ABSTRACT
The primary production and fish production patterns in temperate and tropical ponds under different management practices are described. An attempt is made to understand the relation between the two under the given conditions. With a more or less narrow range of primary production, varying fish production and conversion efficiencies were recorded, indicating the influence of a combination of environmental and management factors. The constraints involved in establishing the relationship are discussed.
1. INTRODUCTION
There have been a number of attempts at correlating the fish yields with limnological factors influencing the productivity of lakes (Rawson, 1955; Northcote and Larkin, 1956; Ryder, 1965). The direct approach that is receiving greater attention in the recent times is the correlation of fish yields with primary production (Smith and Swingle, 1938; McConnell, 1963; Hrbacek, 1969; Wolny and Grygievek, 1972; Sreenivasan, 1972; Melack, 1976; McConnell et al., 1977; Oglesby, 1977; Noreiga-Curtis, 1979; Liang et al., 1981). However, most of these studies pertain to natural waters and those on fish ponds are only a few.
Sufficient data on the primary production and fish yields of culture ponds at different management levels have been collected in the recent years while working on their ecosystem structure and functioning, as a part of the investigations carried out at the Fisheries Research Institute, Hungary. Similar work on the basic production and yields of the tropical, undrainable, rural fish ponds of Orissa, India, had been initiated in 1982 and considerable information is available from 18 ponds. It may be pointed out that in these ponds, the productive potential is often not utilized to the maximum due to the understocking and it is the actual relation between the primary production and the fish yields as recorded by the farmers which is considered. The present paper evaluates the data from these systems with significantly varying management practices and compares the efficiencies of energy conversion from primary levels to that of fish production.
2. CLASSIFICATION OF MANAGEMENT PRACTICES
2.1 Stocking natural waters
Fish stocking in natural waters is a common practice to increase either fish yields or the angling success. It is intended to maximize the fish production through an enhanced utilization of the natural fish food organisms present.
The effect of simple stocking on fish production efficiency was tested in two small water bodies of 0.14 ha each, with an average depth of 1 m at the Fisheries Research Institute, Szarvas, Hungary in 1976. The ponds were drained in the previous autumn, dried thereafter, filled and stocked at the end of March 1976. The stocking structure and density were as follows (mean weights g, density ha-1): silver carp 180, 370; bighead carp 190, 450; common carp 210, 200; grass carp 200, 150. With no feeding and organic or inorganic fertilization during the growing season, fish were harvested in October (6 months).
2.2 Stocking and inorganic fertilization
The next possible management level is stocking and inorganic fertilization. At this management level, it is aimed to raise the quantum of natural fish food resources with an optimum inorganic nutrient input.
The production efficiency of this management level was tested in two fish ponds of 0.14 ha each with an average water depth of 1 m (1977). Ponds were drained in the previous autumn, dried thereafter, filled and stocked at the beginning of April 1977. Stocking structure and density are (mean weights g, density ha-1) silver carp 40, 1 900; bighead carp 50, 1 200; common carp 190, 3 800; grass carp 50, 600. Fish were harvested in October. During the growing season, a total amount of 20 kg P in the form of superphosphate and 150 kg N in the form of NH4NO3 were supplied in weekly rations.
2.3 Stocking and organic fertilization
The twin effects of the organic fertilization are to enhance primary production and the direct utilization of the organic matter through bacterial and detrital food chains. This principle forms the basis of many fish culture technologies including integrated fish farming. The primary production and related fish yields at this management level were tested in three trials.
The first experiment was carried out in domestic sewage oxidation fish ponds in Hungary applying a technology elaborated earlier (1975). Sedimented raw domestic sewage was introduced daily through rotary sprinkler into the fish ponds (1.6 ha; average depth 1 m) at the rate of 100 m3 ha-1. All the six ponds were drained and dried during winter and fish were stocked in the beginning of April and harvested in October. Stocking structure and density (mean weights g, density ha-1) were silver carp 190, 1 500; bighead carp 180, 800; common carp 200, 1 400; grass carp 170, 300.
