R.J. Connor1
1. INTRODUCTION
1.1 The Crisis in Africa
Animal trypanosomiases are serious diseases of livestock in many parts of the tropics and sub-tropics. In Africa they assume a continental dimension, since the causative parasites are cyclically transmitted by tsetse flies, which infest some 10 million km2 south of the Sahara. In other parts of the world the transmission of trypanosomes pathogenic to animals is believed to be non-cyclical, or mechanical, and is effected mainly by blood-sucking arthropods. The essential difference between cyclical and non-cyclical transmission is that, in the former case, tsetse flies form a reservoir of infection, a factor which complicates the epidemiology and control of the African animal trypanosomiases. This report concentrates on tsetse-transmitted animal trypanosomiases.
African animal trypanosomiases should not be seen simply as a group of diseases, but must be seen in the broad context of livestock production and rural development. Sub-Saharan Africa is poor and becoming poorer. It is affected more severely than many other parts of the world by the two closely linked crises of high human population growth rates and environmental degradation. Food shortages are estimated to affect one quarter of the population of sub-Saharan Africa (excluding South Africa and Namibia) or about 100 million people (World Bank, 1989). To overcome these shortages, to feed the growing population (reckoned to double over the next twenty years) and to reduce dependence on food imports, food production must be increased by 4 per cent per annum. Suitable policies need to be put into practice and improved and appropriate technologies must be adopted to achieve this expansion. Of crucial importance is the greater use of animal traction to enable increased cultivation and to provide transport in rural areas for crops, fertilizer and other goods. A major constraint on achieving this goal is tsetse-transmitted trypanosomiasis.
1.2 Trypanosomiasis and Management
The presence of tsetse excludes livestock from large areas of considerable agricultural potential, by virtue of the severity of the diseases caused by tsetse-transmitted trypanosomes. Animals have a central role in most African societies, and provide milk, meat, manure, hides and skins as well as valuable draught power. Additionally, because livestock represent a means of accumulating and distributing wealth, they have great social significance.
Trypanosomiasis depresses every aspect of production : fertility is reduced; young stock growpoorly; milk yields are low; carcase quality is poor; affected animals lack stamina and strength; and mortality rates are high.
In tsetse-infested areas, trypanosomiasis is a herd problem, and against the background of reduced productivity other animal health problems occur. Trypanosomiasis cannot therefore be viewed in isolation. In many parasitic conditions, host and parasite establish an equilibrium, and the presence of the parasite does not seriously disadvantage the host. This is true in animal trypanosomiasis and is best exemplified by the wild animal hosts of tsetse which form a huge reservoir of trypanosomes. The trypanotolerant breeds of domestic animals also display an ability to control trypanosomal infections, and they are often to be found in areas where other breeds cannot survive. Even so, there is evidence that so-called trypanosusceptible animals can establish a delicate balance with potential pathogens. Trypanosomal infections are often protracted, and chronic trypanosomiasis is much more common than acute disease in enzootic areas. In all animals, disturbance of the equilibrium precipitates or exacerbates disease. The disturbance takes the form of stress, which may be due to the occurence of another disease, malnutrition, water deprivation, heat stress or a production stress such as pregnancy, lactation or work. The significance of this point is that management determines to a large extent the well-being of animals and may do much to mitigate the effects of trypanosomiasis. However, the pathogenicity of the different species of trypanosomes in the different species of livestock varies (Table 1), and within a trypanosome species there is a range of virulence.
1.3 Perceptions of Trypanosomiasis
Trypanosomiasis cannot be separated from livestock management or the control of other diseases, and must be seen in this context. However, the perspective varies with the standpoint. A rural person's perception of trypanosomiasis is quite different from that of a rancher or a veterinarian. It is the perception which determines the action taken to overcome the problem. The smallholder farmer wants to keep more animals because he expects, and accepts, high mortality rates. The veterinarian, on the other hand, is trained to approach the problem clinically; he has first to recognize the signs of disease, confirm diagnosis and then provide treatment. He will also consider more widespread control. In common with the control of many other diseases there is often a pyramidal, top-down approach to the control of trypanosomiasis. The action taken usually depends on a decision at headquarters or provincial level which is fed down to district and then to village level. Control of trypansomiasis may be aimed against either the tsetse or the trypanosome and in the absence of adequate funds for large-scale tsetse control, trypanocides have been widely used. At the farmer's level trypanocides provide a way for the individual to take action to control the disease. Very often only sick animals are treated because they constitute the problem perceived by the farmer. Herd prophylaxis is favoured by fewer livestock owners, and has been largely applied by veterinary services.
1.4 Policy and Priority
With few exceptions, throughout Africa governments have lacked the resources to continue to provide effective veterinary services to control trypanosomiasis, among other diseases. The initiative launched through the Pan African Rinderpest Campaign (PARC) promotes cost recovery for veterinary services, to ensure sustainability. This principle dominates decision-making today and any approach to the diagnosis, treatment and prevention of animal trypanosomiasis must be made with this in mind. Furthermore, control measures must be cost effective and should, where appropriate, combine different methods, which are not necessarily mutually exclusive. The present priority in Africa is to apply knowledge and methods already available to promote food production in the undernourished continent. It is against this background that the following consideration of the diagnosis, treatment and prevention of trypanosomiasis is made.
Table 1. Pathogenicity1 of sallvarian trypanosomes in livestock
| Trypanosome subgenus | Trypanosome species | Cattle | Goats | Sheep | Pigs | Horses | Donkeys |
| Trypanozoon | T.brucel2 | + | ++ | ++ | + | +++ | ++ |
| T.evansl3 | ++ | + | + | ++ | +++ | ++ | |
| T.equiperdum4 | - | - | - | - | +++ | ++ | |
| Nannomonas | T.congolense | +++ | ++ | ++ | + | ++ | ++ |
| T.simiae | - | + | + | +++ | - | - | |
| Duttonella | T.vivax | +++ | ++ | ++ | - | ++ | + |
| Pycnomonas | T.suis5 | - | - | - | ++ | - | - |
Notes: 1. Under usual field conditions, but which is modified by many factors.
3. Mechanical transmission by biting files other than tsetse.
2. DIAGNOSIS OF TRYPANOSOMIASIS
2.1 Difficulties of Diagnosis
The diagnosis of trypanosomiasis is notoriously difficult. Not only are there no specific clinical signs, but the intermittent and usually low parasitaemias make detection of the trypanosomes difficult. Furthermore, infection is not synonymous with disease: many subclinically affected animals live in delicate balance with potentially pathogenic trypanosomes. An element of clinical judgement, therefore, enters into the diagnosis of trypanosomiasis. Comparisons of the different diagnostic methods have been made by several authors (Cunningham and van Hoeve, 1965; Killick-Kendrick, 1968; Nantulya, 1990) and detailed descriptions of parasitological methods of diagnosis are available (Boyt, 1984).
Important considerations in the diagnosis of trypanosomiasis are that firstly, the number of detectable parasites is not necessarily directly related to the severity of the disease; and, secondly, in trypanosomiasis-enzootic areas the disease is a herd problem. In most tsetseinfected areas clinical signs of trypanosomiasis are well recognized; farmers and veterinary personnel commonly resort to treatment of sick animals and use the response to therapy to provide retrospective diagnosis. In these areas, a history of the presence of tsetse and the use of trypanocidal drugs, when considered with presenting clinical signs are sufficient to make a tentative diagnosis. However, the presence of concurrent disease may mask trypanosomiasis and complicate the clinical picture. Thus, the only way to confirm a diagnosis in clinically affected animals is to demonstrate and identify the parasites in body fluids.
2.2 The Need for Diagnosis
Before considering briefly the diagnostic methods themselves, the need for diagnosis deserves mention. At the rural level, confirmation of diagnosis is often retrospective; but blood samples taken at the time of treatment should be used to monitor the incidence of infection. On larger farms, routine sampling for diagnosis is needed to assist management decisions. At district or provincial level, diagnosis is essential for disease surveillance and for monitoring control programmes. At each level, the appropriate diagnostic method must be used (Table 1 and Annex 1).
2.3 Direct Diagnostic Methods
The body fluid most commonly examined is blood, either capillary blood from the tail tip, or venous blood from the jugular or from the ear. Lymph, aspirated from a punctured superficial lymph node (usually the prescapular gland), provides useful supplementary diagnostic material, and is indispensable in theileriosis-enzootic areas. Whereas in the diagnosis of human sleeping sickness, cerebro-spinal fluid (CSF) is routinely examined for the presence of trypanosomes, this is exceptional in veterinary medicine. Fresh blood is examined microscopically, as a wet preparation, with a x25 or x40 objective lens. Live, motile trypanosomes are seen as they bore their way between blood cells. This method is rapid and is best suited to screening herds in the field although it is the least sensitive method. For routine diagnosis dry thick and thin blood smears are usually made. The smears are stained with Giemsa's stain and examined under an oil immersion lens (x50 or ×100). The dehaemoglobinized thick smear allows approximately 120 times more blood to be scanned than a thin smear but it may be necessary to examine 700 fields to find a single trypanosome (Cunningham and van Hoeve, 1965). The thin smear permits accurate speciation of the parasites, which being fixed, have more distinct morphology than those in the unfixed thick smear. Although the examination of blood smears alone is a relatively insensitive way to detect infection, the method is simple and, of great practical significance, it is also used to diagnose anaplasmosis, babesiosis and theileriosis.
The most sensitive direct method to detect Trypanosoma congolense and T.vivax infections is by examination of wet preparations of the microhaematocrit buffy coat, under phase contrast illumination. For T.brucei, the subinoculation of bovine blood into mice reveals a greater proportion of infections than direct examination of the buffy coat. For practical reasons sub-inoculation of blood into laboratory animals is not used for routine diagnosis. Furthermore, since rodents are refractory to T.vivax, and not all T.congolense and T.brucei infections become established in the new host, even this method has serious limitations. Mixed trypanosomal infections may also remain undetected. In the diagnosis of human trypanosomiasis a miniature anion-exchange technique has been described for field use, and a double centrifugation technique has been recommended for examination of CSF (WHO, 1986). However, these techniques are impractical for routine veterinary purposes. The application of the various tests (Table 2) in veterinary medicine is based upon cost effectiveness and the availability of suitably trained manpower. Although at the village level a veterinary auxiliary may be competent to measure the haematocrit and examine the buffy coat, it is prohibitively expensive to provide the necessary equipment widely for use under such conditions (Annex 1).
