In going about his duties, an observer must first and foremost be unbiased. Samples should accurately reflect parameters of the catch such as length composition, sex, and maturity ratios, and cover the full range of species caught. If these basic conditions are not met the work becomes useless. The locations in which an observer will probably have to sample will often seem primitive in comparison to shore laboratories. The dampness and motion of the vessel will compound the difficulty of the observer's task. However, a great volume of detailed measurements of fish physiology is not asked for. The observer's duty is to take a reasonable quantity of basic weights and sex determination of fish over the course of a trip. Detailed analysis is handled on shore in a laboratory using preserved samples brought in by observers. In brief, while an observer may find sampling a chore at times, it will seldom prove impossible.
The basic objective of catch sampling is to obtain an accurate estimate of the numbers of fish at age which are caught in any particular fishery. This information forms the basis for virtual population analysis (VPA) or cohort analysis which is the most common stock assessment tool used today. When combined with independent estimates of stock abundance such as catch per unit effort and research vessel surveys various aspects of the population dynamics and life histories of the species involved may be determined. This enables biologists to assess the size of the fish stock and make recommendations on how the resource may be harvested without endangering the stocks.
The objective of this discussion is to outline the strategy of catch sampling.
Given that the objective of sampling is to find out the numbers at age removed during a fishery, it is obvious that each fish caught cannot be aged. Therefore the various catches must be sampled to make estimates of what is in the catch. The samples must be taken at random to eliminate bias that may misrepresent the total catch and enough of the catch must be sampled to account for variation between samples.
Within the Observer Program we have elected to use length frequencies as the basis of the sampling program. The reason for this is that length samples are relatively easy to gather and other vital parameters such as age and weight are well correlated with length. It has been demonstrated that the age composition of removals in a fishery may be estimated by gathering relatively few ages and a much larger number of length measurements. Therefore the first step in getting the removals at age of a given species is to determine the length composition of the catch of that species.
To be successful as an observer, one must quickly learn to accurately estimate sizeable quantities of fish. Estimated total catch by species, as recorded by the observer, forms an integral part of the overall data base of the Observer Program. Often, information from the observer's catch composition data provides the only reliable estimate of removals in certain fisheries. The ability to determine quantities of incidental catches (or by-catch) is indispensible as the traditional recording methods such as logbooks have generally tended to yield incomplete or untrustworthy data. The management of entire fisheries, such as the eastern Canadian Silver Hake fishery, is based on minimizing by-catch levels. The observer program, by providing accurate catch information is a means of assessing a given management regime. The catch composition data recorded on Card Type 4 is used by the observer to monitor compliance to regulations relating to misrecording in logbooks, prohibited species, by-catch limitations and discarding, as well as to complete his trip report and to relay his situation reports.
The first and often very difficult problem encountered by the observer is how to make a reliable estimate of total catch when the fish is in the codend on the trawldeck following the haulback. An estimate may be obtained by knowing the total capacity of the codend and guessing the percentage of it filled with fish, or perhaps by taking a volumetric measure. Both these methods are either highly subjective or cumbersome. The best estimate is usually obtained by considering the area between the lateral strengthening bands of the codend, determining how much fish is contained in such a section and multiplying by the number of bands. The captain and trawl master can usually provide a useful approximation of the amount of fish held between bands. When using this method the observer must take into consideration such factors as the species caught and whether the codend tends to bulge nearer the cod line with larger catches. Squid for example, will always pack more densely in a codend than most fish species. Naturally, a greater weight of squid will be contained between strengthening bands and the estimate must be increased accordingly.
The second opportunity to obtain a good estimate of total catch is when the fish is in the holding pen or bunker below decks. In order to do this, the volume of the bin is determined by multiplying length by width by height, in metres (L × W × H). This yields a volume in cubic meters. The capacity of the bunker for a given species is calculated using the formula:
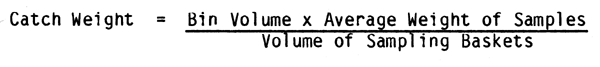
Estimating the weight of approximately 200 fish, the usual sample size, is a simple matter. If the basket is rectangular, the same volumetric equation is used as for the overall bunker volume. If a circular basket or bucket is used, the volume is calculated using the following equation:
Volume (V) = πr²h
where π = 3.14
r = radius (1/2 diameter)
h = depth of basket
For example: If the bin is 5 metres long, 10 metres wide and 2 metres high the volume would be:
L(m) × W(m) × H(m) = V(m³)
or 5 × 10 × 2 = 100m³
The bunker capacity in tons would be:
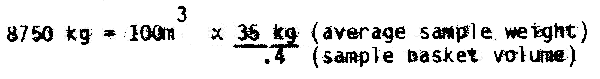
The bunker capacity would be 8.75 tons.
The factory room foreman will probably know the approximate volume of the bunker but might not always be able to tell how much this overall weight will vary by species. It would be a good policy to verify the capacity for a particular species using the above method.
In cases where there is an open pen, such as on domestic vessels, or on the occasional foreign vessel where catch is emptied into a holding pen on deck prior to processing, this calculation may be possible for every set, if height is taken as the level of fish in the bunker. In practice, on most foreign factory vessels determining total catch every set through a volumetric calculation will prove to be too difficult. Many foreign vessels have large, enclosed, irregularly shaped bunkers whose volumes may only be calculated through a complex series of calculations. In instances such as this, the observer should consider a more approximate capacity and estimate the catch by determining the percentage of the bunker filled with fish.
Some domestic wetfish vessels have irregularly shaped open pens that employ planks to retain the catch. In cases where the catch is small, boards are removed while if the catch is large, boards are added. If the observer can determine the weight of fish at a level one plank will retain, a good catch estimate can be arrived at by multiplying by the number of boards.
A third chance to calculate total catch is when the catch is being packaged. Counting the number of boxes of freezer trays of processed fish and multiplying this figure by the weight of a single box or tray (usually constant) will yield an unconverted catch weight or weight of the finished product. The round weight is calculated by using a conversion factor, a coefficient that takes into account the parts of the fish removed in processing. Although this method is quite accurate, it is probably too tedious to be used on a regular basis, especially when catch rates are high. As with estimating fish while in the bunker, this method becomes less reliable in cases where fish or trays from different sets have become intermixed.