The second experiment was conducted in liquid pig manure oxidation fish ponds in Hungary (1982). Liquid pig manure with a mean dry weight of 10 percent was introduced daily into fish ponds of 0.14 ha area and an average depth of 1 m through a rotary sprinkler with flexible rubber openings, at the rate of 2 m3 ha-1. All three ponds were drained and dried during winter and fish were stocked at the end of March and harvested in October. The stocking structure (mean weight g, density ha-1) was silver carp 190, 3 500; common carp 150, 1 800.
Further, the primary production levels were determined and fish yield data collected from 18 undrainable rural fish ponds in Orissa, India. Most of these village ponds were not put to fish culture till 1977. Fish culture was later initiated with the technical guidance of the Freshwater Aquaculture Research and Training Centre (FARTC), Bhubaneswar. Most of these ponds were stocked with the three Indian major carp species, catla (Catla catla), rohu (Labeo rohita) and mrigal (Cirrhinus mrigala) and common carp (Cyprinus carpio var. Communis). In some ponds, the stocking was supplemented with silver carp (Hypophthalmichthys molitrix). The initial stocking weight in smaller ponds was around 2–3 g and ranged between 20–60 g in larger ponds. The stocking density varied between 2 800 and 7 000 ha-1. Some of the rural fish ponds studied are as old as 100 years, with deep organic-rich soft sediment. Most of them, however, are new or reconstructed with an age of 2 to 8 years (Table I). The majority of the surveyed rural fish ponds have a water surface area below 1 ha, with the average pond size being less than 1 000 m2. The largest pond, No. 4, has an area of 2.13 ha. The water depth varies from as low as 48 to 225 cm, the average being around 1 m. Supplementary feed is rarely given in these ponds and when given, it has a low nutritive value and functions rather as an organic fertilizer. These small ponds serve many purposes in the life of the villages and organic enrichment is realized through these activities too. Thus the magnitude of organic fertilization relates to the size of human population and animals associated with the particular pond. The human population ranged between 10–1 250 ha-1 and the animal livestock between 5–641 ha-1. They are the point-source of organic enrichment during dry season and diffuse source during the monsoon. Thus the management level of the rural fish ponds corresponds to the organic fertilization technology.
2.4 Stocking fertilization and supplementary feeding
The analysis of the relation between primary production and fish production is still relevant at this management level, where the applied lowprotein, incomplete food only supplements the natural food. Altogether 23 fish ponds were tested under a project designed to optimize the inorganic fertilization technology in the Hungarian polyculture. Using different dosages of nitrogen and phosphorus fertilizers, it was possible to regulate the primary production levels and establish a gradient from 1.27 to 5.00 gC m-2 day-1. The two-year project (1977–1978) was conducted in ponds of 0.14 ha each with an average depth of 1 m. The ponds were drained and dried during winter and filled. They were stocked at the beginning of April and harvested by the end of October in both the years. The stocking structure and density (mean weights g, density ha-1) were silver carp 200, 1 500; bighead carp (Aristichthys nobilis) 180, 1 000; common carp 210, 4 000; grass carp (Ctenopharyngodon idella) 220, 500. The dosage of fertilizers were a combination of 0, 20, 60, 120 kg ha-1 year-1P in the form of superphosphate and 0, 50, 150, 300 kg N as NH4NO3, applied weekly in equal portions during the growing season. A total amount of 5–6 t ha-1 wheat was fed to each pond as supplementary food during the season, according to the increasing biomass of the fish.