2.4 Indirect Diagnostic Methods
Because of the difficulty of detecting trypanosomes in the blood (due largely to the phenomenon of antigenic variation) numerous indirect tests have been applied to the diagnosis of trypanosomiasis. The non-specific diagnostic tests to detect raised protein levels in the serum of animals suspected to have trypanosomal infections, have no role in diagnosis today. Although a range of serological tests has been applied to the diagnosis of animal trypanosomiasis, a major problem arises from the lack of specificity of antigens which have been used. Even when anti-trypanosomal antibodies are detected they do not distinguish between current and past infection, and cross reactions occur between some trypanosome species. The CATT card agglutination test adopted to assist diagnosis of West African human sleeping sickness, relies on the presence of anti-trypanosomal antibody to agglutinate intact, stained, preserved trypanosomes. Whilst, with some modifications, this system has been found useful in the diagnosis of T.evansi infections in water buffalo, the non-specific agglutination caused by dust, and the evaporation in a hot, dry climate render this method unsuitable for field diagnosis of T.evansi infections in camels.
Table 2. Suitability of diagnostic tests at different functional levels
| LEVEL | DIAGNOSTIC METHOD | ||||||
| DIRECT | INDIRECT | ||||||
| Wet blood | Dry blood and lymph | Microhaematocrit buffy coat | Mouse Inoculation | Antibody IFAT | Antibody ELISA | Antigen ELISA* | |
| 1. Village | +++ | + | - | - | - | - | |
| 2. Veterinary centre | ++ | +++ | - | - | - | - | - |
| 3. Commercial farm | +++ | +++ | + | - | - | - | - |
| 4. District | + | +++ | ++ | + | - | - | - |
| 5. Provincial | - | +++ | ++ | + | + | + | - |
| 6. Surveillance | - | +++ | +++ | ++ | ++ | +++ | + |
| 7. Research | (+) | + | +++ | +++ | +++ | +++ | ++ |
+ Suitable method but not likely to be used on grounds of cost or efficiency
++ Suitable method but not the most appropriate
+++ The most suitable test/Resources available at this level to perform the test
(+) Examination of wet films of mouse tail blood
* Suitability in its present form.
Note: 1. The tests most suitable for use at the producer level have the greatest potential for positive impact on production.
2. Surveillance should combine a range of tests to ensure maximum sensitivity.
The most successful serological adjuncts to diagnosis of trypanosomiasis are the indirect fluorescent antibody test (IFAT) and the enzyme linked immunosorbent assay (ELISA). The micro-ELISA compares favourably with the IFAT and has been found to give results which correlate with the local history of trypanocide usage. However, these antibody assays cannot be used practically to confirm a diagnosis of animal trypanosomiasis. Each test system requires expensive equipment (Ultra-violet light source, microplate reader) and at best the result of a single determination is only indicative of exposure to the pathogen. These tests do nevertheless have a role in surveillance of the disease, either before or after a control operation, when a population of animals may be screened. This is more easily achieved with the ELISA which lends itself to automation (Table 3).
2.5 Recent Developments
The recent development of monoclonal antibodies (MoAbs) that distinguish T.brucei, T.congolense and T.vivax (Nantulya, Musoke, Rurangirwa, Saigar and Minja, 1987) opens a new avenue for the diagnosis of African trypanosomiases. When these MoAbs are used in an antigen-trapping ELISA, diagnosis is sensitive and specific enabling many latent infections to be detected. Validation of this promising method is in progress; the test gives some false negative results, apparently due to the low levels of circulating antigens early in infections. This will necessitate the use of other diagnostic tests in conjunction with the antigen-trapping ELISA. Furthermore, each serum sample is tested separately with each of the three MoAbs. Another problem with this system is its vulnerability to slight alterations in the quality of reagents and buffers. Even when these obstacles are overcome and the test is standardized and validated, there remains the problem of the interpretation of results.
Many trypanosome-infected animals appear to be clinically normal, and even if subclinical disease is present, it may not be economical for treatment to be given. The wide application of an antigen detection ELISA, either in its present microplate form or as a more practical “strip test”, may cause more confusion than clarification at certain levels. The greatest use of this system would seem to be in disease surveillance, in much the same way that PARC uses an ELISA to monitor the immune status of cattle to rinderpest.
2.6 Field Diagnosis
For the foreseeable future, field diagnosis will rely upon the use of the least expensive direct parasitological methods. The buffy coat technique will be increasingly used for surveillance and this has the added advantage of providing haematocrit readings - a key criterion in the selection of trypanotolerant animals (Trail, d'Ieteren, Colardelle, Maille, Ordner, Sauveroche and Yangari, in press).
At village level, diagnosis will have to be intensified to improve treatment and disease surveillance. Blood smears should be taken from every animal which receives treatment, in order to justify the use of drugs, for which the farmer will henceforth pay. The repeated sampling needed to confirm a diagnosis of trypanosomiasis in many cases will seldom be practised. At best, thick and thin blood smears will be taken at the time of treatment for subsequent examination.
Table 3. Guide to equipment and materials required for diagnosis of trypanosomiasis
| DIAGNOSTIC METHOD | MAIN ITEMS OF EQUIPMENT | CONSUMABLES | REMARKS | ||
| 1. | Blood and lymph smears Yet/stained | a. | Compound microscope, oil immersion. With mirror for ambient light | Lascets, slides, coverslips, stain, ethanol, filter paper, buffered water, immersion oil, lens tissue, xylene. Record books. | 2000 stained smears per year, Smears delivered by bicycle. |
| 2. | Dark ground/buffy coat | a. | Compound microscope, oil immersion. Phase contrast/bright field light source. x 25/ x 40 phase objective lens | All of the above items. | |
| b. | Microhaematocrit centrifage, reader, diamond pencil | Heparinized capillary tubes, tube sealant, spare rin gaskets racks | Mobile team incurs transport and subsistence costs. 50 specimens per day x 3 days per week x 40 weeks = 6000 specimens p.a. | ||
| c. | Electric power source/Generator | Cold box, freezer packs | |||
| d. | Carrying boxes | ||||
| 3. | Mouse inoculation | a. | Mouse cages, scissors | All of items in 1. above. Mice, feed, syringes, hypodermic needles | |
| 4. | Serology | a. | Cold chain : Cold boxes, freezer packs, refrigerator, freezer | Blood collection bottles, hypodermic needles, | One team collects 100 samples daily × 3 |
| marker pens, record sheets | days per week x 40 weeks = 12 000 p.a. | ||||
| b. | Serum separation : Bench centrifuge | Pipettes, bulbs, serun storage bottles and racks | |||
| c. | Serum testing : IFAT-UY light source for microscope, micropipettes | Pipette tips, glassware, salts, reagents | One technician tests 50 sera per day x 3 days per week x 40 weeks = 6000 p.a. | ||
| ELISA | |||||
| Plate reader, plate shaker. micropipettes | Microtitre plates, pipette tips, buffer salts, reagents, glassware | One technician tests 400 sera per day x 3 days per week x 40 weeks = 48 000 p.a. | |||
Note: 1. Four full-time field teams sampling 48 000 animals per annum could engage one full-time technician on antibody detection ELISA's for disease surveillance.
Antigen detection ELISA requires three tests per serum sample.
2. Cost of each test must reflect costs of sampling team, transport, separation, storage, testing and retesting.
3. Microscopy enables diagnosis of anaplasnosis, babesiosis, covdriosis and theileriosis, which reduces cost of trypanosoniasis diagnosis.
On individual farms, a “diagnosis and treatment” policy would benefit from the use of the buffy coat technique. However, the initial cost of purchasing a centrifuge and microscope, as well as the recurrent cost of employing a skilled microscopist, represents a deterrent to all but the larger farms.
Accurate diagnosis is crucial in the initial surveys to justify and plan control programmes, and to monitor their success or otherwise (Annex 1). The examination of samples from a proportion of animals in a herd or a population may yield positive diagnoses, and the haematocrit profile will be informative. However, the interpretation of results is difficult in areas of low tsetse density when livestock movement is not controlled. Serological surveys then have a useful role.
3. TRYPANOCIDES
3.1 The Present Range
The chemotherapy of animal trypanosomiasis has been comprehensively reviewed during the last two decades (Finelle, 1973; Williamson, 1976; Leach and Roberts, 1981; Tacher, 1982; Holmes and Scott, 1982). Over this period the number of compounds marketed has dwindled as some compounds have been withdrawn because of the emergence of drug resistance. The last trypanocide was developed in 1961 and, despite the importance of the animal trypanosomiases, the chemotherapeutic armoury is pathetically depleted. This worrying state of affairs has developed largely for economic reasons. Animal trypanosomiases mainly affect the livestock in poor countries which renders the development of new trypanocides commercially unattractive. The stringent drug registration regulations necessitate extensive and costly testing of all new chemotherapeutic compounds and the development costs are unlikely to be recovered with acceptable profit margins from markets in poor countries alone. There is no new trypanocide on the horizon, neither is there a vaccine.
The high costs of vector control operations and inadequate methods to protect areas reclaimed from tsetse infestation have lead to widespread dependence on trypanocides, of which only four compounds are now available (Table 4). Diminazene, homidium and isometamidium are used mainly in cattle, goats and sheep. The fourth compound, quinapyramine was withdrawn from the market in 1977 because of the emergence of widespread resistance among trypanosomes in cattle. It was reintroduced in 1985 mainly to treat T.evansi infections in camels and horses. It is also useful in the treatment of African trypanosomiases in horses and pigs. Although suramin - the oldest trypanocide still marketed - is used to treat early cases of human sleeping sickness as well as T.evansi infections in camels, it is ineffective against the other species of trypanosomes. Similarly, the other trypanocides also differ in their activity against the different species of parasite. Diminazene is active against T.congolense and T.vivax at a dosage rate of 3.5 mgkg -1, but must be used at 7.0 mgkg -1 or more to remove T.brucei infections. The phenanthridinium related compounds homidium and isometamidium are active against T.congolense and T.vivax but less so against the brucei group. There are, however, strain differences, and in some cases isometamidium is effective against T.evansi.
