 |
| Gastrula | 6 | 30 |
| Neurula | 8 | 30 |
| Early embryo with eye vesicle | 11 | 20 |
| Heart function, free movement of tail | 15 | 50 |
| Embryonic development stage | ||
| Breaking of eggshell by hatching | 17 | 30 |
Source: Maneewongsa and Tattanon 1982a
Table 5
SURVIVAL RATE OF SEABASS LARVAE AT DIFFERENT SALINITIES
| Salinity (ppt) | Survival rate (%) |
| 0 | 0 |
| 5 | 24.0 |
| 10 | 28.0 |
| 15 | 28.0 |
| 20 | 68.0 |
| 25 | 22.0 |
| 30 | 18.0 |
| 35 | 10.0 |
Source: (Maneewongsa and Tattanon, 1982b)
Table 6
STOCKING DENSITY AND EXPECTED SURVIVAL RATE OF
SEABASS FRY AT DIFFERENT AGE INTERVALS
| Age (days) | Stocking density/m3 | Expected survival rate (%) |
| 0–2 | 100 000–150 000 | 90 |
| 3–7 | 40 000–50 000 | 75 |
| 8–14 | 10 000–20 000 | 50 |
| 15–21 | 5 000–10 000 | 20 |
| 22–30 | 2 000–5 000 | 15 |
Table 7
TYPES AND RELATIVE AMOUNTS OF FOOD GIVEN AT
DIFFERENT STAGES OF THE SEABASS LARVAE
| Age (days) | Chlorella % | Rotifer % | Artemia % | Daphnia or Moina % | Acetes % | Minced fish % |
| 3–7 | 10 | 90 | - | - | - | - |
| 8–15 | 10 | 75 | 15 | - | - | - |
| 16–20 | - | - | 50 | 50 | - | - |
| 21–30 | - | - | - | 80 | 10 | 10 |
| 31–40 | - | - | 50 | - | 25 | 25 |
| 41 + | - | - | - | - | - | 100 |
Source: Maneewongsa and Tattanon, 1982b
Table 8
PORE SIZE OF GRADER AND RETAINED SIZE OF FISH
| Pore size (ø mm) | Minimum size of fry retained (mm) |
| 2 | 3–4 |
| 6 | 10 |
| 10 | 25 |
| 15 | 50 |
| 20 | 750 |
Table 9
NORMAL GROWTH OF SEABASS FRY IN 30 DAYS
| Age (days) | Total length (mm) | Remarks |
| 0 | 1.50 | newly hatched |
| 1 | 2.20 | |
| 7 | 3.60 | |
| 14 | 4.40 | |
| 20 | 8.10 | |
| 30 | 12.00 |
Table 10
DISEASE TREATMENT OF SEABASS FRY IN NURSERY TANK
| Product Names | Suggested Uses | Suggested Dose and Treatment |
| 1. Chloramphericol (Chlormycetin) | Bacteria protozoans some virus | Food: 1 g/kg of food or 50–100 mg/kg of food/day/for 5 days |
| 2. Furazolidone (nf-180-Furoxone) | Bacteria | 0.25 mg/kg of fish in feed for 14 consecutive days |
| 3. Nitrofurozone (Furacin, Nitrofural) | Antimicrobial | Food: 7.5 g/kg of food daily for 2 weeks |
| 4. Oxytetracycline HCL (Terramycin) | Antimicrobial | Food: 1.8 mg/g of food to approximately 3% of body weight/day for 8 days |
| 5. Sulfamethazine | Antimicrobial | Food: 5 g/45 kg of food/day for 10–15 days |
| 6. Sulfaguaridine | Antimicrobial | Food: 6 g sulfaguaridine plus 12 g sulfameragine/45 kg of food/day for 3 days followed by 4 g sulfaguaranidine plus 6 g sulfamerazine/45 kg fish/day for 7 days |
