A toxicity problem is different from a salinity problem in that it occurs within the plant itself and is not caused by a water short-age. Toxicity normally results when certain ions are taken up with the soil-water and accumulate in the leaves during water transpiration to an extent that results in damage to the plant. The degree of damage depends upon time, concentration, crop sensitivity and crop water use, and if damage is severe enough, crop yield is reduced. The usual toxic ions in irrigation water are chloride, sodium and boron. Damage can be caused by each, individually or in combination.
Not all crops are equally sensitive to these toxic ions. Most annual crops are not sensitive at the concentrations shown in Table 1 but the majority of tree crops and woody perennial-type plants are. Toxicity symptoms, however, can appear on almost any crop if concentrations are high enough. Toxicity often accompanies or complicates a salinity or infiltration problem although it may appear even when salinity is low.
The toxic ions sodium and chloride can also be absorbed directly into the plant through the leaves moistened during sprinkler irrigation. This occurs typically during periods of high temperature and low humidity. The leaf absorption speeds the rate of accumulation of a toxic ion and may be a primary source of the toxicity.
Many trace elements, in addition to sodium, chloride and boron, are toxic to plants at very low concentrations. Fortunately most irrigation supplies contain very low concentrations of these trace elements and are generally not a problem. Suggested maximum concentrations for these unusual trace elements are given in Section 5.5. These concentrations are based upon limits established to protect the soil resource from contamination if continuously irrigated with water which contains them.
The most common toxicity is from chloride in the irrigation water. Chloride is not adsorbed or held back by soils, therefore it moves readily with the soil-water, is taken up by the crop, moves in the transpiration stream, and accumulates in the leaves. If the chloride concentration in the leaves exceeds the tolerance of the crop, injury symptoms develop such as leaf burn or drying of leaf tissue. Normally, plant injury occurs first at the leaf tips (which is common for chloride toxicity), and progresses from the tip back along the edges as severity increases. Excessive necrosis (dead tissue) is often accompanied by early leaf drop or defoliation. With sensitive crops, these symptoms occur when leaves accumulate from 0.3 to 1.0 percent chloride on a dry weight basis, but sensitivity varies among these crops. Many tree crops, for example, begin to show injury above 0.3 percent chloride (dry weight).
Chemical analysis of plant tissue is commonly used to confirm a chloride toxicity. The part of the plant generally used for analysis varies with the crop, depending upon which of the available interpretative values is being followed. Leaf blades are most often used, but the petioles of some crops (grapes) are sometimes used rather than leaves. For irrigated areas, the chloride uptake depends not only on the water quality but also on the soil chloride, controlled by the amount of leaching that has taken place and the ability of the crop to exclude chloride. Crop tolerances to chloride are not nearly so well documented as crop tolerances to salinity. Table 14 gives the known tolerances of several crops to chloride in the saturation extract or in the applied water. These values may need to be changed where local experience indicates that different levels cause damage. For example, tobacco, although tolerant to chloride, acquires progressively more undesirable burning characteristics of the leaf as well as reduced storage life if chloride levels in irrigation water increase above a few milliequivalents per litre. This greatly affects its market value.
| Crop | Rootstock or Cultivar | Maximum Permissible Cl-without Leaf Injury2 | |
|---|---|---|---|
| Root Zone (Cle) (me/l) | Irrigation Water (Clw)3 4 (me/l) | ||
| Rootstocks | |||
| Avocado (Persea americana) | West Indian | 7.5 | 5.0 |
| Guatemalan | 6.0 | 4.0 | |
| Mexican | 5.0 | 3.3 | |
| Citrus (Citrus spp.) | Sunki Mandarin | 25.0 | 16.6 |
| Grapefruit | |||
| Cleopatra mandarin | |||
| Rangpur lime | |||
| Sampson tangelo | 15.0 | 10.0 | |
| Rough lemon | |||
| Sour orange | |||
| Ponkan mandarin | |||
| Citrumelo 4475 | 10.0 | 6.7 | |
| Trifoliate orange | |||
| Cuban shaddock | |||
| Calamondin | |||
| Sweet orange | |||
| Savage citrange | |||
| Rusk citrange | |||
| Troyer citrange | |||
| Grape (Vitis spp.) | Salt Creek, 1613-3 | 40.0 | 27.0 |
| Dog Ridge | 30.0 | 20.0 | |
| Stone Fruits (Prunus spp.) | Marianna | 25.0 | 17.0 |
| Lovell, Shalil | 10.0 | 6.7 | |
| Yunnan | 7.5 | 5.0 | |
| Cultivars | |||
| Berries (Rubus spp.) | Boysenberry | 10.0 | 6.7 |
| Olallie blackberry | 10.0 | 6.7 | |
| Indian Summer Raspberry | 5.0 | 3.3 | |
| Grape (Vitis spp.) | Thompson seedless | 20.0 | 13.3 |
| Perlette | 20.0 | 13.3 | |
| Cardinal | 10.0 | 6.7 | |
| Black Rose | 10.0 | 6.7 | |
| Strawberry (Fragaria spp.) | Lassen | 7.5 | 5.0 |
| Shasta | 5.0 | 3.3 | |
A chloride toxicity can occur by direct leaf absorption through leaves wet during overhead sprinkler irrigation. This occurs most frequently with the rotating type sprinkler heads and is discussed in Section 4.3.
