There are a number of different criteria by which the distribution of acid sulfate soils can be recognized, including soil profile descriptions of colour and mineralogy, field pH measurements in moist soils, pH measurements following extended periods (up to several months) of drying and air oxidation of soil samples, complete oxidation of pyrite with reagent chemicals followed by pH and/or sulfate measurements, major element chemical analysis of soil samples and determination of mineral percentages in soil samples. The source of acidity in acid sulfate soils is the existence of appreciable pyrite and the lack of a comparable quantity of carbonate minerals to neutralize the acidity resulting from pyrite oxidation. Formation of pyrite in reducing marine and brackishwater sediments is a pervasive and well understood process (Berner, 1970; Pons, 1973). Microbial reduction of the abundant sulfate in seawater to hydrogen sulfide is followed by rapid formation of iron monosulfides (FeS) by reaction with reduced iron, and then slow conversion to pyrite (FeS2) involving reactions with elemental sulfur (Berner, 1963). At temperatures of 20–30°C the conversion of iron monosulfides to pyrite can require a few years to occur, assuming continuously reducing conditions. The organic load created by mangrove forest detritus, mostly retained by a complex root system, favours the onset of anaerobic conditions where sulfate reducing bacteria thrive. The total quantity of pyrite formed is related to the rate of supply of organic detritus to the sediments, temperature, and the existence of a continuous supply of dissolved sulfate. Warm, near-shore marine sediments with a large supply of organic particles, such as exists in mangrove forests, are ideal for production of pyrite by the sediment microbial community. Such sediments often contain more than 5 percent pyrite by weight. The percentage of pyrite in nearshore sediments is generally highest for low latitude areas with high rates of primary production in the coastal zone and active rooted plant communities such as mangrove forest and thus the potential for acidic conditions is also greatest there as well.
The pyrite in sediments is stable as long as reducing conditions are maintained, but as soon as oxygen (as well as moisture) is present, this mineral begins to be converted by another community of microorganisms to oxidized iron and sulfur minerals and dissolved species. The mineral products of pyrite oxidation include a number of iron minerals such as jarosite, a pale yellowish potassium iron sulfate, goethite, a brownish iron oxide, hematite, a reddish iron oxide and several greenish iron phases which usually are not as well characterized by X-ray diffraction because of their instability in the presence of oxygen. These mineral assemblages can be readily recognized in the field by trained soil scientists and are some of the principal indicators of the distribution of developed acid sulfate soils in which considerable pyrite oxidation has already occurred.
The chemical conditions of soil and sediment represent only one class of critical factors for brackishwater aquaculture, but clearly must be carefully considered if high yields of culture organisms are to be obtained. Oxidation of pyrite can play a major role in altering chemical conditions of brackishwater ponds, overwhelming the buffering capacity of the natural dissolved carbonate system and increasing acidity drastically, mobilizing iron and aluminium as toxic dissolved species, sequestering inorganic phosphorus, and generating large amounts of iron hydroxide precipitates. The chemical reactions which accomplish pyrite oxidation are largely mediated by microbial communities, and involve a number of sequential steps, much of which can be summarized by the following set of chemical reactions involving pyrite and oxygen (Stumm and Morgan, 1981):
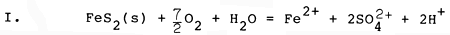
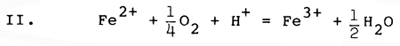
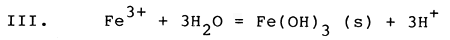
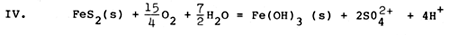
The first reaction (I) illustrates the conversion of solid mineral pyrite to dissolved iron (+2 oxidation state) and dissolved sulfate. Two hydrogen ions (acid) are generated for each dissolved iron ion. In the presence of oxygen, the relatively soluble form of iron (+2 oxidation state) is converted (II) to an extremely insoluble form of iron (+3 oxidation state), and in the process removes one of the hydrogen ions generated by reaction I. When the insoluble form of iron precipitates (III) as a hydroxide, three additional hydrogen ions are generated for each dissolved iron ion which precipitates. The net result of these three reactions (IV) is to remove pyrite, to form solid iron hydroxide and to form dissolved sulfuric acid, generating 4 hydrogen ions (acid) for each iron atom initially present as pyrite. Most of the acidity generated results from precipitation of ferric hydroxide, which has very low solubility.