On domestic vessels, the total processed catch may be estimated in the ice pens. If the observer knows how much a typical ice pen holds, and what portion of the pen has been filled for a particular tow, then a reasonable estimate may be obtained. The icemen are good sources of information to the observer as they are often the persons with the best knowledge of what fish is actually aboard the vessel.
Accurate determination of by-catch is one of the most difficult skills an observer needs to acquire. While estimating total catch is seldom an easy task, to deduce the species breakdown of the catch is almost impossible from the trawl deck. The only opportunity to get an approximation of the species present is to observe the catch as it is dumped into the bunker. Often, a deck crew will hoist the codend directly above the bunker opening, causing the fish to empty in a great rush. If this is the case, to attempt to visually assess the by-catch may be useless. The observer's best strategy is to ask the master if it is possible to dump the catch more slowly onto the edge of the bunker by hoisting the codend at an angle. In this way the crew can more effectively remove prohibited species such as lobsters or debris such as rocks while the observer has a better opportunity to record the by-catch. This method also overcomes the tendency of certain species to stratify in a codend due to size or a peculiar aspect of their physiology. As the catch is slowly pushed into the bunker, those fish that have risen to the top of the codend or cling to the mesh will not go unnoticed.
This tendency of the various fish species to either rise or sort to the bottom of the pile will cause problems when the fish is in an enclosed bunker. It is best to wait until the catch is allowed to flow from the bunkers before a second attempt at estimating catch composition is made.
Crew members are almost always willing to match their own estimating skills with those of the observer, and because of their superior knowledge of the vessel's gear and performance, they are well worth listening to. They are not always attempting to deceive the observer. Although their estimates may seem unbiased, there is however a trap the unwary observer can easily fall into. Many crew members will look at a bag of fish bearing in mind how much product it will yield and how much money they will receive for it. The observer of course needs an estimate of the catch weight before any cutting or gutting has been done. He should always know what product form a crewman is estimating before allowing himself to be influenced by such an estimate. If indeed the crew are estimating a codend full of fish in terms of its landed value, the observer should consider using an accepted conversion factor in order to compare their estimate with his own.
On some vessels caution will be required when using information from the logbooks. In the past, masters and senior officers have occasionally demonstrated a tendency to tailor catch estimates so that they may fit the restrictions imposed by the vessel's licence. For example, many captains have been reluctant to admit the presence of an amount of fish that would place the vessel in violation of by-catch regulations. Violation or not, the lack of such an entry in a logbook would certainly not be conducive to good biological information, and the observer in such a situation should obviously trust his own estimates over those of a master showing tendencies to bias the catch data. Of course, not all captains aim to deceive in the use of their logbooks but nevertheless, it is of supreme importance for the observer to have confidence in his ability to estimate catches. When observer estimates vary sharply from those of the crew or logbook, there is no reason to panic.
The observer is asked to observe 80% of a vessels sets so as to provide a consistently unbiased record of the vessel's fishing performance. If for example a vessel averages ten sets daily and the observer witnesses nine of them, he is maintaining an excellent coverage. It is only asked that an observer not fall into the habit of missing a set roughly the same time every day. The routine should be varied so that late night and early morning sets receive equal attention with afternoon and evening sets. It is of extreme importance that observers are in a position to observe any changes in catch or catch compositions that may be attributable to diurnal changes in a particular species behaviour.
The observer's prime concern in choosing a sampling location is access to the fish. A place near the main bunker or conveyor would be ideal. The further away from the main source of fish the observer places himself, the greater the inconvenience from having to carry the often very heavy samples. Although there may be a chance of an observer interfering slightly with the processing crew, many foreign fish factory foremen will accept this as unavoidable and make an extra effort to facilitate an observer's work. If however, there proves to be unusual resistance to the observer's requests and no suitable sampling position is offered, the observer should first stress to the master that sampling is a necessary part of his role that must be fulfilled. If there is still no satisfaction, then the program authorities should be contacted and the problem elaborated to them. Figure 7 illustrates some of the options available to the observer.
fig. 7 Factory of a large freezer trawler.
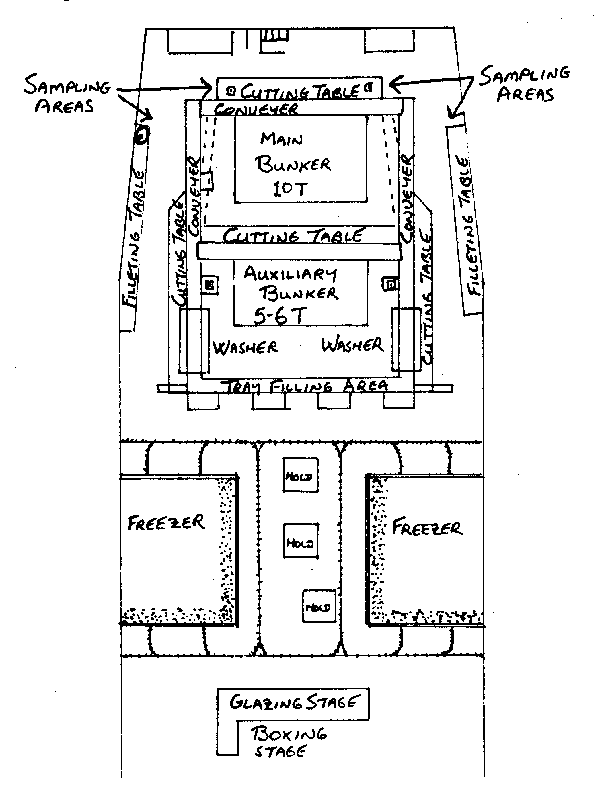
On this very common foreign vessel type the observer has a choice of several excellent places to sample. The location on the cutting table behind the main bunker is ideal. The observer can practically reach into the conveyor from the main bunker to gather fish. On some vessels however, this space may be occupied by extra cutting stations or there might not be a suitable place to hang a scale. If this is the case, the observer can consider moving to the middle of the table, or even to one of the filleting tables along the sides of the vessel. Scarcely less convenient than the first location, these side tables have the added advantage of offering nearby portholes, and perhaps a better place to hang a scale. In a large, roomy factory of this type, the observer should have no problems.