Table 4. Generic and trade names of trypanocides for the treatment and prevention of African animal trypanosomiasis
| Compound | Manufacturer | Action | Range of dosage rates mgkg-1 | Route of administration | Remarks | |
| Generic name | Trade name | |||||
| Diminazene aceturate | “Berenil” | Hoechst A.G., Germany | T | 3.5 – 7.0 | I/m s/c | Also babesicidal. Toxic to horses, donkeys, dogs and camels |
| Homidium bromide | “Ethidium” | CAMCO Animal Health, U.K. | T(P) | 1.0 | I/m | |
| Homidium chloride | “Novidium” | Rhone-Merieux, France | T(P) | 1.0 | I/m | |
| Isometamidium | “Samorin” | Rhone-Merleux, France | P/T | 0.25 – 1.0 | I/m,(i/v)*** | Toxic above 2.0mgkg-1 Highly Irritant. Avoid subcutaneous administration |
| “Trypamidium” | Rhone-Poulenc Sante, France | P/T | 0.25 – 1.0 | I/m, (i/v)*** | ||
| Quinapyramine sulphate* | “Trypacide” | Rhone-Merieux, France | T | 3.0 – 5.0 | s/c | Rest animals before and after treatment |
| Quinapyramine prosalt* | “Trypacide” prosalt** | Rhone-Merieux, France | P/T | 3.0 – 5.0 | s/c | Dosage calculated as sulphate |
* Re-introduced in 1985 to treat mainly T.evansi infections
** Prosalt is a mixture of sulphate and chloride salts of quinapyramine
*** Source : Dowler, Schillinger and Connor, 1989
T = Therapeutic action,
P = Prophylactic action
(P) = short prophylactic activity
i/m = intramuscular
i/v = intravenous
s/c = subcutaneous
3.2 Toxicity
Trypanocides have narrow therapeutic indices which further restrict their use since their toxicity varies with the species of animal. For example, diminazene is toxic to equines, camels and dogs, but may be used safely in cattle, goats and sheep. In addition to systemictoxic effects which occur with overdosage, there may be marked local side effects. This is most marked in the case of isometamidium, which is highly irritant, and, if the solution is inadvertently injected subcutaneously, a severe local reaction occurs. Trypanocides are relatively inexpensive drugs. They are supplied in powder form (isometamidium; quinapyramine), granular form (diminazene) or as a tableted preparation (homidium). None is supplied in solution for the African market. Each trypanocide has therefore to be made up into solution (or suspension, in the case of quinapyramine prosalt) with either distilled or boiled, cooled water before use. Severe reactions can occur at the injection site if normal aseptic precautions are not observed in preparing solutions and giving injections.
As a consequence of their toxicity, the limited range of compounds available and the concern over drug resistance, the use of trypanocides, although controlled by legislation in many countries, is often not strictly enforced.
3.3 Pharmacokinetics and Activity
The pharmacokinetics of trypanocides are incompletely understood, but the rate of excretion of the different compounds is known to affect their activity. Diminazene, which is rapidly excreted, is used only for its therapeutic effect, whereas isometamidium is slowly excreted and is the most effective prophylactic compound currently available (Kinabo and Bogan, 1988). Homidium is excreted more slowly than diminazene, but more rapidly than isometamidium and thus has limited prophylactic activity. Whilst slow excretion is advantageous for prophylaxis, it is disadvantageous because of the drug residues produced; the trypanocides have recently been scrutinized by the Joint FAO/WHO Expert Committee on Food Additives (WHO, 1989).
Although diminazene is rapidly excreted it has been shown to have a prophylactic effect, against intravenous challenge with T.congolense, of 12 days (Wellde and Chumo, 1983). Isometamidium, which is widely considered to be the drug of choice in the prevention of bovine trypanosomiasis, is administered by deep intramuscular injection. The usual sequel to the correct injection of isometamidium is that an encapsulated lesion forms within the muscle, from which the drug is slowly released to give prolonged protection from infection by trypanosomes. The duration of protection appears to be largely dose related; the higher the dosage rate, the longer is the period of protection (Peregrine, Ogunyemi, Whitelaw, Holmes, Moloo, Hirumi, Urquhart and Murray, 1988).
Homidium is mainly used for its therapeutic effect, but it does have prophylactic activity for several weeks (Dolan, Oketch, Alushula, Mutugi, Stevenson, Sayer and Njogu, 1989). It too is administered by deep intramuscular injection but is less irritant than isometamidium.
Quinapyramine sulphate is soluble in water and is administered by subcutaneous injection to treat T.evansi and T.brucei infections. The prosalt formulation is a combination of the soluble dimethyl sulphate and the insoluble chloride salt of quinapyramine. After subcutaneous injection of the prosalt suspension, the soluble salt exerts a therapeutic effect, whilst the chloride provides a depot from which the trypanocide is slowly absorbed, providing prophylaxis.
3.4 Attempts to Improve Efficacy
The intravenous administration of isometamidium as a 1% w/v solution has been used successfully to treat trypanosomiasis in cattle (Dowler, Schillinger and Connor, 1989). Care is needed with this technique to avoid acute systemic toxic effects and perivenous leakage of the solution. Whilst this method of administration overcomes the problem of carcase damage arising from repeated intramuscular injections, the period of prophylaxis is likely to be reduced in the absence of a depot of drug at the injection site.
Attempts have been made to overcome the severe local effects of isometamidium injection by complexing isometamidium with the polyanion dextran sulphate (James, 1978; Aliu and Chineme, 1980). The local tolerance and prophylactic activity of isometamidiumdextran complex was assessed in cattle (Schillinger, Cheruyiot, Connor, Karanja, Maloo and Rottcher, unpublished data). Although, compared to isometamidium, the dextran complex produced less severe local reactions in cattle, the period of prophylaxis under natural tsetse challenge was reduced.
Diminazene, homidium and isometamidium have been incorporated into liposomal formulations with a view to prolonging their activity and improving tolerance (Fluck and Hopkins, 1988). No adverse effects were reported after administration of the phospholipid complexes as multilamellar liposomes. The reaction at the intramuscular injection site to liposomal isometamidium was markedly less than the reaction to a solution of isometamidium. Even when liposomal isometamidium was injected subcutaneously at a dosage rate of 2.0 mgkg-1, the local reaction was much less severe than that produced by a solution of isometamidium administered at a dosage rate of 0.25–0.5 mgkg-1. However, available evidence indicates that the liposomal preparations did not substantially enhance the duration of prophylaxis of the three compounds.
The advantage of improved tolerance of liposomal isometamidium by cattle has to be weighed against several disadvantages. Of these, the higher cost of such a formulation, if it were to be marketed, and its stability (or shelf life) are critical considerations. For much the same reasons responsible for the minimal effort to develop new trypanocides, there is little activity to develop formulations to improve the efficacy of existing compounds. The higher cost of any new formulation would have to be offset by significant improvements in the drug's efficacy. At present to such development is forthcoming.
3.5 Use of Sanatives
Trypanocides are used either to treat or to prevent trypanosomiasis. However, their use is also determined to some extent by drug resistance. The use of a sanative drug to eliminate trypanosomal infections is of great importance. Cross-resistance between diminazene and isometamidium, although not common, has been reported. Consequently, the alternate use of these two compounds as a sanative pair is widely practised. A herd sanative treatment with diminazene is often integrated into a prophylactic regime to remove infections which have become established as isometamidium levels decline and no longer provide protection. Caution is needed when using diminazene and isometamidium to avoid cumulative toxic effects. Isometamidium should not be given less than one week after diminazene, and at least one month should lapse after treatment with isometamidium before diminazene is injected. Failure to take these precautions leads to high mortality rates.
3.6 Treatment of Trypanosomiasis
The satisfactory treatment of trypanosomiasis requires more than a correctly administered trypanocidal injection, and the rate of an animal's recovery is largely determined by the plane of nutrition and amount of exercise during convalescence. Wellrested and well-fed animals recover more rapidly after trypanocidal therapy than undernourished animals which have to trek long distances to pasture and water. However, chronic trypanosomiasis often fails to respond to therapy; the ferrokinetic disturbances and accompanying dyshaemopoeisis appear to be irreversible, and affected animals may remain thin and anaemic despite trypanocidal treatment.
It is common practice for many owners to treat their animals with trypanocides on a presumptive diagnosis of trypanosomiasis based solely on recognition of clinical signs. In such cases sub-curative treatment is often given, the effect of the trypanocide being merely sufficient to restore the host-parasite equilibrium and effective premunity. Clinical recovery often follows such treatment but the exposure of trypanosomes to low concentrations of trypanocide increases the risk of drug resistance developing. Nevertheless, this enables livestock to be maintained in trypanosomiasis-enzootic areas at minimal cost, although it is not easy to defend the practice scientifically.
The therapeutic approach to the control of animal trypanosomiasis is mainly practised when the incidence of infection is low. Diagnosis may be confirmed before treatment in some cases although it is more usual to take specimens at the time of treatment for subsequent examination. Diminazene and homidium are the commonly used drugs. An accurate estimation of liveweight is important for curative treatment, however, the pack sizes in which these compounds are presented can encourage underdosing. There is a temptation not to use a second sachet or tablet to treat an animal requiring only slightly more than the dose contained in one sachet or tablet.
The treatment of individual animals to control trypanosomiasis can be very successful but requires a high level of surveillance for which diagnostic equipment and materials are needed (Table 3). Under low tsetse challenge, the cost of diagnosis and treatment may be considerably less than prophylactic treatment of the whole herd (Wissocq, Trail, Wilson and Murray, 1983; Dowler, Schillinger and Connor, 1989). An advantage of this approach to the control of trypanosomiasis is that it permits changes in disease incidence to be monitored. However, when isometamidium is used intravenously to treat individual animals, the residual drug levels in the tissues probably provide some protection, thus obscuring the true challenge. For purposes of monitoring the incidence of infection diminazene is to be preferred.