Sodium toxicity is not as easily diagnosed as chloride toxicity, but clear cases of the former have been recorded as a result of relatively high sodium concentrations in the water (high Na or SAR). Typical toxicity symptoms are leaf burn, scorch and dead tissue along the outside edges of leaves in contrast to symptoms of chloride toxicity which normally occur initially at the extreme leaf tip. An extended period of time (many days or weeks) is normally required before accumulation reaches toxic concentrations. Symptoms appear first on the older leaves, starting at the outer edges and, as the severity increases, move progressively inward between the veins toward the leaf centre. Sensitive crops include deciduous fruits, nuts, citrus, avocados and beans, but there are many others. For tree crops, sodium in the leaf tissue in excess of 0.25 to 0.50 percent (dry weight basis) is often associated with sodium toxicity.
Leaf tissue analysis is commonly used to confirm or monitor sodium toxicity but a combination of soil, water and plant tissue analyses greatly increases the probability of a correct diagnosis. When using only leaf blade analysis to diagnose sodium toxicity, it is advisable to include analyses of leaf blades from damaged trees as well as separate analyses from nearby undamaged ones for comparative purposes.
Sodium toxicity is often modified or reduced if sufficient calcium is available in the soil. Whether an indicated sodium toxicity is a simple one or is more complicated involving a possible calcium deficiency or other interaction is presently being researched. Preliminary results indicate that for at least a few annual crops, calcium deficiency rather than sodium toxicity may be occurring. If confirmed, these crops should respond to calcium fertilization using material such as gypsum or calcium nitrate. For a discussion of possible calcium deficiency, see Section 5.6 on Nutrition and Water Quality.
Many crops do show sodium toxicity. The toxicity guidelines of Table 1 use SAR as the indicator of the potential for a sodium toxicity problem which is expected to develop following surface irrigation with a particular quality of water. Table 15 gives the relative sodium tolerance of several representative crops. The data in the table are given not in terms of SAR but of soil exchangeable sodium (ESP). Estimates of soil ESP that are expected to result from long-term (several years) use of water of given SAR can be made using the nomogram in Figure 1. (Refer to Section 3.2.1 for a discussion of the impact of erroneous interpretations of SAR-ESP relationships in presence of gypsum.)
Adapted from data of FAO-Unesco (1973); Pearson (1960); and Abrol (1982).
The approximate levels of exchangeable sodium percentage (ESP) corresponding to the three categories of tolerance are: sensitive less than 15 ESP; semi-tolerant 15–40 ESP; tolerant more than 40 ESP. Tolerance decreases in each column from top to bottom. The tolerances listed are relative because, usually, nutritional factors and adverse soil conditions stunt growth before reaching these levels. Soil with an ESP above 30 will usually have too poor physical structure for good crop production. Tolerance in most instances were established by first stabilizing soil structure.
Particular care in assessment of a potential toxicity due to SAR or sodium is needed with high SAR water because apparent toxic effects of sodium may be due to or complicated by poor water infiltration. As shown in Table 15, only the more sensitive perennial crops have yield losses due to sodium if the physical condition of the soil remains good enough to allow adequate infiltration. Several of the crops listed as more tolerant do show fair growth when soil structure is maintained and, in general, these crops can withstand higher ESP levels if the soil structure and aeration can be maintained, as in coarse textured soils.