3. METHODS
A major drawback in trials to relate the primary production to fish production is the diverse methodology used to measure the plant photosynthesis in water. It was measured 14C or oxygen changes inside the dark and light bottles with short incubation time (Melack, 1976; Noreiga-Curtis, 1979) or sometimes as long incubation as a day (Liang et al., 1981). Another approach is the direct measurement of diel changes of oxygen concentration in the whole water body (McConnell et al., 1977). These methods approximate the gross and net primary productions. In the hypertrophic, highly productive fish-rearing ecosystems, the light and dark bottle method, especially with a long incubation time, underestimates the primary production. In these waters, the most suitable approach is the direct measurement of the diel oxygen concentrations. In the present study, this in situ method of measuring the concentration with an oxygen electrode was used. The mean magnitudes of primary production were calculated using an earlier developed model with seven-measuring points during a diel cycle (Olah et al., 1978). In the case of 18 undrainable rural fish ponds, diurnal variations of dissolved oxygen were monitored with three measuring points and the McConnell (1962) equation was used to calculate the primary production.
Most of the earlier studies have obtained their data on fish production from commercial estimates (Melack, 1976; Oglesby, 1977). In the present work, all the fish ponds were completely harvested. While the harvesting was simultaneous in all the ponds at the end of the growing season in October with draining in the Hungarian experiments, repeated netting was carried out by the owners of the rural fish ponds in India. In all the cases, total annual fish production was used and the unit of gC m-2 day-1 has been chosen instead of that per year, considering the differences in durations of growing season in the temperate and tropical zones. The conversion efficiencies from primary production to fish production in terms of carbon were calculated as ratios and presented as precentages.
4. RESULTS
With a mere stocking manipulation, although with a comparatively high density, a tenfold increase in the percent utilization of primary production has been observed in the two experimental ponds (Table II), as compared to the mean efficiency ratio for natural lakes. A good efficiency level of 2–3 percent may be a result of the relatively low average primary production values of 1–1.7 gC m-2d-1 measured in the experimental ponds. The fish density seems to have exerted a strong grazing pressure on the natural fish food resources.
The average value of primary production in ponds with stocking and inorganic fertilization was as high as 5.3 gC m-2d-1. The fish production was more than double as compared to the previous management level (Table II), but the percent utilization remained low. The decrease of production efficiency at high levels of primary and fish productions may well be explained by the logistic curve describing the full range of GP-FP relations (Liang et al., 1981).
The ponds with raw domestic sewage manuring maintained a high primary production besides the high sustaining bacterioplankton. The wide range of fluctuations in the mean primary production values (2.02 to 6.39 gC m-2 d-1; Table III) among the six experimental ponds may be a result of residual effects of the experiment with different organic loadings carried out in the preceding years. The fish production however, was more uniform and the variations did not follow the trend of primary production. A definite relation between primary production and fish production was not observed, thus indicating the importance of the bacterial food chain in organic enriched systems.
In the experimental ponds with daily introduction of liquid pig manure, a high and constant level of primary production was maintained. Also, the highest average primary production for all systems was measured in these ponds (Table IV). The daily fish production ranged between 14 and 21 kg ha-1. The average percentage of the primary production as fish production was 3.04.
Similarly, there was no close relation between the primary and fish production in the rural fish ponds of Orissa, India (r = 0.15).The highest daily fish production of 15.8 kg ha-1 was observed in pond 11 with low primary production and pond 1 with extremely high primary production produced a moderate fish yield of 8 kg ha-1 (Table V). This particular fish pond (1) is typically representative of rural ponds with organic-rich thick sediment layer, nutrient deficient water column and a permanent bloom of Microcystis spp. In these 18 rural fish ponds, the magnitudes of fish production and conversion efficiency are regulated by a combination of factors such as primary production, stocking density and organic fertilization (Tables I and V).
A close correlation between primary and fish productions was observed in the 23 experimental ponds with supplementary feeding (r = 0.87). The primary production was fluctuating in a wide range according to the doses of inorganic fertilizers applied. Although the supplementary feed was applied in a nearly equal amount in all the ponds, the fish production was varying according to the fluctuating pattern of the primary production. This is due to the incomplete dietary composition of wheat for normal fish growth and their further dependence on the natural food. Fish ponds with moderate primary production ranges of 1–27 to 2.10 gC m-2d-1 maintained an average daily fish production in the range of 16.4 and 27.1 kg ha-1 (Table VI), with high fish production efficiencies of 10.89 to 14.34.In the fish ponds with the high primary productions in the range of 3.77 and 5.00 gC m-2d-1, the average daily fish production was also high and ranged between 27.3 and 40.8 kg ha-1. The fish production efficiency was lower than in the previous ponds, but remained in the range of 6.03 to 10.05.