At high concentrations of hydrogen ion (very acid conditions), both aluminium and iron become much more soluble than at near neutral hydrogen ion concentrations. They also form very insoluble phosphate compounds (Watts, 1968) and thus will remove dissolved phosphate. A high fraction of inorganic phosphate fertilizer added to brackishwater aquaculture ponds in acid sulfate soil environments will thus be stripped from solution and have no stimulating effect on the plant community.
The presence of pyrite in an aquaculture system can be considered as transient since it will react and be removed eventually. This happens in the mounds of mud lobster, Thalassina anomala, which may be very old and may contain only traces of jarosite and pyrite in their core (Andriesse, van Breeman and Blokhins, 1973) if compared with the surrounding soil. However, the amounts of acid production which are associated with the removal are so large as to present a major problem. In an area of 1 ha, assuming a dry soil density of 1.5 g/ml and a pyrite content of 5 percent by weight, there will be 75 t of pyrite for every 10 cm of soil depth. The equivalent weight of lime required to neutralize all the acid from each 10 cm depth internal of soil is about 150 t/ha.
If all of the carbonate and bicarbonate ions in a brackishwater pond of 1 ha and 1 m depth reacts completely with acid, it would take approximately 150 complete exchanges of water to neutralize the acid from each 10 cm depth interval of soil. Thus the supply of acid from pyrite oxidation in a brackishwater aquaculture pond system could be expected to overwhelm the natural buffer capacity in the water for a long period.
The chemical reactions described above have complex negative effects on the flora and fauna of the ponds where they occur. They affect not only individual organisms but also the trophic relationships in pond food chains. The oxidation of pyrite in traditionally constructed and managed ponds has effects on the following: (i) bacteria, (ii) plankton composition and biomass, (iii) meiobenthos composition and biomass, (iv) fish and shrimps intentionally stocked in the pond, and, (v) macrophytes (submerged, rooted vegetation and grasses planted on pond dykes).
The mechanisms through which the pond biocenosis is affected by the oxidation of pyrite fall broadly into two major categories: chemical ones, which could be considered the primary mechanisms, and physical ones which are derived from the first group. Although summarily explained below using a reductionistic approach to render explanation easier, these effects cannot be isolated, but instead are intermingled, with some factors being more important, or better understood, for different groups of organisms.
The most obvious chemical change is the reduction of pH to very low values. Although initially impacting organisms exposed to dike surface runoff after rainfall, it has an effect on meiofauna at the time of flooding the ponds. When traditional practices for rearing of “lab-lab” are followed, only a very shallow layer of water is allowed into the pond after a period in which the bottom of the pond has been dried and has cracked. This shallow layer of water (5–10 cm) becomes extremely acid resulting in the death of almost all meiofauna.
Fish and shrimps intentionally stocked in ponds are also affected by low pH levels and avoidance reactions as well as lethal effects are commonly observed, especially after heavy rains. Gills are especially affected, being the most sensitive part of these organisms exposed to water. Although most of the literature on effects of low pH on fish refer to freshwater ecosystems, many of the same findings should also be applicable to estuarine or marine species when exposed to acid waters. In reaction to acid water, gills tend to increase production of mucus to protect the epithelium, thus reducing gas exchange capacity. Also uptake ovf H+ through gills lowers blood pH, resulting in chemical suffocation due to the lower carrying capacity for O2 (known in fish physiology as the “Bohr and Root” effect). Osmoregulation capacity, another important role of the gill, is also affected as both water and ion transport through gills are modified due to the changed ionic composition of the water (Evans, 1975).