Boron, unlike sodium, is an essential element for plant growth. (Chloride is also essential but in such small quantities that it is frequently classed non-essential.) Boron is needed in relatively small amounts, however, and if present in amounts appreciably greater than needed, it becomes toxic. For some crops, if 0.2 mg/l boron in water is essential, 1 to 2 mg/l may be toxic. Surface water rarely contains enough boron to be toxic but well water or springs occasionally contain toxic amounts, especially near geothermal areas and earthquake faults. Boron problems originating from the water are probably more frequent than those originating in the soil. Boron toxicity can affect nearly all crops but, like salinity, there is a wide range of tolerance among crops.
Boron toxicity symptoms normally show first on older leaves as a yellowing, spotting, or drying of leaf tissue at the tips and edges. Drying and chlorosis often progress toward the centre between the veins (interveinal) as more and more boron accumulates with time. On seriously affected trees, such as almonds and other tree crops which do not show typical leaf symptoms, a gum or exudate on limbs or trunk is often noticeable.
Most crop toxicity symptoms occur after boron concentrations in leaf blades exceed 250–300 mg/kg (dry weight) but not all sensitive crops accumulate boron in leaf blades. For example, stone fruits (peaches, plums, almonds, etc.), and pome fruits (apples, pears and others) are easily damaged by boron but they do not accumulate sufficient boron in the leaf tissue for leaf analysis to be a reliable diagnostic test. With these crops, boron excess must be confirmed from soil and water analyses, tree symptoms and growth characteristics.
A wide range of crops was tested for boron tolerance by using sand-culture techniques (Eaton 1944). Previous boron tolerance tables in general use have been based for the most part on these data. These tables reflected boron tolerance at which toxicity symptoms were first observed and, depending on crop, covered one to three seasons of irrigation. The original data from these early experiments, plus data from many other sources, have recently been reviewed (Maas 1984). Table 16 presents this recent revision of the data. It is not based on plant symptoms, but upon a significant loss in yield to be expected if the indicated boron value is exceeded. Table 17 presents recent data on citrus and stone fruit rootstocks and are listed in order of increasing boron accumulation.
Obviously, the most effective method to prevent occurrence of a toxicity problem is to choose an irrigation water that has no potential to develop a toxicity. But if such water is not available, there are often management options than can be adopted to reduce toxicity and improve yields.