5. DISCUSSION
Production efficiency levels computed in different fish ponds during the present study were compared with the published results from various sources, covering the temperate and tropical lakes and reservoirs (Table VII). Among the 54 fish ponds tested in the present survey, those with stocking and inorganic fertilization and others with oxidized liquid pig manure exhibited stable primary production levels in the high ranges of 5.29 to 6.49 gC m-2d-1. The average primary production in the tropical undrainable rural ponds was low, but the range of variations was wide being 1.76 to 12.3 gC m-2d-1, the latter in pond 1 which is very near the theoretical upper limit of primary production for standing waters. The variation is a result of the differences in organic load, age, size and management practices.
The fish production in 27 water bodies of the temperate zone change between three orders of magnitude, the low values of 2.75 and 2.83 g ha-1d-1 being in Lakes Superior and Huron (Oglesby, 1977) and the largest value of 3.9 kg ha-1d-1 in Lake Dalneye (Krohun, 1969). The range of variations in the tropical water bodies was smaller and the average fish production remained at a rather constant level in managed ponds except rural fish ponds (2.8 to 15.8 kg ha-1d-1).These values were 3.5 kg ha-1d-1 in simply stocked ponds, 10.4 in the stocked and inorganic fertilized ponds, 11.0 in the stocked and domestic sewage fed ponds, 18.0 in the liquid pig manured ponds and 28.1 in the stocked, inorganic fertilized and supplementary fed ponds.
The fish production efficiency varied over a wide range in both the temperate and tropical lakes and reservoirs. The mean value was larger for water bodies in the temperate zone. By applying the simple management of stocking, the efficiency increased more than ten times and the range was narrow. At the level of inorganic fertilization with stocking, primary and fish productions were high, but the efficiency decreased. High efficiency was obtained for both types of organic fertilized fish ponds. The high efficiency was, however, the result of the bacterial food chain which has great importance in these organic loaded systems.The highest efficiency was observed in fish ponds with supplementary feeding. Even with supplementary feeding, it may be emphasized that the natural food contributes considerably to a proper nutrition as well as utilization of the lowprotein feed applied.
Plotting the primary production and fish yield data from all the 27 water bodies of the temperate zone, two groups of water bodies could be distinguished. Twenty one gave an eye-fitted line with a steep slope, indicating increasing fish production in a narrow range of primary production (Fig. 1). For this large group of temperate lakes and reservoirs, a smaller rate of primary production is characteristic, which may explain the higher efficiency. The second group consisted of six lakes whose fish production ranges were corresponding to the primary production levels. Three of them, Lake Superior, Lake Huron and Lake Ontario, had low fish yields with moderate primary production and the other three - Lake Leven, Lake Neagh and River Tjeukemer had high primary production with moderate fish yields (Oglesby, 1977). A similar slope was obtained for tropical lakes and reservoirs, but with a higher fish production at the same ranges of primary production as earlier.
The comparison of relation between primary production and fish production in fish pond ecosystems seemed to corroborate the conclusion drawn by McConnell et al., (1977). There are principal real differences among different types of fish rearing ecosystems and a uniform equation cannot be elaborated and applied for all the waters. Even the logistic curve describing the whole range of primary production and fish production relations proposed by Liang et al., (1981) seems to be inadequate for a satisfactory explanation of the relation in different management levels. Also the data on the management level with organic fertilization confirmed the conclusion of Noriega-Curtis (1979), that primary productivity alone is not sufficient to account for the high yields attained in organic fertilized systems. The significance of the bacterial food chain has high priority in these allochthonous systems. The insignificant correlation between primary and fish productions in the domestic sewage fed or liquid pig manured fish ponds and in the undrainable rural fish ponds is thus explained. Hence, waters receiving substantial organic inputs as compared to those producing autochthonously should be excluded from models describing the relation between primary and fish productions as was stated by Oglesby (1977).
