As a result of bacterial activity, nitrate in a system is reduced to nitrogenous gases during denitrification. This specific process as described by the latest findings (Payne, 1973; Focht and Verstraete, 1977) is the following:
NO3 - → NO2 - → NO → N2O → N2
Denitrification is a respiratory process in which nitrate is the hydrogen acceptor.
The measurement of denitrification has a great significance in the quantitative expression of nitrogen cycle and can give data as to the loss resulted by the nitrogen release in the ecosystem.
Due to the lack of reliable and sensitive quantitative methods, denitrification was determined by indirect approaches up to the 1970s. The rate of denitrification was estimated from the number of bacteria (Brezonik and Lee, 1968). The decrease of nitrate concentration in the undisturbed hypolimnion was also used as a value to estimate the rate of denitrification in deep lakes (Brezonik and Lee, 1968; Larsen, 1974).
Methods based on the indirect measurement of N2 release generally need long incubation, thus the possibility of nitrogen contamination from the atmosphere may interfere. The application of stable 15N labelled nitrogen is rather limited because of the need of an excessive amount of substrate and a 15N analyser with a mass spectrometer. Nevertheless, for both water (Goering and Dugdale, 1966; Koike et al., 1972) and sediment (Kenney et al., 1971; Chen et al., 1972) there are procedures elaborated to apply the stable nitrogen isotope method.
The method using 13N is one of the most suitable ones for this purpose (Gersberg et al., 1976; Tiedje, 1978) though the short, 10 minutes, half life time of the labelling and the need of a cyclotron for processing will limit the application. Fedorova et al. (1973) found that the biological reduction of dinitrogen-oxide to dinitrogen form is inhibited by acetylene.
This opened a new possibility for measuring the rate of denitrification. In the system treated with acetylene, N2O accumulates gradually, as it is an intermediate. In several bacteria it may be also the end of product of the denitrification process (Payne, 1973). Sorensen (1978) applied this method for the first time. Dinitrogen oxide was measured by gas chromatography.
Denitrification process is terminated by acetylene in the last step:
NO-3 → NO-2 → NO→ N2O → N2
Dinitrogen oxide accumulated during the incubation time should be recovered from the water saturated with acetylene, and measured with gas chromatograph.
Reaction flask: nurser bottle of 300 cm3 graduated up to 150 cm3
Rubber teat
Balloon for gas storing: a football bladder with rubber tubing closed with a glass stopper. The vacuumed balloon is filled from gas cylinder rinsing first with the gas. In this balloon the gas can be transported safely for in situ measurements and the necessary amount of gas can be withdrawn with needle and syringe
Syringe-tuberculin of 1, 20 and 50 cm3, respectively with well fitting pistons
Perlon string
Lead sinker
Equipment for gas recovery (of. Fig. 6) consists of the following parts: flask(s) with round bottom; a magnetic stirrer; a rinsing flask with KOH; a gas drying tube with CaCl2; a gas acceptor spiral; a thermos with wide opening
Gas chromatograph: glass column (2.1 m long, 4 mm in diameter) packed with Porapak N and equipped with an electron-capture detector.
Acetylene gas
Dinitrogen oxide (chromatographic purity)
Saturated HgCl2 solution
KOH
CaCl2 (H2O free)
Liquid nitrogen
A 150 cm3 water sample (Vsample) is poured into a reaction flask, and closed with a rubber teat. Then 20 cm3 air is withdrawn with a needle and syringe and replaced by the same amount of acetylene gas from the gas storing balloon.A perfect solution of acetylene is promoted by vigorous shaking for 10 sec. The reaction flask, after shaking, is suspended by the Perlon string at a water depth corresponding to the water sample and incubated in situ for 4 h.A 1 cm3 HgCl2 solution is injected after incubation into the reaction flask to prevent any further microbial activity.
Since N2O can be found in the reaction flask both in the water and the gas phase above it, measurements can be taken in two steps: 1 cm3 gas mixture is withdrawn (Vo sample) through the rubber teat with needle and syringe and directly injected into the gas chromatograph; then the column is run at 45°C applying helium gas as carrier at a flow rate of 43 cm3 min-1. An electron capture detector of 320°C is used. Chromatographic peaks are registered and the height of the peaks (Hsample, gas phase) is measured.