Past experience in the program has shown that while some vessel types may prove difficult, there are very few foreign ships that have factory layouts that totally prevent biological sampling. In the rare case that an observer does encounter such a vessel, the only course of action remaining would be to try to find a safe location on the trawldeck and gather fish to be sampled before the catch is dumped. Of course certain allowances in the sampling program would have to be made for such factors as weather and trawl operations.
The choice of a place to sample on most domestic vessels is quite limited but the small size of the factory can work to the observers advantage. In most cases where there is absolutely no place close to the fish bunker, a hatch cover or conveyor not in use at the opposite end of the factory can be used. The following diagram is of the factory of a domestic vessel.
fig. 8 Factory of a wetfish trawler.
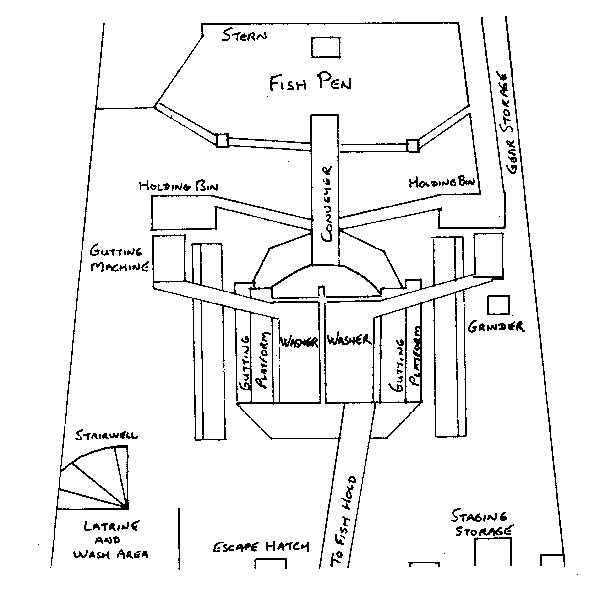
The observer's chief obstacle on a vessel of this type would be the gutting machines and holding bins which occupy much of the limited space ahead of the fish pens. A tall observer may be able to sample on top of the gutting machines, but this is a far from ideal solution. Unfortunately, on a vessel of this type, an observer will always have to do a lot of climbing and lifting, regardless of what sampling location is chosen and access to fish will be more difficult than on foreign factory vessels. With this incovenience in mind, the observer might choose to use the relatively flat escape hatch or make a suitable surface in the staging storage area, in spite of their distance from the fish. The gear storage area might also be considered. If catches are small or there is no inconvenience to the crew, an observer may be lucky enough to find a place on a spare gutting platform or in the open area between the holding bins and the fish pen. For the most part however, these areas will be occupied by crewmen processing fish. Occasionally the fish pen may be sectioned off enabling the observer to sample in amongst the fish. Of course caution should be used when walking through a pile of fish. Wolffish, which are frequently caught in eastern Canadian waters can inflict a painful bite, while blue sharks can do far more serious damage.
In general, finding a suitable sampling location on a domestic vessel can be difficult, so the observer should try several locations prior to choosing the place that suits him best. As with all other aspects of the observer's job, cooperation with the crew is crucial, and nothing will ruin a good working relationship faster than an observer who fails to clean up the mess after his sampling. The observer on a domestic vessel should fall into the practice of ripping and gutting the fish he has measured after a sample or at least offer to step in and process a quantity of fish similar to the amount sampled. Status as an observer does not entail that a person is superior in rank to the ordinary seaman. On the contrary, an observer should treat all crew with due respect and courtesy, whether they be captain or cabin boy.
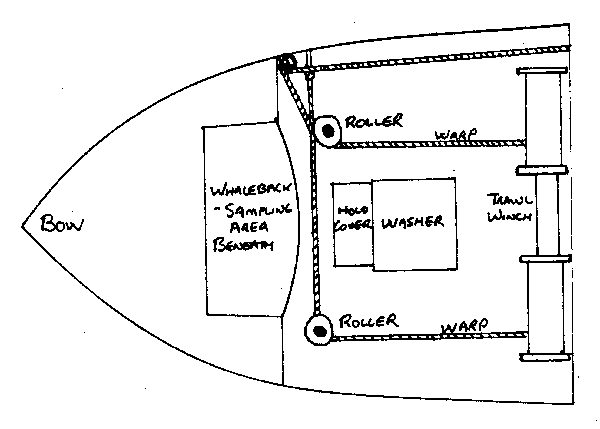
Both Canadian trawler companies and some foreign countries still operate side trawlers and observers can periodically expect to be deployed on them. These vessels pose a unique set of problems in carrying out a sampling program. Such ships are seldom roomy enough to permit sampling below decks in the fish room or in a cabin off the trawl deck. Invariably, work must be done in the fresh air and salt spray of the open deck. There are some useful guidelines to follow. No matter where he sets up, the observer is never far from the towing warps so it is a good idea to confine sampling to periods when the net is out, between shooting and hauling, to avoid interfering with the crew as well as for safety. More often than not, the observer will be forced to sample in the bow of the vessel where there is considerable motion. The taking of otoliths and sample weights should be limited to calmer weather, not only to make the job easier but for the sake of accurate information as well.
A typical deck plan of a side trawler is given in the following diagram. Note that the best and probably only sampling area on the vessel is located in an area known as the whale back in the bow of the vessel ahead of the forward trawl frame. Occasionally this area is used for storing spare trawls and twine which can serve as a secure surface for a measuring board.
fig. 9 Deck layout of a side trawler
The observer's prime concern when gathering a sample is to achieve as random a sample as possible. The slightest bias in the method used to collect the sample can distort the overall length range of the species selected. A point to remember is that all fish of the species to be sampled must have an equal chance of selection. The subconscious human tendency to select only larger individuals must be resisted.
The observer's task is complicated in view of the large catches often encountered in the foreign fisheries. Although it is only asked that the observer gather 200 fish for a representative sample, he might occasionally have to choose 200 fish out of a catch of 30,000 kilos in a random manner! How is it done?