| Very Sensitive (<0.5 mg/l) | |
| Lemon | Citrus limon |
| Blackberry | Rubus spp. |
| Sensitive (0.5 – 0.75 mg/l) | |
| Avocado | Persea americana |
| Grapefruit | Citrus X paradisi |
| Orange | Citrus sinensis |
| Apricot | Prunus armeniaca |
| Peach | Prunus persica |
| Cherry | Prunus avium |
| Plum | Prunus domestica |
| Persimmon | Diospyros kaki |
| Fig, kadota | Ficus carica |
| Grape | Vitis vinifera |
| Walnut | Juglans regia |
| Pecan | Carya illinoiensis |
| Cowpea | Vigna unguiculata |
| Onion | Allium cepa |
| Sensitive (0.75 – 1.0 mg/l) | |
| Garlic | Allium sativum |
| Sweet potato | Ipomoea batatas |
| Wheat | Triticum eastivum |
| Barley | Hordeum vulgare |
| Sunflower | Helianthus annuus |
| Bean, mung | Vigna radiata |
| Sesame | Sesamum indicum |
| Lupine | Lupinus hartwegii |
| Strawberry | Fragaria spp. |
| Artichoke, Jerusalem | Helianthus tuberosus |
| Bean, kidney | Phaseolus vulgaris |
| Bean, lima | Phaseolus lunatus |
| Groundnut/Peanut | Arachis hypogaea |
| Moderately Sensitive (1.0 – 2.0 mg/l) | |
| Pepper, red | Capsicum annuum |
| Pea | Pisum sativa |
| Carrot | Daucus carota |
| Radish | Raphanus sativus |
| Potato | Solanum tuberosum |
| Cucumber | Cucumis sativus |
| Moderately Tolerant (2.0 – 4.0 mg/l) | |
| Lettuce | Lactuca sativa |
| Cabbage | Brassica oleracea capitata |
| Celery | Apium graveolens |
| Turnip | Brassica rapa |
| Bluegrass, Kentucky | Poa pratensis |
| Oats | Avena sativa |
| Maize | Zea mays |
| Artichoke | Cynara scolymus |
| Tobacco | Nicotiana tabacum |
| Mustard | Brassica juncea |
| Clover, sweet | Melilotus indica |
| Squash | Cucurbita pepo |
| Muskmelon | Cucumis melo |
| Tolerant (4.0 – 6.0 mg/l) | |
| Sorghum | Sorghum bicolor |
| Tomato | Lycopersicon lycopersicum |
| Alfalfa | Medicago sativa |
| Vetch, purple | Vicia benghalensis |
| Parsley | Petroselinum crispum |
| Beet, red | Beta vulgaris |
| Sugarbeet | Beta vulgaris |
| Very Tolerant (6.0 – 15.0 mg/l) | |
| Cotton | Gossypium hirsutum |
| Asparagus | Asparagus officinalis |
1 Data taken from Maas (1984).
| Common Name | Botanical Name | Level of Boron accumulation |
|---|---|---|
| Citrus | ||
| Alemow | Citrus macrophylla | Low |
| Gajanimma | Citrus pennivesiculata or Citrus moi |  |
| Chinese box orange | Severinia buxifolia | |
| Sour orange | Citrus aurantium | |
| Calamondin | X Citrofortunella mitis | |
| Sweet orange | Citrus sinensis | |
| Yuzu | Citrus junos | |
| Rough lemon | Citrus limon | |
| Grapefruit | Citrus X paradisi | |
| Rangpur lime | Citrus X limonia | |
| Troyer citrange | X Citroncirus webberi | |
| Savage citrange | X Citroncirus webberi | |
| Cleopatra mandarin | Citrus reticulata | |
| Rusk citrange | X Citroncirus webberi | |
| Sunki mandarin | Citrus reticulata | |
| Sweet lemon | Citrus limon | |
| Trifoliate orange | Poncirus trifoliata | |
| Citrumelo 4475 | Poncirus trifoliata X citrus paradisi | |
| Ponkan mandarin | Citrus reticulata | |
| Sampson tangelo | Citrus X tangelo | |
| Cuban shaddock | Citrus maxima | |
| Sweet lime | Citrus aurantiifolia | High |
| Stone Fruit | ||
| Almond | Prunus dulcis | Low |
| Myrobalan plum | Prunus cerasifera | |
| Apricot | Prunus armeniaca | |
| Marianna plum | Prunus domestica | |
| Shalil peach | Prunus persica | High |
1 Data taken from Maas (1984).
The potentially toxic ions sodium, chloride and boron can each be reduced by leaching in a manner similar to that for salinity, but the depth of water required varies with the toxic ion and may in some cases become excessive. If leaching becomes excessive, many growers change to a more tolerant crop. Increasing the leaching or changing crops in an attempt to live with the higher levels of toxic ions may require extensive changes in the farming system. In cases where the toxicity problem is not too severe, relatively minor changes in farm cultural practices can minimize the impact. In a few cases, an alternative water supply may be available to blend with a poorer supply to lower the hazard from the poorer one.
Alternatives for management of toxicity and to maintain production are discussed in the following sections.
A parallel can be drawn between salinity and toxicity. The toxic ions (chloride, sodium and to a lesser extent boron) are an appreciable part of the normal salinity accumulation in the root zone and, as with salinity, leaching is the only practical way to reduce and control these toxic ions in the crop root zone. A toxicity can develop within a few irrigations or within one or more growing seasons, depending upon the toxic ion concentrations in the irrigation water and the leaching fraction accomplished.
Leaching can be used either to prevent a problem or to correct the problem after it has been recognized from plant symptoms or damage to the crop. Plant symptoms along with soil, plant and water analyses are very useful for monitoring for both potential toxicity and the adequacy of present leaching practices and crop management. If the toxic ion is coming from the irrigation water, emphasis should be placed on prevention through adequate leaching. In continuously irrigated areas, reclamation should not be necessary unless leaching has been inadequate and excess toxic ions have built up to concentrations that affect crop production.
