Identification and evaluation of embryos is one of the most challenging aspects that confronts the embryo transfer practitioner, especially the beginner. Embryo quality and poor handling techniques can directly affect pregnancy rates. A stepwise procedure for embryo searching is presented at the end of this section.
ENVIRONMENT
Once removed from the stable, protective environment of the uterus the embryo should be handled with respect regarding its temperature, pH, osmolarity and contaminants-factors which may affect viability.
Embryos may be maintained at room temperature for several hours without decreasing pregnancy rates significantly, provided the embryos are transferred to fresh holding medium every two hours. Storing embryos in an ice-water bath is recommended for long-distance transportation. There is no obvious decrease in pregnancy rates after storage at this temperature for 12–24 hours. Embryos do not tolerate temperatures above body temperature (39°C) very well.
The physiological range of proper pH for embryos is from 7.1 to 7.5. Thus, the pH of flushing and holding media needs to be within this range. Phosphate buffered saline (PBS) is commonly used because under atmospheric conditions its pH changes very little. However, if bicarbonate (NaHCO3) buffered media (Ham's F-10, MEM, TCM-199, etc.) are used, care must be taken to avoid large shifts in pH (rising pH) when the embryo is exposed to an atmosphere high in CO2.
Even a slight change in the salt concentration (osmolarity) of the medium can effectively reduce the viability of embryos. If the salt concentration of the flushing or holding media is below that of the uterine environment, embryos will absorb water and swell to reach osmotic equilibrium, which sometimes results in rupture of the cell membrance. Conversely, if the salt concentration is above that of the uterus, the embryo will shrink in size (dehydration) causing a reduction in metabolic activity. Comparing the two situations, although both are deterimental to embryos, shrinkage would be less detrimental. If PBS is prepared from a powdered mixture, care should be taken that the correct amount of water is added. The normal osmolarity of uterine fluid is 270–300 mosm.
Exposing the embryos to ultraviolet rays for a prolonged period may cause cellular death. The use of insecticide sprays in the embryology room should be avoided. Insufficient time of aeration after using ethylene oxide gas for sterilization of equipment is detrimental to live cells. Storage period, different suppliers and batches (lot number) of sera all affect embryo growth differently.
IDENTIFICATION OF EMBRYOS
Identification of the embryo in the uterine effluent is based on several morphological features of the embryo, which is the only practical method to determine suitability for transfer. Such methods are subjective and depend very much on experience.
The embryo is spherical and is composed of cells (blastomeres) surrounded by a gelatin-like shell, an acellular matrix known as the zona pellucida. The zona pellucida performs a variety of functions until the embryo hatches, and is a good landmark for embryo identification. The zona is spherical and translucent, and thus is clearly distinguishable from cellular debris. Because of its shape the embryo tends to roll on the bottom of the (searching) dish.
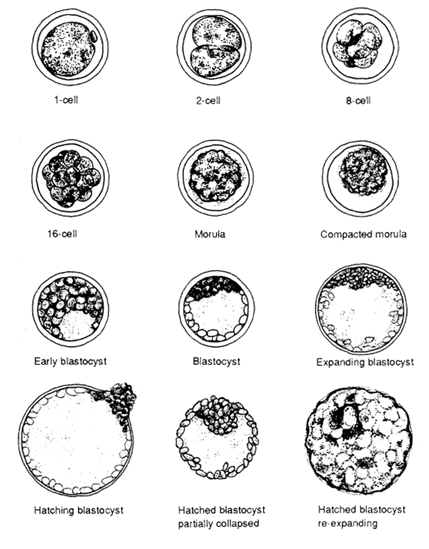
Diagrams of normal bubaline embryos at various stages of development are presented in Figure 4. The stage of development after fertilization must be appropriate for the day on which the embryo is collected, but is not precisely known for buffalo embryos since only relatively few have been recovered to date, with most of them recovered on day 5 to day 7 after the onset of oestrus. The rate of development of water buffalo embryos is faster than that of cattle embryos (Drost and Elsden, 1985). Development from the morula stage to escape from the zona pellucida (hatching) appears to occur in one day in buffaloes. Morphologically water buffalo embryos are similar to bovine embryos and general morphological descriptions can be adapted.