Consider the way fish may accumulate in a codend. As the gear begins fishing, it is probably allowing the smaller individuals to pass through the codend meshes while retaining the larger fish. However, as the codend is filled, these larger fish block or mask the codend meshes allowing greater retention of the smaller fish. It is because of this effect that different length ranges of fish of a single species will accumulate in different parts of the codend. The best way to overcome this obstacle is to gather the sample in stages. As the fish begin to emerge from the bunker at the beginning of a processing run, the observer should gather the first subsample of fish, perhaps half a basket or a full basket, depending upon the size of the container and species sampled. The observer should then finish sampling the fish in this container before sampling another. Meanwhile the crew will have processed the fish that initially emerged from the codend to allow catch from other areas of the codend to be accessible to the observer. The second subsample can be gathered at this stage, completed and a third subsample taken, etc. If catches are extremely large, time may work in the observer's favour. Many vessels will stop fishing in order to clear a production “log jam”, thus enabling the observer to pace his sample to work on fish from a wide variety of areas of the codend and still find time for a coffee break.
In most instances, the observer will have to gather a sample from a conveyor or a section of open pen. The easiest way to assure a random selection is to visualize a section of conveyor, perhaps a metre, or half a cubic metre of pen space, and gather fish from that section only (A conveyor belt should obviously be stopped). Often it may be easiest to gather everything from that section and then sort out unwanted species rather than delay processing by sorting as the sample is removed. Crewmen may occasionally step in to help the observer collect the sample. Because they are seldom versed in the techniques of gathering a random sample, the observer should make sure that they are not exercising any personal tendencies to select larger or smaller fish. On many vessels crewmen will often bring unusual specimens to the observer and while such fish may not be needed for immediate sampling purposes, such interest on the part of the crew should always be encouraged.
Other methods of gathering a sample may be of particular use in obtaining bycatch samples. On many foreign vessels and even some domestic ships, commercially attractive bycatch species are sorted from the main catch and put off to the side in baskets. If the observer can satisfy himself that no fish of the desired species have been discarded or deliberately left on the main production line, then he has an already gathered random sample. On some vessels species such as cod or haddock are thrown on special tables for filleting. The observer might merely have to move his measuring board.
The reduction of fish into meal can occasionally be the source of controversy, but it is always desirable to know exactly what range of fish are being redirected for meal. The observers best bet in getting a reduction sample is to wait by the conveyor leading to the fish meal plant and pull off the fish as they pass. A second, messier method is to gather the fish from the storage hopper. This often means however, that an observer will have to sort round fish from the offal. Also, extreme care should be exercised. An observer should always make absolutely certain that there are no moving screws or cutting blades in the hopper than can remove fingers, hands, or even arms.
A special set of problems may arise when sampling large fish such as cod and pollock. Due to the large sizes such species often reach, sampling the desired 200 individuals may be a difficult task. Good physical condition and time are the best answers to the problem. Occasionally however, the fast production rates typical of these species can cause the amount of fish available for sampling to rapidly diminish. If this is the case, the observer should first ask to have enough fish for a sample set aside and volunteer to process and ice them himself upon completion of the sample. If this cannot be arranged then the only alternative is to settle for a quantity of fish less than 200.
The task of sampling a basket of fish is quite straightforward. The following pointers are offered to make the job even easier.
An observer should first and foremost keep his writing and cutting hand clean and dry. If, for some reason, an observer requires two hands for handling fish, he should consider a loose fitting glove for the writing hand. Hard plastic snorkel gloves will slide on and off with a minimum of tugging. They can be left on the measuring board when not being used and put on without the aid of the other hand when needed.
The observer should acquire the habit of weighting fish before he samples them in order to minimize the loss of body weight that may come about as a result of cutting. It is not a bad idea to sex the entire sample before measuring the fish, and then weigh each sex in separate containers. This way an observer eliminates the need to take off the glove, pick up the knife, put it down, pick up a pencil, put it down, put the glove back on, etc., for every fish sampled. If the species sampled is generally of a small size, silver hake for example, the observer can even do an entire length frequency sample without picking up his knife. As always, the final choice to find the most suitable sampling sequence is the observer's.
When sampling squid it is a good idea to use one hand. Nothing is worse than to be covered in squid ink and have some previously recorded numbers obliterated. The animals are small enough to be held in the left hand while using the right hand to cut them. Scissors will generally cut through the soft mantle with little resistance. Also the observer may consider placing the animal on the measuring board or another hard surface and making a knife cut down the edge of the mantle. In this manner, the entrails and gonads are left intact, while the loose flat of the mantle can be flipped up to allow the observer to take the necessary measurements.
Extra care should be used when sampling redfish or any other fish with sharp spines or teeth (wolffish, monkfish, spiny dogfish, thorny skate, and obviously all sharks). Heavy rubber gloves should be worn at all times when sampling these species. The observer should learn to grab redfish by pinching them between the eyes rather than risk a painful puncture wound as a result of placing a hand on the fish's dorsal spines. The other species should all be handled using one hand just behind, and out of reach of the mouth while the other hand can be used to hold the tail. If a fish such as wolffish starts to thrash too violently while being held, drop it and stay clear!
Obtaining accurate sample weights is often easier said than done. A heavy swell can cause the scale's indicator to sway madly back and forth. The simple answer to this problem is the same as for all other measurements taken in the course of sampling; if no accurate value can be determined, then leave it blank. If the midpoint on the scale is not readily identifiable, don't invent a suitable value. A wild guess makes for a useless sample. The quality of a sample is only as good as the discretion of the observer who took it.
Placing a fish on a measuring board and reading its overall length seems to require little explanation. Still, certain guideliness must be followed and the observer must be wary of particular traps that await him.
The standard measuring board is built for readability enabling the fast measurement of fish. The observer, when placing a fish on the board, should nevertheless assure himself that the fish's snout is resting gently against the head piece of the board. If there is a gap between the snout and board or if the head is compressed, the measurement is obviously no good. At the other end of the fish, the centimetre grouping in which the caudal fork (or tip of the caudal fin) falls, is the measurement to be recorded.