Chloride ions move readily in the applied irrigation water and make up an important part of water and soil salinity. The concentration factors for salinity given in Table 4 also apply for the chloride ion. The concentration factor for a certain leaching fraction (Table 3) multiplied by the concentration of the chloride ion in the water will closely approximate the expected average concentration in the crop root zone. Chloride can be leached and the leaching requirement equation (9) for salinity (Rhoades 1974), as described in Section 2.4.2, is equally appropriate for calculating the leaching requirement for chloride if the chloride tolerance (Cle in saturation extract) and the chloride in the irrigation water (Clw) are known. The LR equation then becomes:
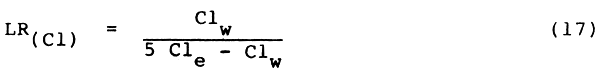
| where: | LR(Cl) | = | the minimum leaching requirement needed to control chloride with ordinary surface methods of irrigation |
| Clw | = | chloride concentration in the applied irrigation water in milliequivalents per litre (me/l) | |
| Cle | = | chloride concentration tolerated by crop as determined in the soil saturation extract, in milliequivalents per litre (me/l) |
Sodium ions cause toxicities to sodium sensitive crops (mostly tree crops and woody ornamentals) at a lower SAR value than would be expected to cause a permeability problem. The sodium ions move less readily with the soil-water than do chlorides. However, research indicates that high leaching fractions (LF) can be effective to maintain a low soil SAR but for SAR values in the water in excess of 9, without added amendments, a leaching fraction of 0.30 or greater may be required. Deliberately adding such large quantities of water in an attempt to control sodium toxicity may not be practical because this may cause problems with soil aeration and drainage. A preferred solution is to add moderate amounts of gypsum or calcium supplying fertilizer materials (acidifying if lime is present; basic or calcium supplying if no soil lime is present). If leaching plus amendments cannot control the sodium toxicity problem, a change to a more tolerant crop may be advisable.
Boron is much more difficult to leach than are chloride and sodium. Boron moves slowly with the soil-water and requires about three times as much leaching water as would be needed to reduce an equivalent amount of chloride or salinity. In many field observations, the boron concentration in the soil saturation extract of the upper root zone usually approaches that in the irrigation water applied. With good irrigation management, it should be possible to reduce and maintain the upper root zone soil at nearly the same boron concentration as in the applied water.
As discussed above, the key to controlling a toxicity problem is to select a good source of irrigation water and then leach as needed to control any toxic build-up which may impair crop production. If the irrigation management is poor and harmful concentrations develop, amendments and reclamation leaching may be needed to restore soil productivity. For reclamation leaching, the same general guides apply for both salinity and chloride (see Section 2.4.6). For boron, the same principles apply but about three times as much water will be needed. Figure 22 shows the relative effectiveness of leaching of boron by sprinklers or by intermittent ponding. Recent research indicates that soil application of sulphuric acid may speed reclamation of a boron affected soil but no extensive field tests or observations are available to confirm this.
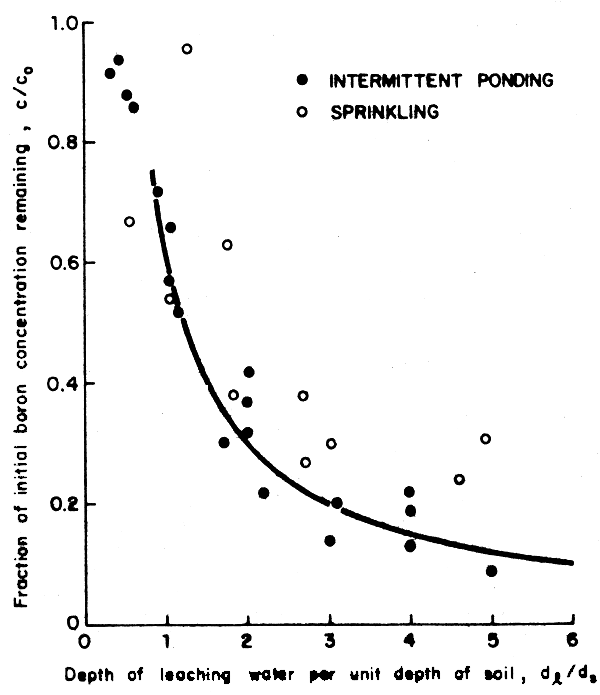
Fig. 22 Depth of leaching water per unit depth of soil required to reclaim a soil inherently high in boron (Hoffman 1980)
Selecting a more tolerant crop offers a very practical solution to a toxicity problem. There are degrees of sensitivity to boron, chloride and sodium just as there are degrees of sensitivity to salinity. Limited information is available on the relative tolerance of crops to toxic ions. Table 14 presents data for chloride, Table 15 for sodium, and Tables 16 and 17 for boron. It must be kept in mind that these are approximations and local farming conditions may modify them. Factors affecting tolerance include climate, irrigation management, leaching fraction, drainage, growth stage of the crop and crop maturity date.