A significant advantage of diagnosis and treatment is that it promotes drug-assisted acquired immunity, which is manifested as the reduced frequency of infection in older cattle (Boyt, Lovemore, Pilson and Smith, 1963; Wilson, Paris, Luckins and Gray, 1976; Logan, Goodwin, Tembely and Craig, 1984; Trail, Murray, Sones, Jibbo, Durkin and Light, 1985). Whilst the acquisition of immunity facilitates maintenance of livestock in tsetse-infested areas, it can interfere with surveillance of trypanosome transmission. Challenge would be underestimated since infections would not become established in animals resistant to certain serodemes. To overcome this problem sentinel animals have to be periodically replaced with trypanosome-naive stock.
There is considerable variation between individual animals in their susceptibility to trypanosomal infection and some individuals are parasitaemic more frequently than others. Since an animal's ability to control parasitaemia and anaemia has a major effect on weight gain, monitoring infection rates and haematocrits of individuals in a herd is recognized as a useful tool for the selection of trypanotolerant breeding stock (Trail, d'Ieteren, Colardelle, Maille, Ordener, Sauveroche and Yangari, in press). Judicious use of trypanocides is indicated in these circumstances.
3.7 Chemoprophylaxis
The diagnosis and treatment of trypanosomiasis is not a practical way to control the disease when the challenge is high. The incidence of infection under these conditions necessitates such frequent treatment of so many animals that a prophylactic approach to control is needed. All animals at risk should be treated to control trypanosome transmission in an area. To maintain protection repeated treatments are needed, the interval between which is mainly determined by the dosage rate used and the level of challenge. Of the trypanocides now available isometamidium is the only true prophylactic.
There are many reports confirming the efficacy of isometamidium in maintaining livestock (mainly cattle) in tsetse-infested areas, and the advantages of herd protection with isometamidium over a therapeutic approach have been well demonstrated (Wilson, Le Roux, Paris, Davidson and Gray, 1975; Blaser, Jibbo and McIntyre, 1979; Logan, Goodwin, Tembely and Craig, 1984; Takken, Taylor-Lewis and Woodford, 1988). Direct comparisions between trials cannot be made: not only did experimental designs differ but different breeds of animals were challenged by different species of tsetse in different parts of Africa. Irrespective of these differences the common decision had to be made as to when to retreat animals to provide continued protection.
3.8 Duration of Prophylaxis
This is a vexing issue, to which there is no easy answer. The duration of protection afforded by isometamidium has been shown experimentally to be as long as five months (Whitelaw, Bell, Holmes, Moloo, Hirumi, Urquhart and Murray, 1986). However, numerous factors modify the duration of protection (Ogunyemi and Ilemobade, 1989). Experimentally, the dose of isometamidium given has been shown to have a significant effect; higher dosage rates confer longer protection. To obtain prolonged protection the dosage rate of isometamidium cannot be increased greatly because of its toxic effects. Normally this drug is used at between 0.5 mgkg-1 and 1.0 mgkg-1; whilst a dosage rate of 2.0 mgkg-1 is not tolerated by all cattle.
Numerous reports from the field indicate that the period of protection is reduced by high tsetse challenge. Experimental evidence does not confirm this common observation, but it has been shown that the sensitivity of the parasites to isometamidium affects prophylaxis. The manner in which high trypanosomal challenge reduces prophylaxis is obscure, but may be related to the presence of a large number of different serodemes with a range of sensitivities to the trypanocide.
The physiological state of animals also influences the duration of prophylaxis. Animals stressed by poor nutrition, intercurrent disease, lactation or physical exertion receive shorter protection than non-stressed animals. This occurrence may reflect altered pharmacokinetics in physiologically disturbed animals, a condition not found in the animals used for the experiments of Whitelaw and others (1986), or those of Peregrine and his colleagues (1988). Under experimental conditions it has been shown that cattle protected by isometamidium do not develop anti-trypanosomal antibodies (Whitelaw and others, 1986; Peregrine and others, 1988) unless under heavy metacyclic challenge. In the field, the host's immune response may well contribute to apparent prophylaxis, especially if infection becomes established when residual levels of isometamidium confer partial protection.
3.9 Interval Between Prophylactic Treatments
This aspect of prophylaxis is obviously directly related to the duration of prophylaxis. In practice it is not easy to determine the precise timing of repeat herd treatments. The need to maintain protective levels of isometamidium at all times to prevent the establishment of trypanosomal infections, has to be balanced against both the practicality and economics of doing so. Each field situation may require a different approach, and in the several studies of maintaining animals by chemoprophylaxis, the criteria determining retreatment varied.
One of the most unequivocal demonstrations of the maintenance of cattle by chemoprophylaxis under tsetse-challenge was provided by the analysis of the data collected from Mkwaja ranch in Tanzania (Trail and others, 1985). Cattle received an average of 4.4 treatments with isometamidium and 0.6 treatments with diminazene per year, and were impressively productive. The decision on when to repeat treatment was based, not only on parasitological parameters, but incorporated an element of clinical judgement. The criteria for deciding when to repeat isometamidium treatments have to be simplified for most situations. Repeat group treatments may be given when the first animal in a group of 20 regularly monitored cattle becomes parasitaemic. This criterion was used by Wilson and others (1975). However, they concluded that it would have been more economical to delay retreatment until three of the 20 animals were found positive. In other cases repeat treatments were made at arbitrary three monthly intervals. In the absence of regular parasitological or entomological monitoring to determine the probable risk or challenge animals face in tsetse-infested areas, the arbitrary intertreatment interval has been widely used. There is a need to relate prophylaxis more closely to challenge in order to achieve high drug levels at a time when challenge is high.
3.10 Strategic Chemoprophylaxis
Local epidemiology determines the design of strategic chemoprophylaxis. For example, cattle entering a tsetse-infested dry season grazing reserve would benefit from prophylactic treatment before exposure to challenge. Similarly, animals in sedentary herds should receive protection immediately before the anticipated onset of high risk. Attempts have been made to calculate challenge in terms of a Berenil index (Rogers, 1985). For practical purposes it is important to know when to expect an increase in risk so that preventive measures such as chemoprophylaxis can be implemented. The incidence of infection often increases soon after the onset of the rainy season in areas with unimodal rainfall and the efficacy of strategic prophylaxis before the onset of high risk has been demonstrated (Njogu, Dolan, Sayer, Wilson and Alushula, 1985; Connor, Mukangi and Halliwell, 1989). A similar approach in which prophylaxis was provided to village cattle over the high risk period, as three treatments with isometamidium at intervals of three months, produced good control of trypanosomiasis and led to improved productivity (Maloo, Chema, Connor, Durkin, Kimotho, Maehl, Mukendi, Murray, Rarieya and Trail, 1988). A sanative diminazene treatment was included in this regime during the period of lowest trypanosomiasis risk.
3.11 Benefits of Chemoprophylaxis
Most studies of the efficacy of trypanocides in the control of animal trypanosomiasis have concentrated on the parasitological aspects. This is to be expected because parasitological parameters have to be examined to determine the trypanocidal effect of the drugs used. It is more important form the economic standpoint to examine the benefits derived in terms of productivity. Few studies have been made of this important aspect. The reduction in mortality under heavy tsetse challenge by isometamidium was amply demonstrated on Mkwaja ranch in Tanzania by Blaser, Jibbo and McIntyre, (1979). Over a period of 31 months three deaths occurred in a group of 37 cattle which received isometamidium every two to three months. In contrast, none of the 18 untreated cattle survived.
As already mentioned, the use of chemoprophylaxis in Boran cattle at Mkwaja enabled a high level of productivity to be achieved, which compared favourably with that of Boran cattle under trypanosomiasis-free ranching conditions in Kenya (Trail and others, 1985). Chemoprophylaxis in East African zebu cattle under tsetse challenge yielded a positive rate of return in productivity (Itty, Chema, d'Ieteren, Durkin, Leak, Maehl, Maloo, Mukendi, Nagda, Rarieya, Thorpe and Trail, 1988). The results of this study indicated the importance of milk production, both in terms of lactation yield and producer prices.
Of great importance to increased food production is the use of animal traction. The presence of tsetse restricts livestock rearing, but work oxen have been maintained successfully under tsetse-challenge by chemoprophylaxis (Bourn and Scott, 1978). Although resistance to isometamidium emerged, the problem was controlled by the sanative use of diminazene at nine-month intervals, whilst continuing to provide prophylactic cover with isometamidium. It was concluded from this study in Ethiopia that if sound veterinary advice and treatment (= prophylaxis) are available, people settling tsetse-infested land can benefit from draught power.
Responses of village goats in tsetse-infested areas of Tanzania to chemoprophylaxis were marked (Hendy, 1988); parturition intervals were reduced, birth weights were higher, kid survival in the perinatal period was increased and growth rates of kids suckling isometamidium-treated dams were higher than those of the kids of untreated control goats. Similar results have been obtained from a study in progress in Zambia (Connor, 1989). No analysis of the cost effectiveness of chemoprophylaxis was made in the former study, but this aspect was considered in other studies. Proof is available to show a positive economic impact on productivity in goats and sheep. Although in one study only mortality and growth rates of protected and unprotected goats were compared (Griffin and Allonby, 1979), the beneficial effect of prophylaxis on fertility was indicated in the second study (Kanyari, Allonby, Wilson and Munyua, 1983). Several breeds of goats were used in these studies and all received anthelmintic treatment on a monthly basis. Thus, the extrapolation of these results to field conditions is not a simple matter. Furthermore, the benefit demonstrated by the economist may not be appreciated by the farmer, to whom the “cost” component of the cost:benefit ratio is a psychological, and often, a material barrier.
3.12 Tactical Chemoprophylaxis
Chemoprophylactic treatment of a whole herd has been found to enhance all production parameters. However, on a cost recovery basis, few owners will be prepared to adopt this approach to the control of trypanosomiasis, even strategically. There is a need to evaluate the efficacy and acceptability of a tactical approach to the use of trypanocides in selected and economically important categories of animals. The overall objective should be to achieve, on a limited basis, maximum benefit from restricted trypanocide usage.