There are many constraints in evolving a model describing the relation between primary production and fish yields. Several trials at this have been inadequate to explain the mode of energy flow or to predict the fish production from different waters (McConnell et al., 1977; Liang et al., 1981). The methodology employed in studying this aspect has been far from being perfect, as already explained earlier. A variety of factors influence the relation, viz., species composition, stocking density, nature of fertilization and feeding intensity. Significant differences in the efficiency have been demonstrated in case of Tilapia feeding on phytoplankton and Gambusia feeding on the next trophic level of zooplankters (McConnel, 1965; Goodyear et al., 1972), the former giving higher productions within a narrow range of primary production. The stocking density influences the utilization of the available natural food resources and the basic production is underutilized in case of low stocking densities. The organic fertilization introduces a complex food web into the fish ponds, with a higher intensity of bacterial activity through detrital food chain (Noriega-Curtis, 1979). Detailed investigations on the relation between primary production and fish yields, keeping in view the above aspects, are needed in natural waters as well as fish pond ecosystems, to provide more satisfactory explanations.
6. SUMMARY
In an attempt to understand the relation between primary production and fish production, data were collected from 54 fish ponds of different trophic levels and belonging to various management practices, beginning from simple stocking to that of fertilization and supplementary feeding in Hungary and India. While primary production was determined by the diurnal oxygen curve method, fish production was by the actual harvests.
The gross primary production ranges in terms of gC m-2d-1 in the different systems were 1.01–1.73 (stocked ponds), 5.29–5.32 (stocked and inorganic fertilized ponds), 2.02–6.39 (domestic sewage oxidation ponds), 5.36–6.49 (liquid pig manure oxidation fish ponds), 1.76–4.79 (undrainable rural fish ponds except one high value of 12.30 in pond 1) and 1.27–5.00 (stocked, fertilized and fed ponds).
The average fish production values were 3.5 kg ha-1d-1 in simply stocked ponds, 10.4 in stocked and inorganic fertilized ponds, 11.0 in the domestic sewage fed ponds, 18.0 in the liquid pig manured ponds, 2.8–15.8 in undrainable rural ponds and 28.1 in the stocked inorganic fertilized and fed ponds.
The fish production efficiency, i.e., fish yield as a percentage of primary production varied widely: 2.31–3.06 (stocked ponds), 1.87–2.04 (stocked and inorganic fertilized ponds, 1.56–4.95 (domestic sewage ponds), 2.61–3.27 (liquid pig manure ponds), 0.65–6.75 (undrainable rural ponds) and 6.03–14.34 (stocked, fertilized and fed ponds).Even the simple fish stocking without any treatment increased the efficiency levels by ten times, and high values obtained in the organic systems were largely due to the bacterial activity adding to higher energy conversions.
With higher levels of management practices, fish production seemed to depend to a lesser degree on the magnitudes of primary production. The latter however, sustained the higher trophic levels to the requirement and regulated the utilization of inputs. A universal relation between primary and fish productions could not be established.
There are many constraints in evolving a model describing the relation between primary production and fish production, including the methodologies employed and a variety of factors influencing the correlation, such as fish species, stocking density, nature of fertilization and feeding intensity.
REFERENCES
Goodyear, C.P., Boyd, C.E. and Beyers, R.J., 1972. Relationships between primary productivity and mosquito fish (Gambusia affinis) production in large microcosms.Limnol. Oceanogr., 17(3): 445–450.
Hrbacek J., 1969.Relations between some environmental parameters and fish yield as a basis for a predictive model. Int. Ver. fur Theor. Angew. Lim. Verh., 17: 1069–1081.
Liang, Y., Melack, J.M. and Wang, J., 1981.Primary production and fish yields in Chinese ponds and lakes. Trans. Am. Fish. Soc., 110: 346–350.
McConnell, W.J., 1962. Productivity relations in carboy microcosm.Limnol. Oceanogr., 7: 335–343.
