The majority of inorganic nitrogen in the atmosphere is in elementary, dinitrogen form. Natural or artificial nitrogen fixation is the only way by which this nitrogen moves into the biosphere. Since the energy demand for the cleavage of N=N binding is as high as 940 KJ mol-1, most of the living organisms cannot utilize it and assimilate the combined nitrogenous molecules of ammonia, nitrite and nitrate from their environment. The primary production resulting from the nitrogen fixation in water ecosystem manifests first of all as eutrophication, which is harmful to environmental protection but useful for fish production.
Until recently, the determination of nitrogen fixation in lake water and sediment was hindered by the lack of a proper method. The increments in total and protein-nitrogen in pure cultures of nitrogen-fixing organisms can be measured with the classic method of Kjeldahl, where the change in nitrogen content is significant. The sensitivity of the method, however, is not satisfactory under natural conditions. The manometric measurement of nitrogen gas fixed in the process might seem to be a good method. This method is not sensitive either, and in addition to reducing the nitrogen, the volume of O2 produced and consumed can considerably influence the accuracy of the measurement. Theoretically, it might also be an alternative to measure the changes in concentration of ammonia from nitrogen gas either indirectly or after microdiffusion. Ammonia, however, changes further entering protein synthesis under unidentified conditions. It is known that the nitrogenase enzyme can reduce a number of other substrates (Burris, 1974; Koros, 1980):
| Substrate | Product |
| N2 - (dinitrogen) | NH3 - (ammonia) |
| N2O - (nitrous oxide) | N2 - (dinitrogen), H2O (water) |
| N3- - (azide) | N2 - (dinitrogen), NH3 - (ammonia) |
| CN- - (cyanide) | CH4 - (methane), NH3 - (ammonia) |
| CH3NH2 - (methylamide) | |
| C2H2 - (acetylene) | C2H4 - (ethylene) |
| CH3NC (methyl-isonitril) | CH3NH2 - (methylamide), CH4 - (methane) |
| CO - (carbon monodixe) | inhibitor |
| NO - (nitrogen oxide) | inhibitor |
Nitrous oxide is converted into dinitrogen gas, the quantitative measurement of which, however, is rather difficult. Nitrous oxide and ammonia are also formed from sodium azide. The measurement of ammonia here could be feasible, the model substrate, however, inhibits several secondary reactions, thereby hindering nitrogen fixation. Methane comes about from cyanide on nitrogenase enzyme treatment, which is measurable by gas chromatography, but cyanide as a general metal protein inhibitor similar to azide, may interfere. Scholhorn and Burris (1966) and Dilworth (1966) have discovered the acetylenereducing capacity of the nitrogenase enzyme and in the following year, it was applied for measuring nitrogen fixation in lakes (Stewart et al., 1967). A great advantage of acetylene gas is its solubility in water by which the enzyme readily saturates with the model substrate. Ethylene as an-end product of the reduction by nitrogenase enzyme, has relatively poor solubility in water. Therefore, with its early quantitative recovery, it can be measured gas chromatographically. For the determination of nitrogen fixation, the use of nitrogen stable isotope (15N) is also suitable. Its main advantage is that it does not need any conversion coefficient. The shortcoming of this method, however, is its laborious procedure and special instruments required (15N analyser and mass spectrometer). Moreover, as compared to the acetylene reduction method, its sensitivity is 103–104 times less (Hardy et al., 1973).
Nitrogenase enzyme reduces dinitrogen with the transport of 6 electrons:
N2 + 6H+ + 6 e-→2NH3,
while the reduction of acetylene is completed with the transport of 2 electrons:
C2H2 + 2H + + 2e-→ C2H4
To obtain the amount of nitrogen fixed by nitrogen-fixing organisms in the lake water, ethylene produced during incubation by saturating nitrogenase enzyme with acetylene is measured chromatographically. In calculations, it should be considered that reduction of 1 mol N is equivalent to that of 3 mol acetylene.
Reaction flask: nurser-bottle of 300 cm3 (graduated up to 150 cm3) closed with rubber teat. Preferable to use bottle made of thick glass
Balloon for gas storage: a football bladder closed with rubber tubing and glass stopper. The vacuumed balloon is filled directly from the acetylene cylinder after rinsing with the gas several times. In this balloon the acetylene gas can be carried conveniently and taken when necessary in situ with a needle and syringe
Syringe:20–30 cm3 with well fitting piston
Perlon string
Lead sinker
Jar for gas sample: 30–50 cm3 size jar with rubber stoppers. The gas sample is pumped with syringe, displacing an equal volume of NaCl saturated water through a valve
Gas chromatography: glass column (2.1 m long, dia 4 mm) packed with Porapak-N and equipped with a flameionizing detector.