The overall diameter of the bovine embryo has been estimated to be 150–180 μ including a zona pellucida thickness of 12–15 μ. The diameter remains constant until expansion of the blastocoele begins. The colour of the morula and (early) blastocyst also facilitates identification because the embryo is usually darker than other uterine debris. Knowing the age of the embryo (days post oestrus) is also very important in locating the embryo in the searching dish. The fully expanded blastocyst possesses a thinner zona pellucida and is pale (translucent) in colour. A spontaneously hatched embryo is very hard to identify because the embryonic mass, without the zona pellucida, is morphologically similar to uterine debris. If the hatched embryo has collapsed, it may still be identifiable but with considerable difficulty. In summary, the important criteria in identifying embryos are: (1) shape of the embryo; (2) presence of a zona pellucida; (3) size; (4) colour; and (5) knowledge of the age of the embryo.
During embryonic development, cell numbers increase geometrically (1- 2-4-8-16- etc.). Synchronous cell division is generally maintained to the 16-cell stage in embryos. After that, cell division becomes asynchronous and finally individual cells possess their own cell cycle. The cells composing the embryos are termed blastomeres and are easily identified by the 16-cell stages as spherical cells. After the 32-cell stage (morula stage), embryos undergo compaction. As a result, individual cells in the embryo are difficult to identify beyond this stage. The most obvious morphological manifestation during compaction is the loss of a concise cellular outline. The outline of the cells becomes smooth as the cells flatten against one another to maximize their surface contact. It has been shown that both intracellular and intercellular activity in mammalian embryos before and after compaction are very different. At the intercellular level, specialized junctions form between individual blastomeres. These specialized junctions are called gap junctions or focal tight junctions. The tight junctions form an interlocking network providing a permeable seal between the core of the embryo and the external environment. At the intracellular level, polarity develops within individual cells, and microvilli become restricted to the outer surface of the cell. The dynamic cell differentiation begins during the formation of the blastocyst after compaction is completed. Two distinct cell types are present at the blastocyst stage. The two types of cells differ in their morphology, biochemistry, developmental potential and eventual expression. The embryo proper develops from the inner cell mass whereas the surrounding trophectoderm primarily gives rise to the chorionic ectoderm of the placenta. The mechanism that underlines this cellular differentiation is still not well understood.
FIGURE 4
Diagrams of normal, excellent quality embryos at different stages of development
HANDLING THE EMBRYOS
Once an embryo is identified in the searching dish, it is immediately transferred to a small Petri dish (35 × 10 mm) containing fresh, filtered (0.22–0.45 μ pore size), sterile medium. As a holding medium, generally phosphate buffered saline (PBS) containing penicillin plus 10–20 percent heat inactivated serum is used. Embryos are tentatively classified simply as good or bad, and may be recorded on the cover of the holding dish. This allows for a quick estimate of the total number of embryos found. Embryos are then serially rinsed through at least three different dishes containing fresh sterile medium using a new sterile pipette for each step. Finally, they are placed in a dish awaiting transfer or cryopreservation. Under certain circumstances, e.g. for export, embryos must be rinsed through ten different dishes containing sterile media. All dishes must be kept covered between searches to avoid contamination, and particularly evaporation, when placed in the incubator. Evaporation of the small volume of medium in a flat dish rapidly leads to hypertonic solutions.
CLASSIFICATION OF EMBRYOS
Embryos recovered five to seven days after oestrus are classified morphologically into the following groups (Figure 4).
Morula
Blastomeres are round in shape and are not tightly connected to each other. Individual blastomeres are difficult to discern from one another. The cellular mass of the embryo occupies most of the perivitelline space.