An obvious category for such an approach is draught animals. Under conditions of light to medium challenge, the protection of all work oxen in the area, just before the onset of the ploughing season, could be expected to achieve a better output from the “work force”. Oxen represent a considerable investment for all farmers, and the threefold improvement in crop yields as a result of using oxen sharpens the farmer's perception of their importance to his household's economy. A sanative diminazene treatment throughout the area would be a wise, if not mandatory, requirement to guard against the rapid emergence of resistant trypanosomes.
Another important category is the breeding cow. Improved calving rates may be achieved by prophylaxis before the breeding season. A study at OGAPROV, Gabon has shown that trypanosomiasis impaired the fertility of lactation-stressed N'Dama cows, particularly those failing to maintain control of anaemia (G.d'Ieteren and G.J.Rowlands, personal communication). Trypanocidal treatment with diminazene, followed two weeks later by isometamidium, prior to the breeding season, improved the average calving rate of cows lactating at the time of breeding. It must be noted that this improvement, confined to lactating cows, may well have been associated with the level of stress to which they were exposed in the particular environment of the station at the time.
The concept of a breeding season is not appreciated by smallholder farmers generally. There have been numerous attempts to upgrade stock by introduction of so-called improved breeds of cattle and goats throughout Africa, sometimes in tsetse-infested areas. Such schemes demand changes to management practices to reduce the risk that susceptible livestock will succumb to disease or environmental stress. Little attempt has been made to modify selected management practices related to indigenous stock on a broad basis, and this ought to be addressed.
Results from a current trial of the influence of chemoprophylaxis on goat productivity under tsetse challenge in Zambia are encouraging (Bealby and Connor, unpublished data). Chemoprophylaxis in female goats of the local breed greatly improved fertility compared with controls. Early results indicate that pre-breeding prophylaxis improves fecundity, resulting in a higher twinning rate. Prophylaxis given only during pregnancy significantly improves birth weight: bigger, stronger kids are born. No anthelmintics are used in these goats which are housed on a raised floor and are herded for browsing. Recommendations to local farmers on the basis of these findings have yet to be made, but need to be clear, simple and acceptable.
Implementation of tactical prophylaxis must be integrated with modifications to management to ensure that the maximum benefit is obtained, and is seen to be obtained. Treatment of oxen without ensuring adequate nutrition may fail to produce the expected result. The treatment of pre-breeding cattle or goats, without a controlled breeding season, ideally using selected males, would not be practical. In the case of pre-breeding treatment of goats high twinning rates could be expected, but under traditional management high neonatal mortality would erase much of the benefit. Trypanocidal control of trypanosomiasis must not be seen in isolation, and requires close monitoring.
4. DRUG RESISTANCE
4.1 Failure of Trypanocides
The use of trypanocides does not always produce the expected cure or protection and there is then a tendency to assume that drug resistance has arisen. Whilst this may be true, there are many other reasons which contribute to their failure (Table 5). Only after carefully investigating the practical points of drug administration and eliminating them as causes of failure, is it valid to investigate the likelihood of there being true drug resistance.
Before trypanocides can be said to have failed there must be parasitological evidence that trypanocidal treatment either has not removed an established infection or has not provided protection against infection or reinfection. Thus, parasitological monitoring is a prerequisite to drug usage. From an analysis of properly kept records of routine monitoring it is possible to determine whether administration of a therapeutic dose of a trypanocide cures an infected animal. There may be complete cure, temporary aparasitaemia followed by a relapse of parasitaemia or no abatement in parasitaemia. A recent report from Ethiopia (Rowlands, Mulatu, Authie, d'Ieteren, Leak, Peregrine and Trail, 1990) has demonstrated the value of a field appraisal to determine the efficacy of trypanocidal drugs in an area where trypanocide failure occurred.
4.2 True Drug Resistance
Resistance to trypanocides is a well known phenomenon (Williamson, 1979; Leach and Roberts, 1981). The introduction of every new trypanocide has been followed by the emergence of resistance to it, and in the cases of dimidium, quinapyramine and prothidium has led to their withdrawal from the market. Resistance seems to develop in a stepwise manner; trypanosomes resistant to a low dose of a trypanocide can be removed by a higher dose of the same compound. The problem is that, because of the narrow therapeutic indices of the trypanocides, there is only limited scope to overcome resistance by increasing the dosage. An effective trypanocidal dose may also kill the animal!
Table 5.
Causes of apparent drug resistance in trypanosomes : failure of trypanocides
| 1. Underdosage | : | underestimated bodyweight |
| : | overdiluted solution of trypanocide | |
| : | Incorrectly calculated dose volume | |
| : | dellberate underdosage to enable treatment of more animals | |
| : | incorrect injection technique | |
| - short needles | ||
| - non-sterility/abscessation | ||
| - early withdrawal of needle | ||
| - elastic recoll of tissue produces excessive “leak-back” | ||
| 2. Incorrect strategy | : | irregular treatment |
| : | prolonged intervals between treatments (challenge when drug levels have waned) | |
| : | lack of knowledge of seasonality | |
| 3. Stress-induced metabolic changes | : | malnutrition |
| : | lactation | |
| : | intercurrent disease | |
| : | breed-associated stress | |
| : | work and trekking | |
| 4. Increase in challenge | : | seasonal |
| : | changed grazing area | |
| : | absolute increase in tsetse numbers | |
| 5. Relapsing infections | ||
| 6. True drug resistance | ||
The mechanisms of resistance are unclear and probably involve gene amplification and spontaneous shifts in sensitivity. Resistance may appear in an area but may also spontaneously disappear (Whiteside, 1960), which may be linked to unstable genetic components or poor tsetse transmissibility of some resistant strains. On the other hand, transmission of drug resistant strains of trypanosomes in sylvatic cycles is known to occur. Reports of resistance to the four remaining trypanocides are increasingly common, although few reports have been substantiated.
The difficulty in confirming resistance is a result of the numerous factors which may lead to the failure of trypanocides (Table 5), and of the difficulty in obtaining reliable field data. Sometimes the intervals between repeat treatments can be so short that signs of trypanocide overdosage occur before an assessment of drug resistance is made!
4.3 Assessment of Resistance to Trypanocides
An ideal method to assess drug resistance would be accurate, give reproducible results, and should be simple, rapid and inexpensive. None of the available methods meets these criteria. All methods require the isolation of suspect trypanosomes and subsequent testing (Fig 1).
The curative effect of a trypanocide is exerted on bloodstream trypanosomes in the presence of the host's immune apparatus. Prophylaxis is achieved by the action of a trypanocide against metacyclic trypanosomes at the level of the skin (Peregrine and others, 1988). These conditions can only be reproduced in in vivo, and for the determination of prophylaxis animals should be challenged by the bite of infective tsetse. Precise simulation of field conditions is never possible. A single animal may be infected with more than one trypanosome species or serodeme, and animals frequently have some acquired immunity.
Selection of trypanosomes is also inevitable. In a mixed infection one species or serodeme may become more readily established in the new host, especially if a different host species is used; for example, T.vivax and T.simiae are not infective to mice. Furthermore, not all serodemes are transmitted with the same ease by tsetse. The disadvantage of using cattle to assess the sensitivity of trypanosomes to trypanocides is largely related to cost. The purchase and maintenance of a large number of cattle for observation periods of 100 days or longer in fly-proof housing is beyond the resources of most laboratories. Consequently, other laboratory animals are used, and assessment of drug resistance of trypanosomes in mice is widely used. However, the results of tests with drugs administered by the intraperitoneal route to cure syringe-passaged trypanosomal infections in mice, cannot be extrapolated directly to the situation in cattle. Another major difference is that of pharmacokinetics. A dosage rate of 0.5mgkg-1 of isometamidium in a bovine is not the same as 0.5 mgkg-1 in a mouse, which has a much higher metabolic rate. At best, the results obtained from tests in mice can only broadly indicate the sensitivity of a strain or stock of trypanosomes to a drug (Sones, Njogu and Holmes, 1989). Despite the advantage of the lower cost of assessing sensitivity to trypanocides in mice, an observation period of between 30 and 60 days is needed. As a result of these factors, more suitable methods are needed to assess drug resistance and are being sought. The in vitro assay has obvious potential in this respect.
Fig 1
ISOLATION OF TRYPANOSOMES FROM CATTLE TO ASSESS DRUG RESISTANCE
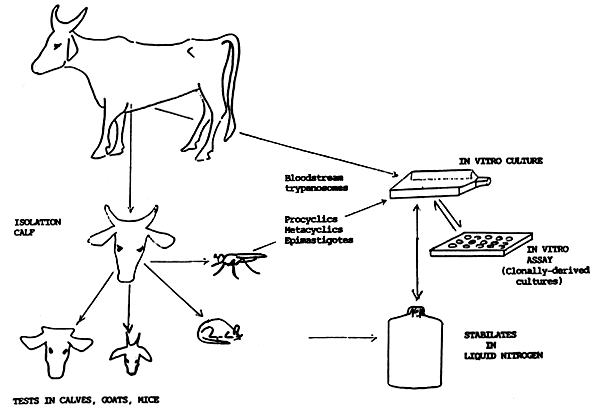
4.4 In Vitro Assay of Resistance
The potential advantages of in vitro techniques to assess drug resistance of trypanosomes relate to savings in time and money compared with in vivo tests. There are, however, significant obstacles still to be overcome before in vitro assays can replace in vivo tests (Kaminsky, 1990). The first difficulty is to isolate trypanosomes representative of the population of parasites in infected animals. Mixed infections commonly occur and parasitaemias are often low. The sub-inoculation of other livestock or laboratory animals with blood from an infected animal does not always result in the establishment of an infection. Similarly, it is not always possible to culture trypanosomes from blood. There is therefore inevitably selection for those trypanosomes which adapt more readily to the culture system, with the faster growing trypanosomes being more abundant.