McConnell, W.J., 1963. Primary productivity and fish harvest in a small desert impoundment. Trans. Am. Fish. Soc., 92: 1–12.
McConnell, W.J., 1965.
McConnell, W.J., Lewis, S. and Olson, J.E., 1977. Gross phto-synthesis as an estimator of potential fish production. Trans. Am. Fish. Soc., 106 (5): 417–423.
Melack, J.M., 1976. Primary productivity and fish yields in tropical lakes. Trans. Am. Fish. Soc., (5): 575–580.
Noreiga-Curtis, P., 1979. Primary productivity and related fish yields in intensely manured fish ponds. Aquaculture, 17: 335–344.
Northcote, T.G. and Larkin, P.A., 1956. Indices of productivity in British Columbia lakes.J. Fish. Res. Bd Can., 13: 515–540.
Oglesby, R.T., 1977. Relationships of fish yield to lake phytoplankton standing crop, production and morphometric factors. J. Fish. Res. Bd Can., 34: 2271–2279.
Olah, J., Zsigri, A. and Kintzly, A.V., 1978.Primary production estimators in fish ponds by the mathematicl evaluation of daily oxygen curves. Aquacultura Hungarica, 1: 3–14.
Rawson, D.S., 1955. Morphometry as a dominant factor in the productivity of large lakes.Int. Ver. Theor. Angew. Limnol. Verh., 12: 164–175.
Ryder, R.A., 1965. A method for estimating the potential fish production of north-temperate lakes. Trans. Am. Fish. Soc. 94: 214–218.
Smith, E.V. and Swingle, H.S., 1938. The relationship between plankton production and fish production in ponds. Trans. Am. Fish. Soc., 68: 309–315.
Sreenivasan, A., 1972. Energy transformations through primary productivity and fish production in some tropical freshwater impoundments and ponds.Pages 505–514 in Z. Kajak and A. Hillbricht-Ilkowska (editors): Productivity problems in freshwaters. Polish Scientific Publishers, Warsaw, Poland.
Wolny, P. and Grygievek, E., 1972. Intensification of fish pond production.Pages 563–571 in Z. Kajak and A. Hillbricht-Ilkowska (editors): Productivity problems in freshwaters. Polish Scientific Publishers, Warsaw, Poland.
Table I. Main characteristics of undrainable rural fishponds in Orissa, India
| Ponds | Age years | Area ha | Water depth, cm | Human population ha-1 | Animal livestock ha-1 | Stocking density ha-1 |
| 1 | 100 | 0.75 | 160 | 166 | 81 | 3400 |
| 2 | 6 | 1.25 | 95 | 36 | 16 | 2800 |
| 3 | 3 | 0.08 | 48 | 125 | 62 | 6000 |
| 4 | 50 | 2.13 | 225 | 56 | 30 | 5000 |
| 5 | 17 | 0.10 | 135 | 127 | 62 | 4500 |
| 6 | 7 | 0.16 | 110 | 1250 | 562 | 5000 |