The selection of tolerant rootstocks or cultivars is another method of changing the crop to cope with the existing conditions. Certain rootstocks or varieties differ in their ability to exclude ions such as chloride (see Table 14) or boron (see Table 17) and produce good crops under less than ideal conditions.
Since leaching is the principal method of toxic ion control, cultural practices to aid in management of irrigation water at the farm level are the keys to success. Cultural practices which offer better control and distribution of water include land grading, profile modification and artificial drainage if natural drainage is inadequate. These steps are complementary to those previously discussed for improved salinity and toxicity control.
The severity of a toxicity problem will increase as the crop withdraws soil-water and the soil dries between irrigations (Figure 4). The ions become concentrated in the smaller volume of soil-water. As the upper soil dries, the crop must withdraw more and more of its water needs from the deeper soil where salinity and toxic ions are usually in greater concentration. Increasing the frequency of irrigation supplies a greater proportion of the water needs from the upper soil as well as diluting the deeper soil-water and should reduce the impact of both salinity and toxic ions. This has been previously discussed in Section 2.4.4.
Fertilization practices are normally thought to offer little benefit to counter salinity, but for a toxicity such as that from boron in a citrus crop, many growers are applying extra nitrogen to stimulate vegetative growth. Boron first accumulates to toxic amounts in the older leaves which then become necrotic and drop, thereby reducing the photosynthetic capability of the tree. In this case, nitrogen is used to stimulate new growth to restore the leaf area and photosynthetic capability. Leaf analysis for nitrogen is the guide to the nitrogen requirement. For example, the recommended nitrogen guideline for the Washington Navel Orange is 2.4–2.6 percent nitrogen (dry weight) in 5 to 7-month old terminal spring cycle leaves from non-fruiting, nonflushing shoots. But, if boron becomes a problem, this guideline is raised to nearer to 2.7–2.8 percent N and fertilization practices are modified to reach it.
It takes time to accumulate boron in the leaves. A crop like walnuts may not accumulate sufficient quantities from moderate amounts of boron (1 to 2 mg/l) in the water to damage the crop before it is harvested. In such a case, toxicity is a potential threat and by the end of the season most leaves will show severe boron toxicity (B = 1500 mg/kg). Even though the quality of crop is not greatly affected, the tree vigour and size may be. Alfalfa grown in the Clear Lake area of California using relatively high boron water (> 10 mg/l) is apparently cut frequently enough to avoid recognizeable problems; similarly, golf course greens at Calistoga, California, irrigated with high boron wastewater (2 to 3 mg/l) have not shown toxicity symptoms, presumably for the same reason (see Section 8.25).
Sodium toxicity (high SAR) from applied water is generally countered by use of a soil or water amendment such as gypsum. In general, where salinity of water is relatively low (ECw < 0.5 ds/m), the beneficial response to a water-applied amendment is much greater than if salinity is high because it is far easier to change the sodium to calcium ratio of a relatively low salinity water than one of higher salinity. Soil amendments rather than water amendments are relied upon to correct a sodium problem related to a highly saline water or to a high ESP soil. It also becomes more difficult to correct the sodium toxicity as the soil clay content increases. Using amendments should not be expected to mitigate chloride or boron problems, unless the amendment improves water infiltration and soil permeability which would permit increased leaching to take place. Amendments are discussed in more detail in Section 3.2.1.
If an alternative water supply is available, but not fully adequate in quantity or quality, a blend of waters may offer an overall improvement in quality and reduce the potential toxicity problem. Blending is especially effective for a sodium toxicity problem since proportions of monovalent (Na+) and divalent (Ca++) cations absorbed on the soil depend on concentration, with dilution favouring adsorption of the divalent calcium and magnesium ions rather than the monovalent sodium. A discussion of a quality change resulting from blending is given in Section 2.4.7 and Section 3.2.2.
Overhead sprinkling of sensitive crops can cause toxicities not encountered when irrigating by surface methods. The toxicity occurs due to excess quantities of sodium and chloride from the irrigation water being absorbed through leaves wet by the sprinklers. Extreme cases have resulted in severe leaf burn and defoliation. Absorption and toxicity occur mostly during periods of high temperature and low humidity (<30 percent), frequently aggravated by windy conditions. Rotating sprinkler heads present the greatest risk. Between rotations water evaporates and the salts become more concentrated in the shrinking volume of water. Slowly rotating sprinklers (less than 1 revolution per minute) cause alternate wetting and drying cycles; the slower the speed of rotation, the greater the absorption. High frequency (near daily) spray irrigation has also created problems in some cases.