| 7. Sulfamerazine | Antimicrobial | Food: 18 g/kg of food/day for 10 days |
| 8. Sulfosoxazale | Antimicrobial | Food: 8–10 g/45 kg of food/day for 7–10 days |
| 9. Copper sulphate | Protozoa | Dissolve in culture medium at concentration of 1 ppm |
Table 11
NUTRIENTS FOR 1-1 STOCK CULTURE OF TETRASELMIS
| Nutrients | Concentration |
| Sodium nitrate | 84.0 mg/1 |
| Any one of the following: | |
| Monobasic sodium phosphate | 10.0 mg/1 |
| Tribasic sodium phosphate | 27.6 mg/1 |
| Calcium phosphate | 11.2 mg/1 |
| Ferric chloride | 2.9 mg/1 |
| EDTA | 10.0 mg/1 |
| Thiamin HCI (B1) | 0.2 μg/1 |
| Biotin | 1.0 μg/1 |
| Vitamin B12 | 1.0 μg/1 |
| CuSO4. 5H2 O | 0.02 mg/1 |
| ZnSO4. 7H2 O | 0.04 mg/1 |
| NaMoO4. 2H2O | 0.02 mg/1 |
| MnCl2.4H2O | 0.013 mg/1 |
| CoCl2.6H2O | 3.6 mg/1 |
Table 12
NUTRIENTS FOR 3-1 CULTURE OF TETRASELMIS
| Nutrients | Concentration (mg/1) |
| Urea 46 | 100 |
| K2HPO4 | 10 |
| FeCl3 | 2 |
| Agrimin | 11 |
| EDTA | 2 |
| Vitamin B1 | 0.005 |
| Vitamin B12 | 0.005 |
Table 13
NUTRIENTS FOR 200-1 AND 1-t CULTURES OF TETRASELMIS
| Nutrients | Concentration (g/t) |
| KNO3 | 100 |
| Na2HPO4. 12H2O | 50 |
| CaHCO3 | 25 |
| FeCl3 | 5 |
Table 14
NUTRIENTS FOR CHLORELLA CULTURE
| Nutrients | Concentration (g/t) |
| Ammonium sulfate | 100 |
| Super phosphate | 15 |
| Urea | 5 |
Table 15
NUTRIENTS FOR MARINE YEAST CULTURE
| Nutrients | Concentration |
| Brown sugar | 15 g/1 |
| Ammonium sulphate | 3 g/1 |
| Potassium phosphate | 1 g/1 |
| Hydrochloric acid | 1 mg/1 |
| Age stage (days) | Size (TL) (cm) | No. of fry per bag | Water temperature | Duration (hours) | Survival rate (%) |
| 7–15 | 0.2–0.3 | 10 000 | 19–23°C | 16 | 90 |
| 20–22 | 0.5 | 5 000 | " | 16 | 90 |
| 1 month | 1–1.5 | 1 000 | " | 16 | 90 |
| 2 months | 2–3 | 500 | " | 16 | 90 |
Source: Tattonon and Maneewongsa, 1982b
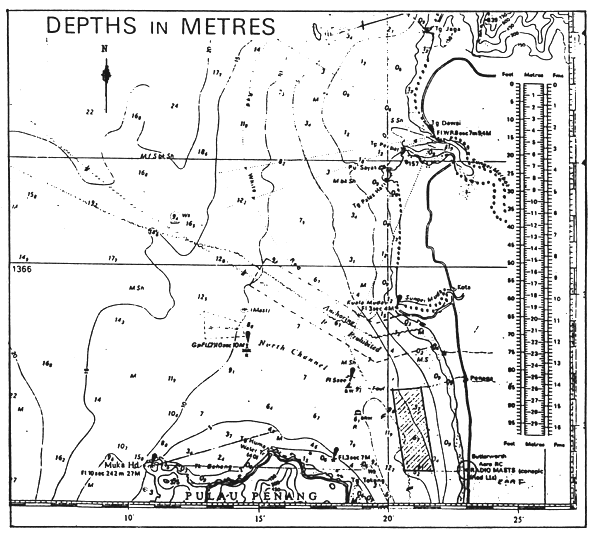
Fig. 1 Seabass spawning ground  seabass spawning ground
seabass spawning ground
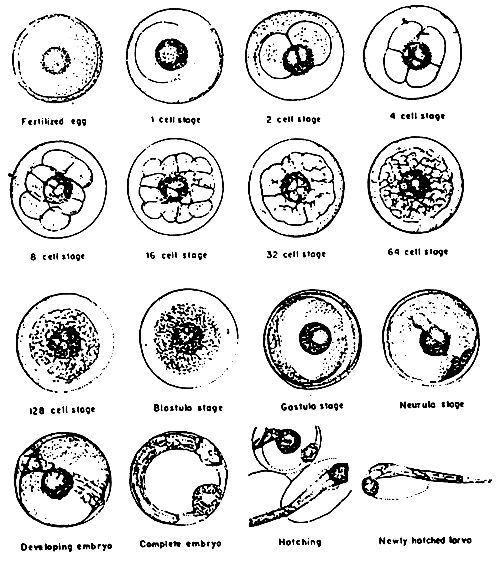
Fig. 2 Embryonic development of seabass
Source: Maneewongsa and Tattanon, 1982a
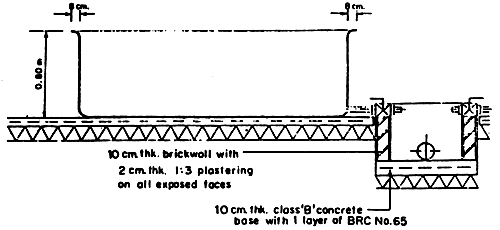
Fig. 3 Seabass nursery tank
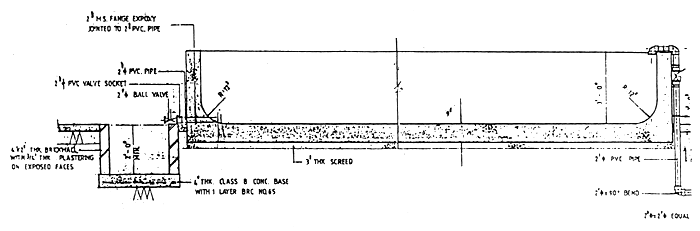
Fig. 4 Seabass fry rearing tank
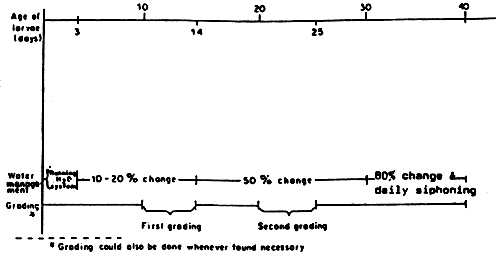
Fig. 5 Water management and grading period for seabass rearing in hatchery within the 40-day period.
Source: Tattanon and Maneewongsa, 1982a
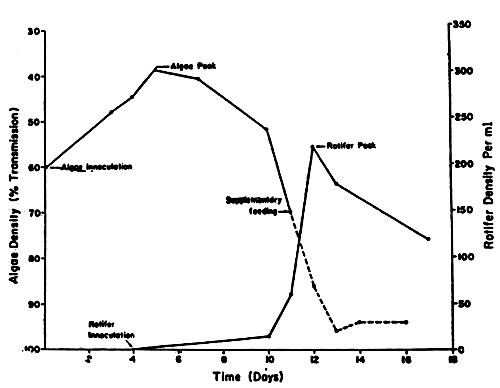
Fig. 6 Rotifer culture showing algae peak, rotifer innoculation and rotifer peak