An offset board should always be used for any species other than illex, mackerel, herring or the tunas (which are too large anyway!).
All of the flounders (not halibuts), red and white hake, cusk, and a number of other species are measured to the tip of the caudal fin. Cod, haddock, pollock, silver hake, redfish, and the halibuts are measured to the base of the caudal fork.
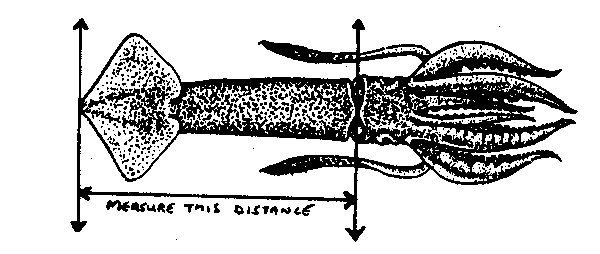
Squid are measured to the nearest half centimetre on a non-offset board, rounded down. Place the apex of the tail against the head piece of the measuring board, and measure to the anterodorsal protruberance.
fig. 10 Squid length measurements
In shrimp samples, only the carapace is measured. Special vernier calipers are used to measure to the nearest half millimetre. Any measurement is rounded down; for example 20.49 is recorded 20, 21.76 is recorded 21.5, 22.13 = 22.0, etc.
Herring and mackerel require a different technique. For herring, use a non-offset board. The tail lobes are pinched together and the length to the end of the lower lobe of the caudal fin should be read to the nearest half centimetre, rounded down (eg: 35.8 = 35.5; 34.4 = 34.0, etc.) Mackerel also require a non-offset board. The length is the caudal fork read to the nearest half centimetre grouping rounded down. Note the following illustration:
fig. 11 Mackerel length measurement
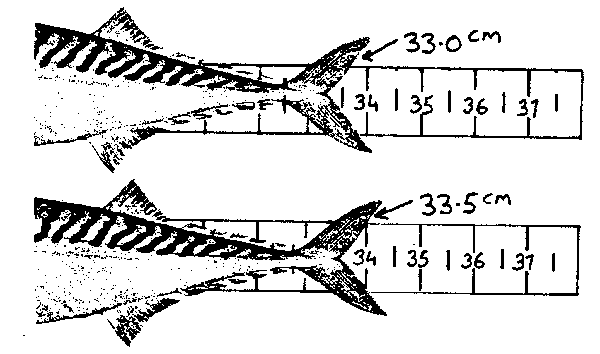
Note that the caudal fork of the first fish does not reach the 3.5 graduation and the measurement is rounded down to 3.0. The caudal fork of the second fish extends beyond the 3.5 mark, so 3.5 is recorded. For other species, the specific approach to measurement should, where possible, follow internationally established procedures.
The separation by sex of fish in a sample is one of the observer's major tasks. Because of the two sexes of certain species grow at different rates, fisheries biologists are especially interested in having length frequencies separated by sex so they can better monitor such growth patterns. Other species such as cod and pollock have shown similar growth rates between the sexes, and therefore observers are not asked to expend extra effort sexing them.
The sexing of most gadoids (cod fish family) and redfish is a relatively simple matter. First, the belly of the fish should be slit, a two to three inch cut paralell to the spine, somewhat ahead of the anal opening, should be suf-ficient. Next, reach inside and move the stomach and intestines aside. The gonads should then be visible against the peritoneum (the membrane that lines the abdominal cavity).
The female gonad, the ovary, is a paired organ that vaguely resembles a pair of orange cigars arranged side by side. It is almost always of a pinkish orange colour and when the fish is sexually mature, is full of eggs. In immature fish, the ovaries maintain a similar colour but are opaque or somewhat granular in appearance.
fig. 12 Overy
The male gonad, the testes, is sometimes less obvious than the ovary, but is not extremely difficult to detect. It can range in appearance from a small crimped string extending from the area of the anal opening in immature fish to a series of leaflike white lobes that yield milt in mature fish. In all but immature fish, the whitish colour and the highly visible capillaries will help to distinguish the testes.
fig. 13 Testes
For a number of groups of tropical fish (eg groupers), the species is a protogynous hermaphrodite: that is, the fish (in this case), is first female, before becoming male at larger sizes. The opposite situation, (protandrous hermaphrodite) occurs, for example, for pandalid shrimps, and some molluscs: in all cases, the advise of an expert will be necessary in deciding on the best sampling strategy.
Flatfish
The gonads of flounder and halibut species are generally similar in appearance to those of the gadoids, but due to the highly unusual anatomy of the flounders, can be quite difficult to locate.
The best method is to locate the soft area of the fish directly behind the operculum (gill opening). This region with few boney structues is the abdominal cavity. The anal opening can be located just ahead of the origin of the fish's anal fin. A circular cut should be made on the ventral side of the fish (not extend far back into the body cavity on both sides of the fish whereas the testes are confined to the general area of the gut cavity. Another distinction is that the ovaries have a rounded edge whereas the testes have a distinctly sharp “knife edge”. This distinction is especially applicable to immature fish. the eye side) allowing the observer to lift up a flap of skin exposing tissue in both the abdominal cavity and the adjacent harder tissue behind. If the fish is mature, the familiar milt or orange eggs should be immediately apparent. If not, the observer will have to do some probing. In all flatfish the ovaries extend far back into the body cavity on both sides of the fish whereas the testes are confined to the general area of the gut cavity. Another distinction is that the ovaries have a rounded edge whereas the testes have a distincly sharp “knife edge”. This distinction is especially applicable to immature fish.
The technique of “candling” can be especially useful as a clue to the sex of flounder. Most of the flounders common to eastern Canadian waters are thin and opaque enough that internal organs may be viewed if the fish is held up to a strong light source. Using this method, the posterior extension of the ovaries can be quite obvious. The fish should still be cut however, for a more exact sex determination. Note that if, after employing any of the above techniques, guaranteed sex determination is still not possible, then the fish should be recorded as unsexed.
Argentine
This species is probably the most difficult to sex of all the fish an observer is likely to encounter in eastern Canadian waters. Unlike the gadoids and flounders, argentine gonads do not have the familiar colours and can often be obscured by layers of fat tissue. Care is needed at all times when attempting to sex argentine.