The leaf burn and resulting crop damage seems to be due to uptake from the applied water of either sodium or chloride. In some instances both sodium and chloride have been absorbed and both accumulate. Toxicity to sensitive crops occurs at relatively low sodium or chloride concentrations (> 3 me/l) and, in general, crops sensitive to sodium or chloride are thought to be most sensitive to foliar absorption. Most annual crops are not sensitive but they will be damaged if concentrations are high enough. Crop tolerances to sodium and chloride in sprinkler-applied irrigation water are not well established due to limited data and the pronounced influence of climatic conditions, but Table 18 gives estimates based upon recent field investigations. They should be used as a first approximation of the potential hazard and any situation which approaches the sodium or chloride values given should be further evaluated by field testing before full implementation of the application system.
| Na+ or Cl- concentrations causing foliar injury3 me/l | |||
|---|---|---|---|
| <5 | 5 – 10 | 10 – 20 | >20 |
| Almond | Grape | Alfalfa | Cauliflower |
| (Prunus dulcis) | (Vitis spp.) | (Medicago sativa) | (Brassica oleracea botrytis) |
| Apricot | Pepper | Barley | Cotton |
| (Prunus armeniaca) | (Capsicum annuum) | (Hordeum vulgare) | (Gossypium hirsutum) |
| Citrus | Potato | Corn (maize) | Sugarbeet |
| (Citrus sp.) | (Solanum tuberosum) | (Zea mays) | (Beta vulgaris) |
| Plum | Tomato | Cucumber | Sunflower |
| (Prunus domestica) | (Lycopersicon lycopersicum) | (Cucumis sativus) | (Helianthus annuus) |
| Safflower | |||
| (Carthamus tinctorius) | |||
| Sesame | |||
| (Sesamum indicum) | |||
| Sorghum | |||
| (Sorghum bicolor) | |||
1 Data taken from Maas (1984).
2 Susceptibility based on direct accumulation of salts through the leaves.
Toxicity has occurred in California citrus areas on leaves wet by sprinklers with water at concentrations as low as 3 me/l of either sodium or chloride. With furrow and flood irrigation this same water causes no toxicity or leaf burn. Slight damage has been reported on alfalfa using water with ECw = 1.35 ds/m and 6 me/l sodium and 7 me/l chloride, but this was under high evaporative, possibly windy conditions, using rotating sprinklers (Table 19). In contrast, water as high as ECw = 4.4 ds/m with 24 me/l sodium and 37 me/l chloride showed little or no damage when evaporative conditions were low (Table 20). The sensitivity also depends upon the crop. Several vegetable crops tested were fairly insensitive to foliar effects even at very high concentrations and in semi-arid areas.
Foliage can be damaged by salt from ocean spray or from drift from sprinklers accumulating on the leaf surface. This has occurred along the Pacific Coast of California as well as in downwind drift areas from sprinklers. Other less frequent problems also occurring with sprinklers include reddish deposits on leaves due to iron content of the sprinkler-applied water and white deposits from bicarbonate or other deposits from water solubles such as gypsum. While these are not toxicities, they can reduce the marketability of a foliage crop or the acceptability of a crop such as table grapes (see Section 5.3).
Where foliar absorption or deposition is a problem, certain management practices have been successful to counter it. Each particular problem will need to be evaluated separately. Some practices may require minor changes in management while others will require more elaborate alterations including holding reservoirs or replacing the irrigation system.
| Rate of Application (mm/hr) | |||
|---|---|---|---|
| 1.8 | 2.7 | 4.0 | |
| Alfalfa plants with leaf burn (percent) | 92.5 | 5.0 | 2.5 |
1 Data taken from Robinson (1980).
| 2 Irrigation water quality | ECw | = | 1.35 dS/m |
| TDS | = | 875 mg/l | |
| Na | = | 6 me/l | |
| Cl | = | 7 me/l |
| Variety | Day Sprinkled | Night Sprinkled | Surface Irrigated |
|---|---|---|---|
| Short staple | 0.73 | 0.46 | 0.44 |
| Long staple | 0.29 | 0.12 | 0.10 |
1 Data taken from Busch and Turner (1967).
| 2 Irrigation water quality | ECw | = | 4.4 dS/m |
| Na | = | 24 me/l |
Irrigate at night: Night sprinkling is quite effective in reducing or eliminating both sodium and chloride toxicity due to foliar absorption and has also reduced the problem of foliar deposits. As humidity generally rises at night and winds decrease, the rate of evaporation and concentration is reduced. Night irrigation has also been of benefit by lowering night-time temperatures during very hot periods. Table 20 shows differences in sodium content in cotton leaves when night and daytime sprinkler irrigation were compared.