After analysing the gas phase, 50 cm3 (Vo sample water) is withdrawn from the reaction flask through the rubber teat and injected into the stirring flask of the gas extracting equipment. Then helium gas is led through the rinsing flask with KOH solution and through the drying tube loaded with H2O-free CaCl2 to bind the interferring CO2 and H2O. N2O thus fixed is then frozen in a spiral of 5 cm3 immersed in a thermos containing liquid nitrogen. The thermos is removed after 15 minutes and the spiral is immersed into hot water and the N2O thus released is introduced into the gas chromatograph. Chromatographic peaks are registered, and their heights measured (Hsample water).
To determine the initial N2O content of the water sample and the possible non-biological nitrate reduction, a control is prepared by poisoning the water sample. This control sample is handled the same way as described for the sample, except that HgCl2 solution is applied to kill any living organism.
Chromatographic peaks corresponding to N2O are registered at the end of the process and their heights measured (Hcontrol gas phase, Hcontrol water).
A 1 000-fold dilution of N2O gas is prepared for calibration in such a way that 1 cm3 (VN2o) dinitrogen oxide gas is brought up to 1 dm3 with helium. A calibrated glass flask closed with rubber teat is used for this purpose.
Then, 1 cm3 from this diluted dinitrogen oxide gas (Vflask) is injected directly into the gas chromatograph with tuberculin syringe. Gas chromatograph is run according to the sample applied. Peaks obtained are registered and their heights measured (Hstandard).
To calculate the rate of denitrification a calibration co-efficient (F) is defined.
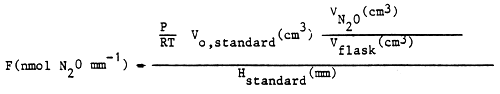
| where: | P = pressure of the diluted standard (Pa) |
| T = temperature of the diluted standard (K) | |
| R = universal gas constants 8.31 10-3 (Pa cm3 nmol-1 K-1) |
The quantity of dinitrogen oxide from denitrification in the gas phase (ngas phase) and in the lake water (nwater), is respectively:
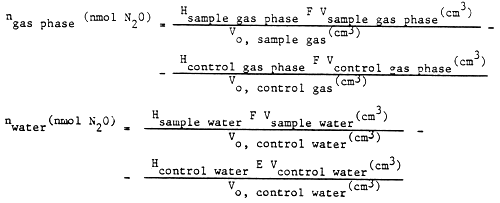
where: Vcontrol gas phase = volume of gas phase above the control sample (cm3)
Vcontrol water = volume of control sample (cm3)
The rate of denitrification (v) as related to a unit volume of lake water is calculated by:

The rate of denitrification (w) as related to the whole water column of the lake is obtained by summing the rates obtained from the partial columns as follows:
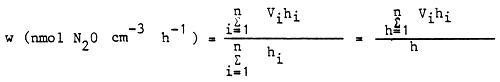
where: hi = the heights of the partial columns
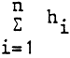 = depth of the water
= depth of the water
The rate of denitrification as related to a unit region of the lake (φ) is:
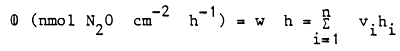
The amount of nitrogen (M) released via denitrification as related to an extended region and longer period of time, is estimated as follows:
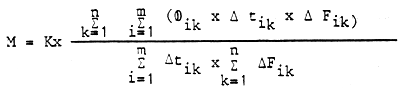
| where: | tik | = | duration of different periods of time |
| n | = | number of different periods | |
| Fik | = | area of different regions | |
| m | = | number of different regions | |
| K | = | constants depending on the units of measurements chosen |
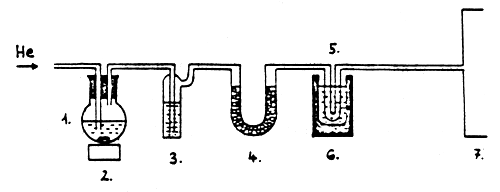
Fig. 6 Procedure for the recovery of dinitrogen-oxide
Flask with round bottom
Magnetic stirrer
Rinsing flask with KOH
Gas drying tube with CaCl2
Gas acceptor spiral
Thermos/liquid nitrogen - hot water
Gas chromatograph