Acetylene gas of high purity
Ethylene gas of chromatographic purity
Mercuric chloride solution, saturated
The procedure is as follows:
150 cm3 of water sample (V-sample, water) is put into the reaction flask and closed with rubber teat. Then, 20 cm3 of air is withdrawn through the rubber teat with the syringe and replaced with the same volume of acetylene gas. The solution of acetylene in the water is is promoted by vigorous shaking (10 sec). This is followed by incubation in situ by suspending the reaction flask at a water depth corresponding to the sample for 4 hours (t-incubation). After incubation and a 10 sec-long vigorous shaking, 20 cm3 is withdrawn from the gas phase (V-gas phase) which is directly pumped into the gas sample jar filled with ion-free water and turned upside down (Fig. 1).
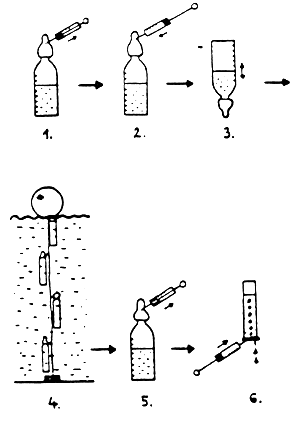
Fig. 1 Measurement of N-fixation in water
Air withdrawal (20 cm3)
Acetylene injection (20 cm3)
Shaking (10 sec)
In situ incubation (4 h)
Withdrawal of gas sample (20 cm3)
Storing of gas sample
The gas sample kept above the water surface in the gas sample jar is taken to the laboratory; 1 cm3 gas mixture is withdrawn from the gas phase with syringe and needle and directly injected into the gas chromatograph (Vo - sample). The column is run at 80°C and nitrogen gas is applied as carrier at a flow rate of 40 cm3 min-1. Peaks belonging to ethylene and acetylene are registered. For calculations, heights of the peaks are measured (H-sample ethylene; H-sample acetylene).
For the next phase, 1 000-times diluted ethylene gas is prepared for calibration from 1 cm3 ethylene gas (V-ethylene) diluted to approximately 1 dm3 with air (V-flask). For this purpose, calibrated glass flasks closed with a rubber teat can be used. From dilution, 1 cm3 (Vo-standard) ethylene gas is injected directly to the gas chromatograph which is run as mentioned in the sample analysis. The chromatographic peak corresponding to the volume of ethylene injected is registered, the height of the peak measured (H-standard, ethylene). Five parallel measures are performed at least. A calibration coefficient (F) is defined which later can be used to determine the rate of N-fixation.

| where | P = pressure of the diluted standard (Pa) |
| T = temperature of the diluted standard (K) | |
| R = universal constants for gas, 8.31 × 10-3 (Pa cm3 nmol-1k)-1 |
A control is prepared with artificial poisoning of the water sample so as to determine the ethylene already present as contaminant in the acetylene and water sample and the incidental, not biological, acetylene reduction during incubation. This control is treated in the same way as the sample with the exception that 2 cm3 saturated HgCl2 solution is added to kill any living organisms before closing the flask with a rubber teat. Chromatographic peaks corresponding to ethylene or acetylene (H-control, ethylene or H-control, acetylene) are registered, and their heights measured at the end of the procedure.
The concentration of ethylene resulting from biological acetylene reduction of the gas sample can be calculated with the following equation:
Csample, ethylene (nmol cm-3) =

The rate of acetylene reduction (u) as related to a unit volume of lake water:
u(nmol cm-3h-1) =
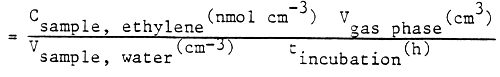
while the rate of nitrogen fixation related to a unit volume of lake water (v) will be:
v (nmol N2 cm-3 h-1) = k u
where k = ⅓ corresponds to the theory that the reduction of 3 mol acetylene equals the reduction (fixation) of 1 mol nitrogen. A practical value of K = γ4 was suggested by Burris (1974). The rate of N-fixation (w) in the whole water column of the lake as related to a unit volume can be calculated by summing the rates (vi) measured in different layer samples as follows:
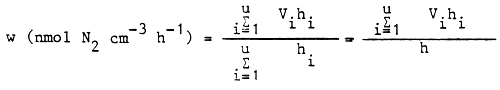
where hi = is the length or part of the column
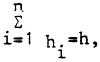 the depth of water
the depth of water
When calculating the nitrogen cycle so as to give the absolute amount of substance moving in a unit period of time, i.e., the multiplication of rate and volume, a constant value is defined in hydrobiology which is the rate related to a unit region of water surface (φ), the so-called surface rate:
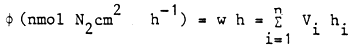
To estimate the amount of N fixed in an extended surface region of the lake (e.g., m2, ha) during a longer time period, N-fixation values belonging to different periods of time (nocturnal, diel, seasonal) and regions (several different parts of the lake) are necessary. With these values (diel and seasonal measurements in different parts of the lake), the quantity of N fixed in different places and time can be obtained by summing the respective surface rate values.
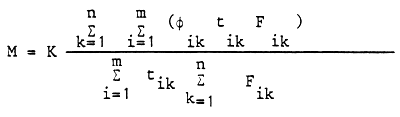
| where: | tik | = | duration of different periods of time |
| n | = | number of different periods of time | |
| Fik | = | area of different regions | |
| m | = | number of different regions | |
| k | = | constants depending on the units of measurements chosen |