No universally standardized culture system is in use. Some systems utilize a feeder layer of, for example, bovine aorta endothelial cells, others have no feeder layer but incorporate serum or serum components. The variation between batches of sera can considerably affect the growth of trypanosomes in otherwise identical culture conditions. Furthermore, trypanosomes may be cultured as epimastigotes or bloodstream trypanosomes, depending on culture conditions; promastigotes and metacyclics may also be cultured and by varying the conditions, transformation through all stages of the trypanosome life cycle can be induced (Brun and Jenni, 1987; Gray, Hirumi and Gardiner, 1987).
Although it is desirable to reduce rigorous selection in the establishment of a culture, the trypanosomes must have stabilized for some types of assays to proceed. That is, there must not be a high death rate compared with trypanosome growth and multiplication. It can take from four to eight weeks to establish an isolate in culture; even then this may not be achieved. Although the adaptation to culture of three stocks of T.congolense did not change their sensitivity (Brown, Ross, Holmes, Luckins and Taylor, 1987), there are indications that the expression of resistance by trypanosomes is not an entirely stable characteristic (Peregrine, Knowles, Fasogbon, Scott and Moloo, in press). It is possible that prolonged culture may be associated with alterations in the level of sensitivity expressed to a particular trypanocide.
4.5 In Vitro Techniques
The medium requires changing at regular intervals of approximately 48 hours. The exposure of trypanosomes to the test compound should be for a time short enough to produce a measurable effect, yet not long enough to select for the more resistant organisms. Solutions of trypanocides are added to culture medium to give final concentrations in the range of 0.01 ngml-1 to 100 ngml-1. Exposure should be in the absence of a feeder layer: these cells may take up the trypanocide. Additionally, an effect of the trypanocide on the cells of the feeder layer may indirectly affect the trypanosomes.
After trypanosomes have been incubated with the trypanocide, the assay is completed by measurement of an end-point, of which several have been used:
The results of these tests will indicate that, compared with laboratory reference stocks, the test trypanosomes are more, or less, sensitive to a particular concentration of a trypanocide. To interpret these results in the light of clinical findings is not straightforward. It is important to be able to state that a trypanocidal effect exerted in vitro at a concentration of, say 1.5 ngml-1 is equivalent to a curative dose of, say, 3.5 mgkg-1 bodyweight. Too few data are currently available for this to be done.
The in vitro technique will be much improved when bloodstream trypanosomes can be adapted rapidly to culture, and exposed to trypanocides (and possibly their metabolites) for a minimum time to give an easily measurable end-point. The result obtained may then be correlated with results of curative treatment. In the case of prophylaxis, metacyclics should be used for the in vitro assay.
It is unlikely that a single assay system will be suitable for all stages of all species of trypanosomes. Studies are therefore needed to compare results from the different systems. The development of in vitro assays to determine drug resistance of trypanosomes is still at an early stage. In view of the potential of in vitro techniques for screening for new trypanocidal compounds, marked improvements in culturing trypanosomes are likely in the near future.
4.6 Assessment of Drug Resistance in the Field
Initially, the sensitivity of trypanosomes to a drug is appraised clinically: the response of trypanosomal infections to treatment, or the prevention of infection is determined. The time taken for infection to relapse, or the duration of protection, are taken as broad indicators of the degree of resistance. Without placing animals in fly-proof accommodation there is the risk of continued challenge, which raises the problem of distinguishing relapse from reinfection.
Isolation of trypanosomes for further testing inevitably requires some degree of selection, and the results obtained have then to be related to the field situation. The assessment of drug resistance seeks to determine the level of trypanocide lethal to trypanosomes, which should be related to a plasma concentration in a treated animal. The closest approximation to these conditions might be achieved by simultaneously demonstrating trypanosomes in an animal and measuring the concentration of the trypanocide, or its metabolites, in the plasma of that animal. A rapid result would then be obtained at low cost which would be easily interpreted, once normal ranges were established. Measurement of trypanocides is not easy because they are active at extremely low levels. High performance liquid chromatography (HPLC) detects isometamidium at levels of 2 ng per gram of tissue (Kratzer, Turkson, Karanja and Ondiek, in press) whilst a competition micro-ELISA to detect plasma levels of isometamidium has a limit of detection of 10 pgml-1 (Whitelaw, Gault, Sutherland, Holmes, Rowel, Phillips and Urquhart, in press). Refinements to sample preparation and pre-analysis extraction improve the level of detection of this ELISA to the femtogram (10-15g) range.
The further development of drug detection ELISA's could offer a simple tool for direct assays of drug resistance. Improvements to the in vitro techniques would not render them as suitable for field use, but would enable baseline data to be collected on the ranges of sensitivity of characterized trypanosomes to drugs. In assessing drug resistance there are strong arguments for working at field level (Table 6).
Table 6. Comparison of suitability of different methods to assess drug resistance in trypanosomes
| CRITERIA | METHODS | |||||
| Field* test | Laboratory test | |||||
| Cattle | Sheep/goat | Mice | “In vitro” | |||
| 1. | Parasite Tb | 5 | 4 | 3 | 2 | 1 |
| adaptation Tc | 5 | 4 | 3 | 2 | 1 | |
Tv | 5 | 4 | 3 | 1 | 2 | |
| 2. | Lowest selectivity | 5 | 4 | 3 | 2 | 1 |
| 3. | Interpretation of result | 5 | 4 | 3 | 2 | 1 |
| 4. | Cost | 3 | 1 | 2 | 5 | 4 |
| 5. | Time to obtain result | 5 | 1 | 2 | 3 | 4 |
| 6. | Minimum imported material | 5 | 3.5 | 3.5 | 2 | 1 |
| 7. | Need for specialist personnel | 5 | 3.5 | 3.5 | 2 | 1 |
| TOTAL RANKING** | 43 | 29 | 26 | 21 | 16 | |
* Requires regular monitoring and distinction between reinfection and relapse in cattle
** Ranking 5 = most suitable, 1 = least suitable
Ranking is not weighted to reflect relative importance and costs of the different criteria.
4.7 Epidemiology of Resistance to Trypanocides
Little is known of the prevalence and incidence of acquired resistance to trypanocides. The appearance of resistance to all trypanocides has been documented, and in view of their severely restricted range, a better understanding of the epidemiology of resistance is needed.
The natural resistance of T.simiae to most trypanocides presents special problems, but it is acquired resistance to trypanocides by other species of trypanosomes which has received greatest attention. The expression of resistance is not entirely stable but the underlying mechanisms are not yet understood. Genetic recombination in the tsetse may contribute to the emergence of resistance, although the dilution of resistant trypanosomes by other populations of trypanosomes in transmission cycles may influence the extent of the problem. Methods to detect and quantify resistance quickly and economically are needed to enable measures to be taken to control the growing menace.
4.8 Control of Drug Resistance
At present, diminazene and isometamidium are used widely as a sanative pair to slow down the development of drug resistance. Nevertheless, there are reports of confirmed multiple resistance in T.congolense and T.vivax to both of these compounds. The efficacy of the sanative pair is also reduced once the resistant trypanosomes enter the tsetse population and become cyclically transmitted. Treatment of, say, an isometamidium-resistant infection with diminazene would soon be followed by repeated challenge with resistant trypanosomes.
The removal of livestock from an area in which resistance is a clinical problem has been used to reduce transmission whilst the resistant trypanosomes die out. However, this does not always happen, and even after two years, the reintroduction of livestock may be followed by the re-emergence of resistance (Gray and Roberts, 1971). Destocking policies are only likely to be used today as a desperate last resort, and other methods are needed.
It is most important to reduce transmission of resistant trypanosomes and, therefore, in the event of resistance emerging as an area problem, vector control is essential. The recent failure of isometamidium on Mkwaja ranch in Tanzania - evidenced by decreasing prophylaxis - has been overcome, as a clinical problem, by vector control (P.Schachenmann, personal communication). Trypanocide use on the ranch has decreased and productivity has improved following a reduction in tsetse challenge. Tsetse control has been achieved by deltamethrin treatment of cattle and limited deployment of odour-baited, insecticide-treated targets, but there is constant reinvasion pressure. Consequently, the sustainability of vector control in this situation is in doubt. In other circumstances, a rapid reduction in tsetse numbers may be sufficient to reduce the problem of resistance to manageable levels.
An important measure to limit the spread of resistant trypanosomes is to restrict livestock movement. The arrival of an animal infected with resistant trypanosomes in a tsetse-infested area may represent a serious risk to other livestock already there and is potentially an expensive problem for livestock owners.
5. CONCLUSIONS
Africa faces the major problem of how to feed its people. The solution to the problem is not simple but undoubtedly one component is the alleviation of the constraint that trypanosomiasis places on agriculture. The question was posed by Ikede (1986) “Is current emphasis misplaced?” in the context of trypanosomiasis and livestock production in Africa. He concluded that the existing knowledge needs to be applied to control trypanosomiasis and improve livestock production. Searching for new diagnostic methods and new trypanocides will not solve the problems faced by farmers today.
Trypanosomiasis control must be integrated with improved management to have maximum impact. Several control methods exist but they all need to be monitored to determine their efficacy and to enable necessary modification. Increased emphasis on diagnosis at the field level and provision of equipment and reagents for the most costeffective method - likely in almost all instances to be examination of blood smears (Annex 1) - would enable improved diagnosis of a range of important diseases. The use of the more sensitive buffy coat method will be limited to larger farms and surveillance teams. Similarly, the overhead costs of transport and refrigeration, together with the greater technical complexity of serological tests, will restrict these diagnostic methods to surveillance of trypanosomiasis by specialist teams, mainly in support of large control operations (Annex 1).
The risk in such an approach is that without regular, close supervision of field staff, the quality of diagnosis and recording will suffer. A comparable need for supervision applies to the use of trypanocides. Staff must be trained in diagnosis, in the estimation of liveweight, the administration of trypanocides and in accurate reporting. The tactical control of trypanosomiasis requires clear, concise directives which should be determined by priorities for production, which will vary from area to area. The success of tactical chemoprophylaxis will depend on well-focused staff training programmes and intensive farmer education in advance of implementation. Protection of work oxen needs to be linked to better nutrition; pre-breeding treatments depend upon acceptance of the benefits of a breeding season. That, in turn, depends on other factors such as the natural breeding season, conflict with other activities during the calving or kidding season and on seasonal nutritional status. Pilot studies would be required in some cases, but sufficient knowledge exists for a start to be made. Modifications would be introduced in the light of experience gained.