| 7 | 7 | 0.20 | 124 | 500 | 325 | 4500 |
| 8 | 8 | 0.02 | 100 | 250 | 100 | 6000 |
| 9 | 22 | 0.20 | 70 | 10 | 5 | 3000 |
| 10 | 12 | 0.50 | 205 | 500 | 240 | 5000 |
| 11 | 4 | 0.02 | 60 | 83 | 641 | 6500 |
| 12 | 2 | 0.01 | 120 | 200 | 15 | 7000 |
| 13 | 2 | 0.01 | 120 | 70 | 7 | 5000 |
| 14 | 6 | 0.08 | 145 | 812 | 200 | 3000 |
| 1- | 3 | 0.03 | 100 | 850 | 50 | 5000 |
| 16 | 3 | 0.08 | 100 | 200 | 37 | 4500 |
| 17 | 2 | 0.02 | 140 | 150 | .20 | 5000 |
| 18 | 2 | 0.10 | 150 | 280 | 115 | 5500 |
TABLE II. Gross primary production (GP), net fish production (FP) and conversion efficiency (FP/GP × 100) in stocked ponds as well as in fish pond with stocking and inorganic fertilization
| Ponds | GP, g C m-2 d-1 | FP, g C m-2 d-1 | FP/GP × 100 |
| Stocked only | |||
| 1. | 1.73 | 0.040 | 2.31 |
| 2. | 1.01 | 0.031 | 3.06 |
| Stocked and fertilized | |||
| 1. | 5.32 | 0.100 | 1.87 |
| 2. | 5.29 | 0.108 | 2.04 |
TABLE III. Gross primary production (GP), net fish production (FP) and conversion efficiency (FP/GP × 100) in domestic sewage oxydation fish ponds
| Ponds | GP, g C m-2 d-1 | FP, g C m-2 d-1 | FP/GP × 100 |
| 1 | 6.39 | 0.100 | 1.56 |
| 2 | 4.02 | 0.117 | 2.91 |
| 3 | 5.34 | 0.114 | 2.13 |
| 4 | 2.02 | 0.100 | 4.95 |
| 5 | 3.57 | 0.123 | 3.44 |
| 6 | 2.06 | 0.108 | 5.24 |
Table IV. Gross primary production (GP), net fish production (FP) and conversion efficiency (FP/GP × 100) in liquid pig manure oxidation fish ponds
| Ponds | GP, g C m-2 d-1 | FP, g C m-2 d-1 | FP/GP × 100 |
| 1 | 5.36 | 0.14 | 2.61 |
| 2 | 6.49 | 0.21 | 3.23 |
| 3 | 5.81 | 0.19 | 3.27 |
Table V. Gross primary production (GP), net fish production (FP) and conversion efficiency (FP/GP × 100) in undrainable rural fishponds of Orissa, India
| Ponds | GP, g C m-2 d-1 | FP, g C m-2 d-1 | FP/GP × 100 |
| 1 | 12.30 | 0.080 | 0.65 |
| 2 | 2.10 | 0.047 | 2.22 |
| 3 | 1.76 | 0.059 | 3.33 |
| 4 | 2.97 | 0.062 | 2.08 |
| 5 | 3.61 | 0.064 | 1.79 |
| 6 | 3.79 | 0.045 | 1.12 |
| 7 | 2.34 | 0.036 | 1.53 |
| 8 | 3.57 | 0.099 | 2.78 |
| 9 | 2.16 | 0.028 | 1.30 |
| 10 | 4.46 | 0.088 | 1.97 |
| 11 | 2.33 | 0.158 | 6.75 |
| 12 | 4.79 | 0.123 | 2.57 |
| 13 | 4.37 | 0.110 | 2.51 |
| 14 | 4.27 | 0.119 | 2.79 |
| 15 | 2.92 | 0.096 | 3.29 |
| 16 | 2.78 | 0.100 | 3.60 |
| 17 | 3.64 | 0.076 | 2.10 |
| 18 | 3.08 | 0.077 | 2.49 |
Table VI. Gross primary production (GP), net fish production (FP) and conversion efficiency (FP/GP × 100) in fish ponds with stocking, fertilization and supplementary feeding