Both the ovaries and testes in argentine are paired organs that appear as narrow white tubes against a black-grey peritoneum in immature fish. When slightly more mature a granular milt, or opaque, colourless eggs may be visible. In very ripe females, the abdominal cavity will be full of loose eggs with no mysentary, or ovary wall, in evidence. Obviously the immature fish cause the most problems. To correctly sex them is both painstaking and time consuming. A magnifying glass might prove to be a valuable aid.
It is best to open the whole gut cavity from the gill area to behind the anal opening. Then lift the internal organs clear and locate the two tubes against the black peritoneum.
fig. 14 Argentine gut cavity
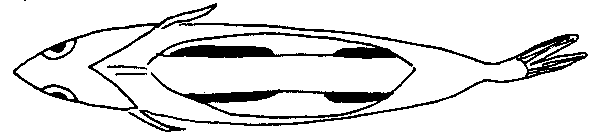
Notice that the gonads (both male and female) consist of two clusters on either side, and are joined by the tube which runs tangential to them (not through them).
In order to sex the fish, take one of the clusters and turn it over. If the underside is convoluted, that is the ovary appears to be divided into longitudinal sections, then the fish is female. The ovary cross section should also be wedge-shaped.
fig. 15 Argentine ovary
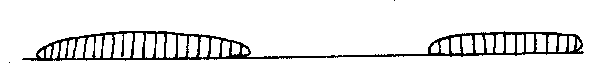
The male testes is flatter and somewhat more granular in appearance. As in other fish species, it is more difficult to detect than the ovary. The best procedure to use may be to routinely check each gonad for eggs, convolutions, and a wedge shape, and in the absence of these, look for the characteristics of the male testes.
fig. 16 Argentine testes
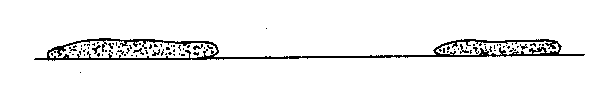
Tunas
The most difficulty in sexing tunas is created by the sheer size of the creatures. It is always best to wait until the crew have gutted a tuna before attempting to sex it. The gonads are generally recognizable. The ovaries are sausage shaped and are often a bright orange-yellow in colour. The testes are flatter and duller in colouration. In many fish a layer of fat will be attached to the gonads but this can quite easily be distinguished from gonad tissue.
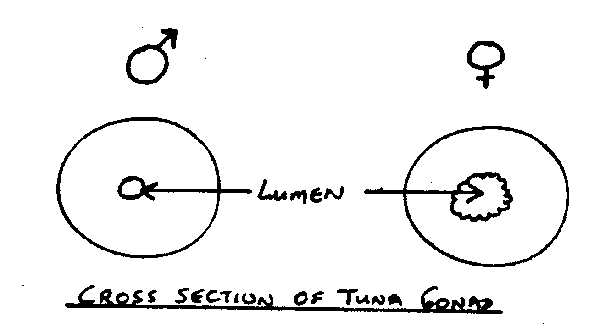
The chief distinctions between the sexes are the cross sectional shape of the gonad, and the size of the lumen (central cavity of the gonad). In female tunas the gonad cross section should reveal a round shape and the lumen should be large and convoluted. The male gonad should be flatter and have a small, smooth lumen. In all cases, squeeze the gonad gently prior to looking for a lumen. This should open the lumen for observation.
fig. 17 Cross section of tuna gonads.
Squid
The sexing of mature squid (Illex Illecebrosus) is relatively simple, but young, small squid will cause problems.
In order to sex squid, the observer can either cut the ventral mid-line of the mantle, or pinch the side of the mantle and cut away a strip allowing him to flip up the remaining tissue to reveal the internal organs. In both methods, care should be used to avoid destroying fragile gonads or digestive organs. Once a suitable cut has been made, the observer should see a configuration resembling the following diagram.
fig. 18 Sampling squid.
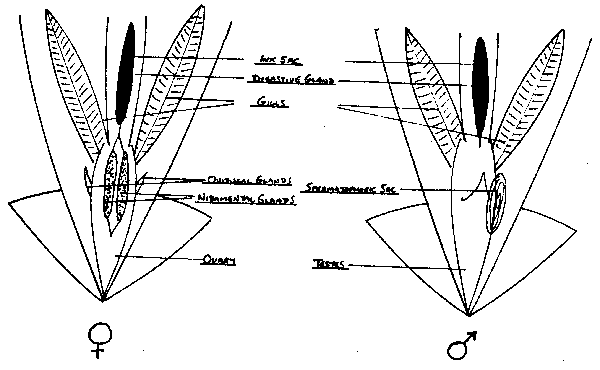
The key distinction between male and female squid are the tube-like nidimental glands in the female and the spiral like striated spermatophoric sac in males. The nidimental glands are opaque thin tubes that lie parallel to each other above the stomach and caecum. The spermatophoric sac lies to the right of the caecum (viewed from above) and may vary from opaque in immature squid to a creamy white swirl in mature animals.
The problem with immature squid is that both of these organs are very clear and seldom obvious when viewed against other organs. One solution is to liberate some ink from its sac and use it to highlight the areas in which the gonads probably lie. A better method is to look for the oviducal glands, two fleshy, pointed, protruberances on either side of the stomach. These can easily be located by gently rubbing the knife against the side of the stomach. If such a protruberance is located on both sides of the squid, it is female. If only one is located on the right side (caecum side) then the squid is most likely male (this single protruberance is known as the spermatophoric gland). The observer should then confirm this identification by locating the round spermatophoric sac in the same general area of the animal.
One of the most important parameters of interest to a fisheries biologist in the study of a sample or catch of fish is the age of the fish taken. Again, observer samples provide this information through the collection of otoliths, or “fish ear bones”. These are bony structures that are located generally beneath the brain in pockets called sacculi and serve as a balance mechanism. When cut (or whole) and placed beneath a microscope, the annuli, (or growth rings) reveal a distinct pattern, allowing the scientist to count the fish's age, in much the same way as a cross section of a tree trunk can reveal a tree's age.