The importance of involving the livestock owners cannot be overemphasized. These are the producers. They have to be convinced of the importance of diagnosis and the importance of correct dosage of their animals with trypanocides - for which they will pay. The farmers' perceptions and expectations have to be respected, and can be put to good use. The value of a trypanocidal treatment costing US$3.20 to protect a pair of work oxen is not difficult to explain to a farmer. Other arguments require greater effort before the farmer is convinced. To merely provide the drugs without having distinct objectives in mind related to improvements in productivity, will not solve the pressing problem of how to improve food production. Trypanosomiasis control is more than a strictly veterinary matter.
However, if trypanocide usage is promoted and diagnosis strengthened at field level, provisions must be made to recognize drug resistance sooner rather than later, to quantify the problem and control it. Identification of resistance is a field activity. Its assessment may be assisted by laboratory studies, but it is ultimately a field responsibility and its control is a matter for field personnel. They will implement departmental directives.
The allocation of resources for the diagnosis, treatment and prevention of trypanosomiasis must be targeted at the field level. Clear departmental policies are needed to focus efforts on the categories of livestock which are most important to their owners. A great deal of groundwork has to be done to convince farmers of the need to pay for trypanocidal treatment or prophylaxis and to modify their management. A suitable basic recommendation is more likely to be accepted and assimilated by farmers than a complex, comprehensive scheme. Adoption of a simple improvement on a large scale will achieve much more than a comprehensive programme with limited appeal.
Acknowledgements
The preparation of this report has been greatly facilitated by my discussions with numerous friends and colleagues. I warmly acknowledge the advice, comments and information given to me by Drs. G. D. M. d'Ieteren and G. J. Rowlands of ILCA, Nairobi, Drs. M.A. Gray, R.Kaminsky, V. M. Nantulya and A. S. Peregrine of ILRAD, and Dr. K. R. Sones of RMB Animal Health Ltd.
I am indebted to Mr. Desmond Lovemore, Regional Coordinator of the EC-funded Regional Tsetse and Trypanosomiasis Control Programme for Malawi, Mozambique, Zambia and Zimbabwe for his encouragement, and to Mrs. M.C. Lovemore who typed this report so efficiently.
REFERENCES
Aliu, Y.O. and Chineme, C.N. (1980). Isometamidium-dextran complex: toxicity and activity against Trypanosoma vivax and Trypanosoma congolense in rats and mice. Toxicology and Applied Pharmacology 53: 196-203.
Blaser, E., Jibbo, J.M.C. and McIntyre, W.I.M. (1979). A field trial of the protective effect of “Samorin” and “Berenil” in zebu cattle under ranching conditions in Tanzania. In International Scientific Council for Trypanosomiasis Research and Control. 15th Meeting, Banjul, The Gambia 1977. OAU/STRC No. 110: 383-386.
Bourn, D. and Scott, M. (1978). The successful use of work oxen in agricultural development of tsetse infested land in Ethiopia. Tropical Animal Health and Production 10: 191-203.
Boyt, W.P. (1984). A field guide for diagnosis, treatment and prevention of African animal trypanosomiasis. FAO, Rome.
Boyt, W.P., Lovemore, D.F., Pilson, R.D. and Smith, I.D. (1963). A preliminary report on the maintenance of cattle by various drugs in a mixed G.morsitans and G.pallidipes fly belt. In Proceedings of the ninth meeting of the International Scientific Council for Trypanosomiasis Research. Conakry, 1962. Commission for Technical Cooperation in Africa. Hertford. Stephen Austin and Sons Ltd. 71-79.
Brown, H.C., Ross, C.A.,Holmes, P.H., Luckins, A.G. and Taylor, A.M.(1987). Adaptation of Trypanosoma congolense stocks to in vitro culture does not change their sensitivity to isometamidium. Acta Tropica 44: 373-374.
Brun, R. and Jenni, L. (1987). Salivarian trypanosomes: Bloodstream forms (trypomastigotes). In “In vitro methods for parasite cultivation”. Eds. A.E.R. Taylor and J.R Baker. London: Academic Press. 94-117.
Connor, R.J. (1989). Final Report of the Regional Trypanosomiasis Expert. Regional Tsetse and Trypanosomiasis Control Programme, Malawi, Mozambique, Zambia and Zimbabwe. December 1989. FGU-Kronberg Consulting and Engineering GmbH. Konigstein, West Germany.
Connor, R.J., Mukangi, D.J.A. and Halliwell, R.W. (1989). Bovine trypanosomiasis in southern Tanzania : investigation into the incidence of infection and duration of chemoprophylaxis. Tropical Animal Health and Production 21 : 135-140.
Cunningham, M.P. and van Hoeve, K. (1965). Diagnosis of trypanosomiasis in cattle. In International Scientific Committee for Trypanosomiasis Research Proceedings of the Tenth Meeting. Kampala 1964. Stephen Austin & Sons Ltd., Hertford. England. 51-53.
Dolan, R.B., Okech, G., Alushula, H., Mutugi, M., Stevenson, P., Sayer, P.D. and Njogu, A.R. (1990). Homidium bromide as a chemoprophylactic for cattle trypanosomiasis in Kenya. Acta Tropica 47 (3): 137-144.
Dowler, M.E., Schillinger, D. and Connor, R.J. (1989). Notes on the routine intravenous use of isometamidium in the control of bovine trypanosomiasis on the Kenya Coast. Tropical Animal Health and Production 21 : 4-10.
Finelle, P. (1973). Chimiotherapie et chimioprevention de la trypanosomiase animale. Acquisitions recentes et situation actuelle. Les Cahiers de Medecine Veterinaire 42 : 215-226.
Fluck, D.J. and Hopkins, J.S. (1988) Chemotherapy and chemoprophylaxis of bovine trypanosomiasis with liposomal trypanocides. In International Scientific Council for Trypanosomiasis Research and Control, Proceedings of the Nineteenth Meeting, Lome, 1987. Publication No. 114 OAU/STRC, Nairobi. : 302-313.
Gray, A.R. and Roberts, C.J.(1971). The stability of resistance to diminazene aceturate and quinapyramine in a strain of Trypanosoma vivax during cyclical transmission through antelope. Parasitology 63 : 163-168.
Gray, M.A., Hirumi, H. and Gardiner, P.R. (1987). Salivarian trypanosomes : insect forms In “In vitro methods for parasite cultivation.” Eds. A.E.R. Taylor and J.R. Baker. London: Academic Press. 118-152.
Griffin, L. and Allonby, E.W. (1979). The economic effects of trypanosomiasis in sheep and goats at a range research station in Kenya. Tropical Animal Health and Production 11 : 127-132.
Hendy, C.R.C. (1988). The effects of trypanosomiasis prophylaxis and anthelmintic treatment in goats under traditional management in southern Tanzania. In Livestock Production in Tsetse affected areas of Africa, Proceedings of a meeting held 23–27 November 1987, Nairobi, Kenya ILCA/ILRAD. Nairobi. 289–309.
Holmes, P.H. and Scott, J.M. (1982). Chemotherapy against animal trypanosomiasis. In Perspectives in Trypanosomiasis Research, Ed. J.R. Baker. Proceedings of the Twenty First Trypanosomiasis Seminar. London, 24 September 1981. Letchworth, Research Studies Press. 59–69.
Ikede, B.O. (1986). Trypanosomiasis and livestock production in Africa : is current emphasis misplaced? Tropical Veterinarian 4 : 1–4.
Itty, P., Chema, S., d'Ieteren, G.D.M., Durkin, J., Leak, S.G.A., Maehl, J.H.H., Maloo, S.H., Mukendi, F., Nagda, S.M., Rarieya, J.M., Thorpe, W. and Trail, J.C.M. (1988). Economic aspects of cattle production and of chemoprophylaxis for control of trypanosomiasis
in village East African Zebu cattle in Kenya. In Livestock Production in Tsetse affected areas of Africa. Proceedings of a meeting held in Nairobi, 1987. ILCA/ILRAD, Nairobi. 360–376.
James, D.M. (1978). Prophylactic activity in rodents of trypanocides complexed with dextran. Transactions of the Royal Society of Tropical Medicine and Hygiene 72 : 471–476.
Kaminsky, R. (1990). “In Vitro” techniques for assessment of drug resistance in trypanosomes. AgBiotech News and Information 2 (2) : 205–210.
Kanyari, P.W.N., Allonby, E.W., Wilson, A.J. and Munyua, W.K. (1983). Some economic effects of trypanosomiasis in goats. Tropical Animal Health and Production 15 : 153–160.
Killick-Kendrick, R. (1968). The diagnosis of trypanosomiasis in livestock : a review of current techniques. The Veterinary Bulletin 38 (4) : 191–197.
Kinabo, D.B. and Bogan, J.A. (1988). The pharmacology of isometamidium. Journal of Veterinary Pharmacology and Therapeutics 11 : 233–245.
Kratzer, R.D., Turkson, P.K., Karanja, W.M. and Ondiek, F.O. (In Press). Studies in cattle on the pharmacokinetics, residues and bioavailability of the anti-trypanosomal drug isometamidium. A paper presented to the 20th Meeting of the International Scientific Council for Trypanosomiasis Research and Control, Mombasa, 1989.
Leach, T.M. and Roberts, C.J. (1981). Present status of chemotherapy and chemoprophylaxis of animal trypanosomiasis in the Eastern Hemisphere. Pharmacology and Therapeutics 13:91– 147.
Logan, L.L., Goodwin, J.T., Tembely, S. and Craig, T.M. (1984). Maintaining zebu Maure cattle in a tsetse infested area of Mali. Tropical Animal Health and Production 16 : 1–12.