| Pond | GP, g C m-2 d-1 | FP, g C m-2 d-1 | FP/GP × 100 |
| 1 | 2.50 | 0.248 | 9.92 |
| 2 | 2.59 | 0.200 | 7.72 |
| 3 | 1.80 | 0.210 | 11.66 |
| 4 | 2.10 | 0.254 | 12.09 |
| 5 | 2.00 | 0.271 | 13.55 |
| 6 | 2.16 | 0.207 | 9.58 |
| 7 | 2.54 | 0.186 | 7.32 |
| 8 | 1.68 | 0.183 | 10.89 |
| 9 | 1.77 | 0.202 | 11.41 |
| 10 | 1.61 | 0.207 | 12.85 |
| 11 | 1.27 | 0.164 | 12.91 |
| 12 | 1.61 | 0.231 | 14.34 |
| 13 | 4.43 | 0.387 | 8.73 |
| 14 | 4.48 | 0.379 | 8.45 |
| 15 | 3.77 | 0.378 | 10.02 |
| 16 | 4.35 | 0.383 | 8.80 |
| 17 | 3.77 | 0.340 | 9.01 |
| 18 | 3.83 | 0.385 | 10.05 |
| 19 | 5.00 | 0.408 | 8.16 |
| 20 | 4.65 | 0.336 | 7.22 |
| 21 | 4.94 | 0.380 | 7.69 |
| 22 | 4.59 | 0.277 | 6.03 |
| 23 | 3.96 | 0.273 | 6.89 |
Table VII. Ranges and mean values of gross primary production (GP), net fish production (FP) and conversion efficiency (FP/GP x 100) in lakes and fish ponds with varying management practices
| Ecosystems | No of water bodies | GP, g C m-2 Range | d-1 Mean | FP, g c m-2 Range | d-1 Mean | FP/GP × 100 Range | Mean | References | |
| 1 | Lakes, reservoirs in temperate zone | 27 | 008–6.54 | 1.55 | 0.00003–0.039 | 0.0029 | 0.0024–1.31 | 0.224 | Oglesby, 1977 Olah, in prep. |
| 2 | Lakes, reservoirs intropical zone | 21 | 0.30–7.78 | 2.57 | 0.0001–0.06 | 0.006 | 0.004–0.85 | 0.13 | Sreenivasan, 1972 Melack, 1976 |
| 3 | Ponds with stocking | 2 | 1.01–1.73 | 1.37 | 0.031–0.04 | 0.035 | 2.31–3.06 | 2.68 | Present work |
| 4 | Ponds with stocking and inorganic fertilization | 2 | 5.29–5.32 | 5.30 | 0.100–0.108 | 0.104 | 1.87–2.04 | 1.95 | Present work |
| 5 | Ponds with stocking and organic fertilization domestic sewage | 6 | 2.02–6.39 | 3.90 | 0.10–0.123 | 0.110 | 1.56–5.24 | 3.37 | Present work |
| 6 | Ponds with stocking and organic fertilization liquid pig manure | 3 | 5.36–6.49 | 5.89 | 0.14–0.21 | 0.18 | 2.61–3.27 | 3.04 | Present work |
| 7 | Undrainable rural ponds with organic fertilization | 18 | 1.76–12.3 | 3.74 | 0.028–0.158 | 0.087 | 0.65–6.75 | 2.49 | Present work |
| 8 | Ponds with stocking in - organic fertilization and supplementary feed. | 23 | 1.27–5.00 | 2.09 | 0.164–0.408 | 0.281 | 6.03–14.34 | 9.78 | Present work |
Prasad/-
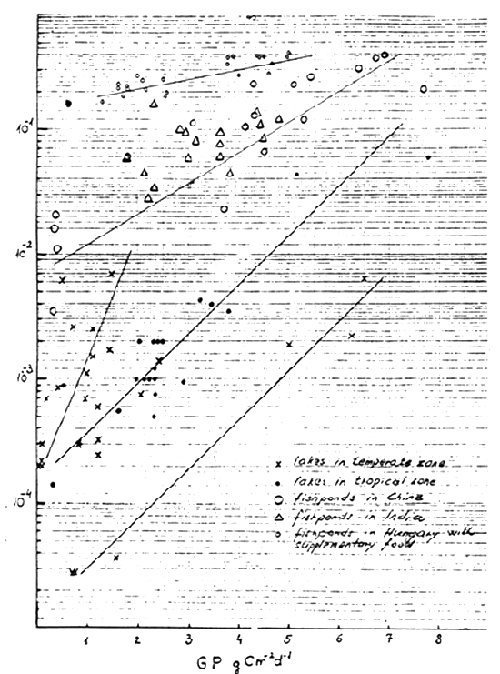
Fig. 1 Relation between gross primary production (GP) and fish production (FP) in different waterbodies