Compact morula (tight morula)
The shape of a tight morula is similar to a golf ball, in that the outer edge is slightly bumpy (scalloped) in appearance because of compaction. Individual blastomeres are no longer distinguishable. Cells on the surface of the mass are polygonal in shape. The embryo mass occupies 60–70 percent of the perivitelline space.
Early blastocyst
A tiny clear space is visible which contains fluid. This area is the beginning of the blastocoele. The embryo occupies 70–80 percent of the perivitelline space.
Blastocyst
The prominent blastocoele cavity comprises more than 70 percent of the volume of the embryo. Two groups of cells are present and clearly recognizable as the trophoblastic layer beneath the zona pellucida and the darker inner cell mass occupying one side of the embryo. The perivitelline space may still be visible.
Expanding or expanded blastocyst
There is no perivitelline space between the layer of trophoblastic cells and the inside of the zona. The zona pellucida becomes thinner as the blastocyst expands. A small (well compacted) inner cell mass positioned on one side of the embryo is observed. The colour of the embryo is pale to clear because of the large amount of fluid present inside.
Hatched blastocyst
Ultimately the blastocyst expands to the point of rupture and the embryo escapes from the disrupted zona. Hatched blastocysts may be spherical with a well-defined blastocoele or they may be collapsed, resembling debris. Identification of embryos at this stage can be difficult for the inexperienced operator. When zona-free, or hatched, blastocysts are collected, there is a greater risk of damage due to handling. Furthermore, hatched blastocysts are “sticky” and may adhere to tubing and glassware. Embryo filters should not be used when there is a possibility that hatched embryos will be recovered (> day 7).
FIGURE 5
Diagrams of good quality embryos
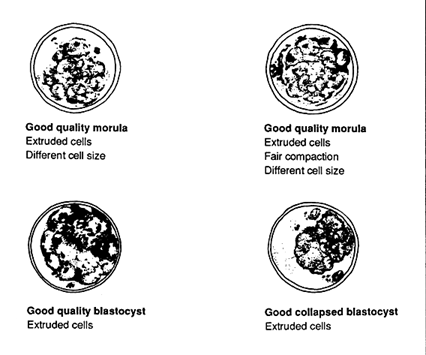
Embryos are generally classified into different groups based on gross morphological appearance (Figures 5, 6, 7).
Excellent embryos have no visible imperfections.
Good embryos have a few recognizable imperfections, such as poor compaction, variation in cell size or a few extruded cells.
FIGURE 6
Diagrams of fair quality embryos
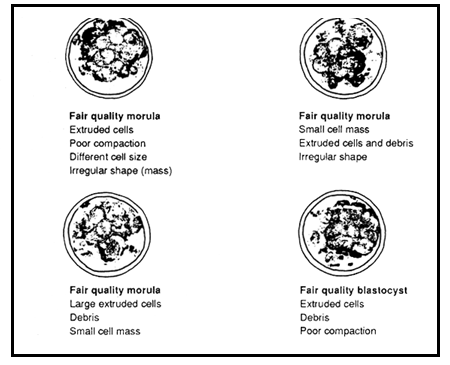
Fair embryos show more disarrangement, such as a small embryonic mass with irregular shape, and large numbers of extruded or dead cells. They are usually one to two days retarded in development.
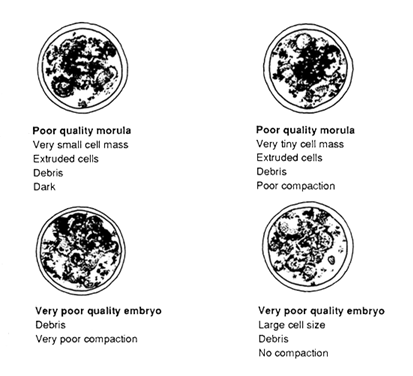
Poor embryos show signs of cellular degeneration, a very small embryonic mass and many extruded cells or disintegrated cytoplasm. Generally, development is retarded by more than two days in these embryos.
Very poor embryos contain either all dead cells or only a few live cells or a very tiny cell mass which is extremely disorganized in appearance. They are composed mainly of debris and generally are not worth transferring.