The observer is required to gather otoliths from a wide range of species. The degree of difficulty in removing them can range from effortless in such species as silver hake, to painstaking, for some of the flatfish species. When removing otoliths from the largest cod and pollock, strength and skill more appropriate to a blacksmith may be required. As a rule of thumb, if an observer always has a sharp knife and can get a good grip on the fish, he should always be able to locate the otoliths.
A major concept used in the collection of otoliths is the stratified sample. In order to get a better idea of fish growth and age length relationships, fisheries biologists require a representation of the ages of all length groupings that may occur in a population. This is achieved through stratification of the sample. Rather than inundating themselves with otoliths from fish of the same size, and probably approximately the same age, one or two pair of otoliths from each length frequency grouping may be aged from within a sample. The result is that the observer avoids needless repetition in the collection of a sample, and the age reader obtains meaningful results.
The removal of otoliths can be accomplished during a normal length frequency sample. The observer can choose to immediately place the otoliths in labelled envelopes as they are removed, or to use an aid such as an otolith board. The advantage of the first method is that the observer has less work upon completion of the sample. The disadvantages to this method are formidable however. The paper envelopes are in constant peril of saturation from dripping machinery and the observer has to be extremely nimble to place otoliths in these envelopes with wet or gloved hands. The second method is to use an otolith board. Placed near the measuring board, an observer uses forceps to place each pair of otoliths in a vacant hole. The observer only has to keep track of the length and sex of the fish the otoliths were removed from. One method is to use strips of masking tape beside each hole on the board. The result appears like this:
fig. 19 Otolith board
Using the above method, the observer is advised to use the length frequency sheet to keep track of length groupings as they are filled. In order to fulfill a requirement of one pair of otoliths per centimetre group per sex, the observer can put a check mark against each length group by sex as it is filled.
A second method is to draw a “map” of the otolith board on a blank piece of paper. This can be kept on the clip board with the Card Type 5 as the sample is being done. The observer should be careful to mark the start hole so that he does not accidentally reverse the board when removing the otoliths.
A third method is to mark “start” on the board and to number each hole on the board in an ascending order from start to finish. As otoliths are taken, the observer can indicate on the length frequency sheet the number of the hole that contains otoliths for a particular length and sex of fish. In the example below, the hole number has been pencilled in the empty 0.5 column beside the length grouping and sex of the fish otoliths were taken from. Once the otoliths have been removed, placed in envelopes and labelled, these numbers should be erased.
fig. 20 Marking otolith numbers.
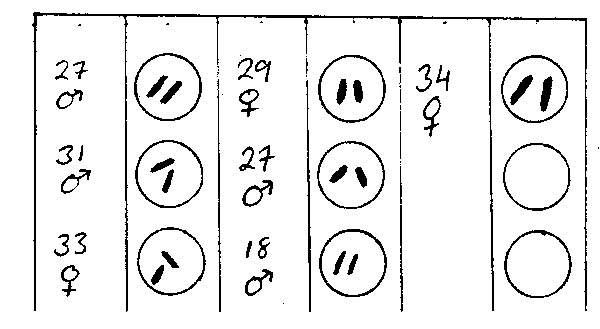
The observer should also keep track of the number of otoliths taken for each length and sex of a given species. The best method is to use stratification sheets which are issued at the program headquarters. The following is an example of a correctly completed stratification sheet:
fig. 21 Stratified otolith sheet
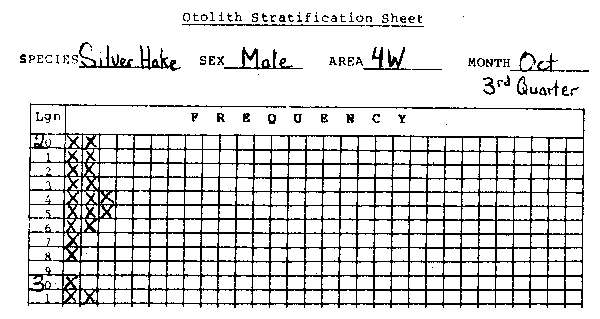
“Quarter” in the above example refers to yearly quarter. Note that the observer was able to get otoliths for all groupings except 29 cm. He was able to fill most of the groupings twice and inadvertantly took a third pair of otoliths for 24 and 25 cm fish. (There is no need to discard any otoliths taken above and beyond normal requirements.)
If an otolith is broken, it should still be included in the envelope, or if one otolith of a pair is not located, the other otolith can still be kept, put in an envelope and labelled.
The otolith envelope should always be labelled as follows:
fig. 22 Otolith envelope

Note that this observer also took individual weights for each fish he removed otoliths from.
fig. 23 Silver hake otoliths
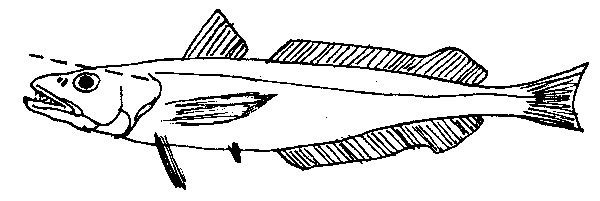
Silver hake have relatively soft skulls that are easily penetrated with a diagonal cut. Such a cut also minimizes the chance of breaking the otoliths. The fish can be held by the eyes or pinched at the gills when making the cut.
fig. 24 Haddock otoliths
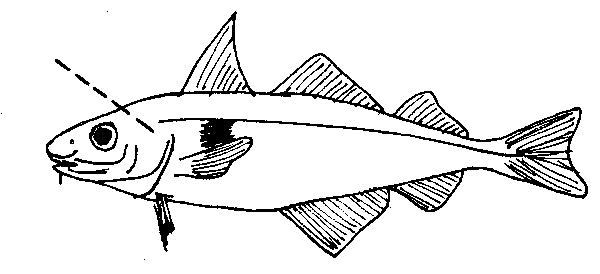
A higher angle cut may be required for haddock because it has a much harder skull than silver hake. This fish should always be held by the eye sockets when removing otoliths.