There is little direct evidence of low pH induced changes in the total phytoplankton biomass. The concurrence of low pH and of stripping of usable P from the water is largely responsible for this lack of information. However, it is clear that species composition may vary as a result of the different tolerances of species to low pH values. Changes in zooplankton may also alter the pressure due to predation on phytoplankton, thus affecting species composition. In addition, sudden variations of pH, typical of weakly buffered systems can inhibit the development of large algal populations.
Bacterial activity in the water column and pond sediment is primarily limited by the low availability of organic matter characteristic of these ponds, and not so much by the low pH. Experiments show that when organic manure is provided to the ponds, bacterial growth is evident (Moriartry, et al., in preparation) as the respiration of the pond may create a negative oxygen balance, leading to anaerobic conditions of the deep waters. When feeds are given, the organic matter added may exceed the digestion capacity of the pond and an anoxic situation may develop in the bottom sediments and part of the water column. As redox potentials of bottom sediments and water become negative, toxic sulphides are produced from sulfates, inhibiting further the growth of benthic organisms, shrimp included.
Another chemical mechanism affecting pond biocenosis during the oxidation of pyrite is the toxicity originated by the release of iron and aluminium into the water. In the case of crustaceans and fish, iron toxicity is evident when gill chambers are examined. When pH levels fall below 4, large amounts of iron go into solution and precipitate later on the gills, forming amorphous iron hydroxide deposits. This impairs the gas exchange capacity of the gill, suffocating the animals. In freshwater acidified lakes aluminium in solution has been shown to be very toxic to fish causing gill necrosis. Benthic algae also get encrusted with iron hydroxide when the pH rises above 4, thus rendering them inedible for the meiofauna. Iron and aluminium, in the concentrations at which they are found in these soils, are toxic for the root system of grasses and other plants which are usually used to prevent dyke erosion.
The release into solution at low pH of large amounts of dissolved iron and aluminium, after heavy rains or from pore waters coming from the dykes, triggers a third process which affects mainly the phytoplankton of the pond. This process is the reaction of phosphate in solution with iron and aluminium to form complex insoluble mineral compounds which are removed to the pond sediment and thus phosphate is not available for algal growth. In fact, low primary production is generally characteristic of ponds built on acid sulfate soils. Consequently, the formation of detrital matter originating from phytoplankton is also reduced and inhibits the growth of meiofauna, such as anellids, nematodes and harpacticoid copepods, which feed on bacteria enriched detritus, and which are important food items themselves for shrimps. This selective binding of phosphorous by iron and aluminium may leave nitrogen and silica substantially in excess, changing the available nutrient N:P:Si ratios, which are required in rather precise ratios by different algal species. Thus, shifts in species composition may result, as well as a reduction of the total biomass.
The entrance of small amounts of acid into a pond is counteracted by the alkaline reserve of pond water, which prevents sudden variations of the water pH. In cases where pond water is not changed frequently chemical reaction with hydrogen ions can consume the entire alkaline reserve. The loss of the carbonate buffer from pond water affects the phytoplankton, which are then unable to develop large populations under conditions of abrupt pH variations. The exhaustion of inorganic carbon supplies for phytoplankton derived from the consumption of carbonate, may lead to extreme situations in which diffusion from air is the only source of CO2 for phytoplankton growth, thus contributing additional limits on algal growth.
Shrimps and crustacea in general, which utilize carbonate for hardening of the exoskeleton after moulting, are also affected by the depletion of carbonate in water, resulting in shrimp populations with a higher percentage of soft shells than in normal ponds.
The last of the major mechanisms affecting pond biota is water transparency. The extreme water clarity results from the very low levels of primary production in these ponds and reduces shelter from bird predators which is normally provided by the reduced visibility of the pond bottom in productive ponds.