Avoid periods of high wind: Hot, dry winds are a major factor in the concentration, absorption and deposition. Avoiding these periods for overhead sprinkling minimizes the problem and avoids possible leaf burn caused by drift to downwind crop areas. In some areas, this may require night irrigation.
Control sprinkler drift: In hot, windy areas, the downwind drift from sprinkler irrigation presents a risk. This drift, if it lands on adjacent plant leaves, is more concentrated than the applied sprinkler water. To minimize the potential leaf burn, movable sprinklers should be moved progressively downwind rather than upwind in order to wash away drifted salts as soon as possible. To avoid drift during high risk periods requires sprinkling during early morning, late evening and night hours when the winds are likely to be less than in the middle of the day. Mist nozzles or high pressure impact sprinklers should be avoided in windy areas where drift is likely to be a problem. Grouping sprinklers in blocks is preferable to long widely spaced single rows if drift is likely to be a problem.
Increase sprinkler rotation speeds: Slowly rotating sprinklers allow appreciable drying on the leaves between sprinkler rotations. More frequent or continuous wetting of foliage allows less drying of leaves and less absorption than intermittent wetting and drying. A sprinkler head rotation of one revolution per minute or less is often recommended, but to achieve this may involve changing the type of sprinkler head and, in some cases, the pressure and design of the system. This alternative may prove costly to implement if the same water use efficiency is to be maintained.
Increase rate of application: If soil water storage capacity and water infiltration rate permit, a higher rate of application may reduce damage by reducing the total period of crop wetting. This would reduce the severity of toxicity due to leaf absorption. Increasing the application rate can be accomplished by enlarging the sprinkler orifices, increasing the pressure, or reducing the spacing on the sprinkler system, but this might require a costly change in sprinkler system design. Table 19 shows the leaf burn associated with different rates of application for the Imperial Valley of California. The data indicate that application rates less than 2.7 mm/hr cause excessive amounts of leaf burn on alfalfa during the high evaporative demand (summer) period in this California desert climate (Robinson 1980).
Change irrigation method: Sprinkler systems which moisten only a little of the foliage can greatly reduce the absorption problem. Low angle or undertree sprinklers wet less of the leaves, but in many cases any lower leaves that are moistened still show symptoms from foliar absorption and in some cases the lower branches may be defoliated. A survey of citrus orchards in California showed that leaf burn and defoliation were associated with the lower leaves that had been wetted by sprinkler spray. Non-sprayed leaves from the upper portions of the trees and leaves from furrow-irrigated trees showed no leaf damage and markedly lower sodium content. In Bahrain (see Section 8.6), similar results have been shown with lemon trees. Furrow, flood, basin or drip irrigation are viable alternatives since they do not wet the leaves.
As demonstrated on some commercial farms in western USA, pivot irrigation sprinkler systems can be modified with drop lines to apply the water to the soil and not to the leaves for many crops.
Increase droplet size: Where a change in sprinkler system design is needed, sprinkler heads that apply a larger droplet size will result in less absorption as small droplets are more subject to evaporation and wind drift. While increasing droplet size may reduce the effect from foliar absorption, a further assessment needs to be made of the effect of droplet size on soil dispersion, sealing and compaction which could cause greater runoff.
Select different crops: In extreme cases it may be necessary to change from the more sensitive crops, such as beans and grapes, if they can no longer be economically produced. Local experience should provide a guide to crops more tolerant to the given conditions.
Plant during cooler seasons: Planting crops during the cooler part of the growing season reduces total water use and the hazards from sprinkler applied water. These cooler season crops can sometimes be harvested before periods of extremely low humidity. Crops planted in the cooler season have a better chance to mature before the sodium or chloride can accumulate to high enough concentrations to cause toxicity damage. Changing the growing season is an extreme alternative which should only be taken after assessment of the market possibilities for the new planting date.