FIGURE 7
Diagrams of poor and very poor quality embryos
Additional criteria may be considered for evaluation of embryos to provide a fair assessment:
There are additional methods for the evaluation of the viability of embryos:
TABLE 4
Effect of embryo quality on the pregnancy rate in cattle
| Researchers | Transfer method | Embryo age (days) | Embryo quality | |||
| Excellent | Good | Fair | Poor/ Very poor | |||
| Drost et al., 1975 | ST | 4 | 60* (23)** | 100 (4) | 14 (7) | - |
| Elsden et al., 1978 | ST | 5–9 | 63 (275) | 58 (152) | 31 (42) | 12 (42) |
| Schneider, Castleberry & Griffin, 1980 | ST | 6–8 | 70 (1 809) | 55 (694) | - | - |
| Wright, 1981 | NST | 6–8 | 64 (1 748) | 45 (438) | 33 (100) | - |
| Linder & Wright, 1983 | NST | 5–9 | 45 (220) | 45 (170) | 25 (85) | 14 (29) |
| Takeda et al., 1986 | ST | 7–8 | 85 (375) | 73 (335) | 70 (161) | 63 (19) |
* Percentage of pregnancies
** Number of embryos transferred
PROCEDURE FOR EMBRYO SEARCHING
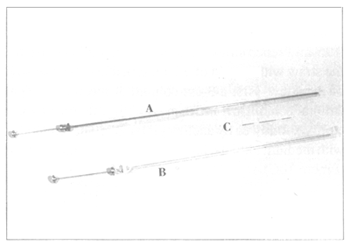
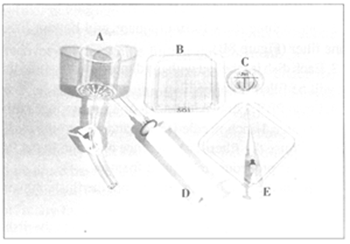
FIGURE 8
Equipment for
embryo searching.
A. Embryo filter;
B. Searching dish
with grid; C. Holding
dish; D. 35-ml
non-toxic syringe
with microfilter
attached; E. 0.5-ml
syringe with
catheter tip.
Alternatively, when a large 500–1 000-ml graduated cylinder is used, the embryos are allowed to settle to the bottom of the cylinder for 20–30 minutes. All but the bottom 75 ml of flushing medium is slowly siphoned off with a small diameter piece of tubing. The final 75 ml of medium is gently swirled and then poured into a searching dish. The cylinder is rinsed two to three times with small volumes of flushing medium (containing 1-percent serum) and emptied into a searching dish. This is followed by steps 7 to 12 as before.
LOADING THE STRAW
Just prior to non-surgical transfer embryos are loaded individually in sterile 0.25-ml French straws. The embryo is aspirated from the holding dish into the straw with the aid of a 1-ml tuberculin syringe attached to the plug end of the straw. First a 3-cm column of medium is aspirated, followed by a 0.5-cm column of air, then a 3-cm column of medium containing the embryo, followed by another air bubble. The remainder of the straw is filled with medium until the initial column of medium wets and solidifies the plug (Figure 10C).
FIGURE 9
Searching
grid-bottomed dish
under
stereomicroscope.
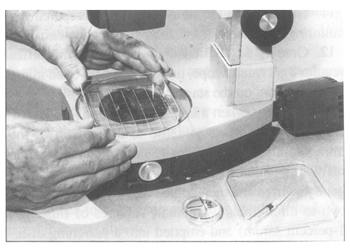
FIGURE 10
Equipment for non-surgical embryo transfer. A. Miniaturized embryo
transfer syringe, length 52 cm, with sheath and covered by a sanitary,
plastic sleeve; B. Standard Al syringe, length 44 cm, with sheath and
covered by a sanitary, plastic sleeve; C. 0.25-ml straw loaded with an
embryo in the centre column of medium flanked by two air bubbles.