fig. 25 Pollock and cod otoliths
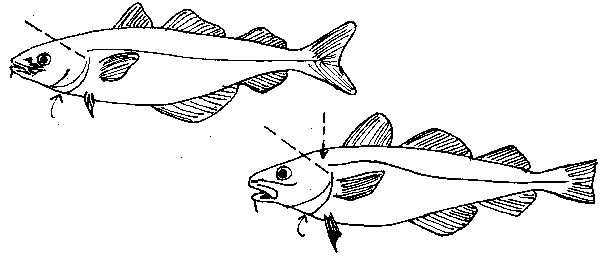
These two species can cause problems. Their large size and extremely hard skulls will often necessitate an almost vertical jabbing cut just to penetrate the skull. Once a small hole has been made, the knife can be worked from side to side to rip the head open. The fish should be tightly gripped by the eye sockets and the fish should be placed on a firm surface. Keep any loose clothing out of the way and make sure the direction of the cut is pointing away from the sampler. If this methods fails, the otoliths may be located by removing the gills and finding the underside of the sacculi, which appear as two bulges against the base of the skull.
fig. 26 Red and white hake and redfish otoliths
The only difficulty with red and white hake are that they are somewhat difficult to get a good grip on. Otherwise they can be treated much like haddock. Observers will seldom be asked to remove otoliths from them.
Redfish have skulls that are more brittle than other fish and therefore a sawing action may be helpful as the cut is made.
fig. 27 Flounder otoliths
The flatfish species are the most difficult of all fish for removing otoliths. The best way is to locate the bony ridge between the eyes and the operculum and make a diagonal cut behind the eyes. The sacculi lie one above the other and the otoliths are generally round in shape but quite small. Much practice will be required before flatfish otoliths can be removed with ease. It is a good idea to remove otoliths from these species when not required for the sake of practice.
1. To remove the stomach, first squeeze down any contents which may be in the oesophagus and snip the pyloric caecum (anterior part of the intestine). Place the cinch tag around the oesophagus, close it as tight as possible and snip the oesophagus as far as possible above the tag.
In cases where the stomach has been cut, or if it is so small that it appears as though the tag may fall off, place the stomach contents and tag with a small amount of formalin in a sealed plastic bag and secure it properly.
2. It is recommended that all samples be placed aside until collecting has finished for the set and then be preserved as a group. Once all the stomachs are collected, inject each stomach with just enough formalin so that they begin to swell slightly. Place them in a plastic bag. This serves to reduce the chance of formalin spillage and possible contamination of the processing and sampling areas.
3. Place the samples for each species with an appropriate label in a separate bag for each set. Squirt some formalin into the bag and tie it off. Place the label in a way that it is readable from outside of the bag.
When assigned to collect stomachs, the observer will be issued a kit containing:
| 2 | buckets with covers | |
| 2 | litres of pre-mixed 10% formalin | |
| 1 | squeeze bottle with a shortened tip for fitting of hypodermic | |
| 1 | vial containing 2 hypodermic needles and a cleaning wire | |
| 65–75 | cinch type stomach tags | |
| 15–20 | sealed plastic bags | |
| 7–10 | large plastic bags | |
| 10–25 | specimen labels |
As part of a detailed fish sample, an observer may be asked to determine the maturity stage of a fish; that is whether a fish is immature, spawning, or spent. This is generally accomplished through close examination of the gonads. An observer will be given prior training in the laboratory or on a research vessel before being asked to determine fish maturities on an observer trip. The following section is intended as a guide for haddock, silver hake, cod, and pollock maturities. The flounder species, argentine and redfish are not currently examined for maturities, and would require different guidelines. Such “maturation states” will need to be defined for each group of fish, in each case by experts on the fish in question.
| CODE | FEMALE | MALE | ||
|---|---|---|---|---|
| Stage I | Immature | 1 | Ovaries small and firm; pale pink or reddish yellow; membrane thin and transparent, eggs invisible to the naked eye. | Testes appear as a clear, crimped and slender string. |
| Stage II | Ripening 1 | 2 | Ovaries slightly larger than I but still small and firm, contents microscopic, yellow or reddish yellow, opaque eggs; blood capillaries showing. | Testes grow grad ually in size; pinkish or flesh coloured; blood capillaries showing. |
| Stage III | Ripening 2 | 3 | Ovaries occupy about half of ventral cavity, reddish with numerous blood vessels; opaque eggs are now visible to the naked eye and give the ovaries a granular appearance. | Testes occupy about half of ventral cavity; begin to turn white with fine, rather con spicuous blood vessels; no milt runs when pressure is applied. |
| Stage IV | Ripe | 4 | Has a few clear eggs at the earliest stage progressing to having mainly clear eggs but eggs to not run freely with slight pressure. Spawning has not started. | Testes distinctly wavy, white and quite distended; large lobes; a small amount of milt may be forced out by pressure. |
| Stage V | Spawning | 5 | As State IV but eggs running freely on slight pressure. | Testes very white and fully distended; ended; milt runs freely at the slightest pressure. |
| Stage VI | Spent | 6 | Ovaries soft, flabby and bloody; practically no eggs remain, purple in colour. | Testes shrunken and reddish; vas deferens promiment against regular surface of testes; some blood left in organ. |
| Stage VII | Recovering | 7 | Membrane purplish and ovaries fairly baggy, no eggs visible. | Testes resume pale ink colour; traces traces of blood in organ; vas deferens deferens and string- like. |
| Stage VIII | Resting | 8 | Looks much like Stage I, but is found in large fish; ovaries larger than in I, also membrane thicker and not as transparent as in I. | Testes larger than in I; surface regular sometimes greyish in colour. |
The art of correctly identifying fish species is not hard to acquire. The first time observer will be taught how to distinguish many of the common species during training. While on a trip however, he may encounter an unfamiliar fish. The best procedure in this case is to consult a key such as those offered in the FAO Species Identification Sheets For Fishery Purposes (e.g. Bianchi and Scott 1981); or other keys offered by FAO for different areas of the world oceans. If this does not result in a positive identification, the observer should try to label and freeze the fish, and return it to headquarters. If the fish is too large for this, a photograph would suffice. Such a fish should be entered as unidentified with an appropriate weight on the catch composition card type (#4).