Maloo, S.H., Chema, S., Connor, R., Durkin, J., Kimotho, P., Maehl, J.H.H., Mukendi, F., Murray, M., Rarieya, J.M. and Trail, J.C.M. (1988). The use of chemoprophylaxis in East African Zebu village cattle exposed to trypanosomiasis in Muhaka, Kenya. In Livestock Production in Tsetse affected areas of Africa. Proceedings of a meeting of the African Trypanotolerant Livestock Network, held in Nairobi, Kenya from 23 – 27 November 1987. English Press, Nairobi, Kenya. 283–288.
Nantulya, V.M. (1990). Trypanosomiasis in domestic animals : the problems of diagnosis. Revue Scientifique et Technique. Office international des Epizooties 9 (2) : 357–367
Nantulya, V.M., Musoke, A.J., Rurangirwa, F.R., Saigar, N. and Minja, S.H. (1987). Monoclonal antibodies that distinguish Trypanosoma congolense, T.vivax and T.brucei. Parasite Immunology 9 : 421–431.
Njogu, A.R., Dolan, R.B., Sayer, P.D., Wilson, A.J. and Alushula, H. (1985). Strategic chemoprophylaxis for the control of bovine trypanosomiasis. In International Scientific Council for Trypanosomiasis Research and Control. Proceedings of the Eighteenth Meeting, Harare, 1985. Publication No. 113 OAU/STRC, Nairobi. 199–204.
Ogunyemi, O. and Ilemobade, A.A. (1989). Prophylaxis of African animal trypanosomiases: A review of some factors that may influence the duration of isometamidium chloride prophylaxis. Veterinary Bulletin 59 (1) : 1–4.
Peregrine, A.S., Knowles, G., Fasogbon, A.I., Scott, J. and Moloo, S.K. (In press). Trypanosoma congolense : clonal variation in expression of resistance to isometamidium and diminazene. A paper presented to the 20th Meeting of the International Scientific Research Council for Trypanosomiasis Research and Control, Mombasa, Kenya 1989.
Peregrine, A.S., Ogunyemi, O., Whitelaw, D.D., Holmes, P.H., Moloo, S.K., Hirumi, H., Urquhart, G.M. and Murray, M. (1988). Factors influencing the duration of isometamidium chloride (Samorin) prophylaxis against experimental challenge with metacyclic forms of Trypanosoma congolense. Veterinary Parasitology 28 : 53–64.
Rogers, D.J. (1985). Trypanosomiasis “risk” or “challenge” : a review. Acta Tropica 42 : 5– 23.
Rowlands, G.J., Mulatu, W.A., Authie, E., d'Ieteren, G.D.M., Leak, S.G.A., Peregrine, A.S. and Trail, J.C.M. (1990). Prevalence of Trypanosoma congolense in East African zebu cattle under high tsetse challenge. Proceedings of the Society for Veterinary Epidemiology and Preventive Medicine. Belfast 1990. 145–152.
Sones, K.R., Njogu, A.R. and Holmes, P.H. (1988). Assessment of sensitivity of Trypanosoma congolense to isometamidium : a comparison of tests using cattle and mice. Acta Tropica 45: 153–164.
Tacher, G. (1982). The use of drugs in the development of livestock production in tsetseinfested areas. World Animal Review 44 : 30–35.
Takken, W., Taylor-Lewis, E.G. and Woodford, M.H. (1988). Field studies on animal trypanosomiasis in Mozambique. Effectiveness of the prophylactic drugs isometamidium chloride and pyrithidium bromide. Tropical Animal Health and Production 20 (4) : 243–255.
Trail, J.C.M., d'Ieteren, G.D.M., Colardelle, C., Maille, J.C., Ordner, G., Sauveroche, B. and Yangari, G. (In press). Evaluation of a field test for trypanotolerance in young N'Dama cattle.
Trail, J.C.M., Murray, M., Sones, K., Jibbo, J.M.C., Durkin, J. and Light, D. (1985). Boran cattle maintained by chemoprophylaxis under trypanosomiasis risk. Journal of Agricultural Science, Cambridge 105 : 147–166.
Wellde, B.T. and Chumo, D.A. (1983). Persistence of Berenil in cattle. Tropical Animal Health and Production 15 : 149–150.
Whitelaw, D.D., Bell, I.R., Holmes, P.H., Moloo, S.K., Hirumi, H., Urquhart, G.M. and Murray, M. (1986). Isometamidium chloride prophylaxis against Trypanosoma congolense challenge and the development of immune responses in Boran cattle. Veterinary Record 118 : 722–726.
Whitelaw, D.D., Gault, E.A., Sutherland, I.A., Holmes, P.H., Rowel, F.J., Philips, A. and Urquhart, G.M. (In press). Measurement of isometamidium in cattle plasma by ELISA. A paper presented to the 20th Meeting of the International Scientific Council for Trypanosomiasis Research and Control. Mombasa, 1989.
Whiteside, E.F. (1960). Recent work in Kenya on the control of drug-resistant cattle trypanosomiasis. In International Scientific Council for Trypanosomiasis Research. 8th Meeting Jos. CCTA Publication No. 62. 141–154.
Williamson, J. (1976). Chemotherapy of African trypanosomiasis. Tropical Diseases Bulletin 73 : 531–542.
Williamson, J. (1979). Chemoresistance in trypanosomes. In Report of the Expert Consultation on Research on Trypanosomiasis, FAO, Rome, 1 – 5 October 1979. Appendix XI, 84–88.
Wilson, A.J., Le Roux, J.G., Paris, J., Davidson, C.R. and Gray, A.R. (1975). Observations on a herd of beef cattle maintained in a tsetse area. I. Assessment of chemotherapy as a method for the control of trypanosomiasis. Tropical Animal Health and Production 7 : 187–199.
Wilson, A.J., Paris, J., Luckins, A.G., Dar, F.K. and Gray, A.R. (1976). Observations on a herd of beef cattle maintained in a tsetse area. Tropical Animal Health and Production 8 : 1–12.
Wissocq, Y.J., Trail, J.C.M., Wilson, A.D. and Murray, M. (1983). Genetic resistance to trypanosomiasis and its potential economic benefits in cattle in East Africa. In International Scientific Council for Trypanosomiasis Research and Control, OAU/STRC Seventeenth Meeting, Arusha, Tanzania 1981. Publication No. 112. 361–364, Nairobi, Eleza Services.
The World Bank (1989). Sub-Saharan Africa. From crisis to sustainable growth. A long-term perspective study. World Bank, Washington D.C.
World Health Organization, 1986. Epidemiology and control of African trypanosomiasis. Report of a WHO Expert Committee. Technical Report Series 739. WHO, Geneva.
World Health Organization, 1989. Evaluation of certain veterinary drug residues in food. (Thirty-fourth report of the Joint FAO/WHO Expert Committee on Food Additives, Geneva, 30 January - 8 February 1989). WHO Technical Report Series, No. 788 : 66pp.
ANNEX 1.
INDICATION OF COSTS OF SELECTED METHODS FOR DIAGNOSIS AND SURVEILLANCE OF
ANIMAL TRYPANOSOMIASIS IN US$ - 1990
Table A.1. EXAMINATION OF STAINED BLOOD SMEARS (2 000 smears p.a.)
| Item | Capital cost | Capital p.a.1 | Consumables p.a. |
| Compound microscope | 2 500 | 500 | - |
| Microscope slides, stain etc. | - | - | 250 |
Total cost p.a. | US$500 +US$250 | =US$750 | |
Cost per test | =US$0.375 | ||
Table A.2. EXAMINATION OF BUFFY COAT (6000 tests p.a.)
| Item | Capital cost | Capital p.a.1 | Consumables p.a. |
| Compound microscope, phase illumination | 3 750 | 750 | - |
| Microhaematocrit centrifuge & reader | 1 200 | 240 | - |
| Portable generator2 | 750 | 250 | - |
| Cold boxes, freezer packs2 | 150 | 50 | |
Capillary tubes, sealant, slides, cover slips, diamond pencil, spare rim gaskets | - | - | 600 |
| Total cost p.a. | US$ 1 290 + US$600 | = US$1 890 | |
| Cost of materials per test | = US$0.315 | ||
Cost of transport 300km/wk × 40wk × US$0.50/km = US$6 000, | per test | = US$1.00 | |
| Total cost per test | = US1.315 | ||
Table A.3. ANTIBODY DETECTION BY MICROELISA (48 000 p.a.)
| Item | Capital cost | Capital p.a.1 | Consumables p.a. |
| Blood collection materials | - | - | 5 000 |
| Cold boxes, freezer packs2 | 900 | 300 | - |
| Refrigerator | 1 000 | 250 | - |
| Bench centrifuge | 800 | 160 | - |
| Serum separation, storage | - | - | 5 000 |
| Freezer | 1 000 | 200 | - |
| MicroELISA reader | 11 000 | 2 200 | - |
| Micropipettes | 2 000 | 400 | - |
| Plate shaker | 700 | 140 | - |
| Sundry accessories | 1 000 | 200 | - |
| Microplates3 | - | - | 2 000 |
| Pipette tips | - | - | 1 500 |
| Glassware, buffer salts, sealers, printer rolls, etc. | - | - | 1 000 |
| Reagents4 | - | - | 1 000 |
Total cost p.a. US$3 850 + US$15 500 | =US$19 350 | ||
Cost of materials per test5 | =US$0.40 | ||
| Cost of serum collection @ 4 teams × 300km/wk × 40wk × US$0.50/km = US$24 000, per test | = US$0.50 | ||
Total cost per test | =US$0.90 | ||
Notes 1. Capital cost equally divided over 5 years
2. Capital cost equally divided over 3 years
4. Not all reagents are commercially available.
Major variables affecting costs and assumptions made :
Cost of blood smears for diagnosis of trypanosomiasis decrease if costs cover other haemoprotozoan diseases.
Costs per test rise if fewer tests are made than budgetted for due to distribution of annual capital costs.
Increasing numbers of tests increase wear and tear on equipment.
No estimates have been made of maintenance costs of equipment or personnel costs (time, salary, subsistence).
Recycling of some consumables may reduce costs marginally.
Costs will vary depending upon liability to duty and taxation.
Transport costs will vary enormously, depending upon terrain, sampling schedules, distances travelled, cost sharing with other duties and running costs. The figures used here are only for illustration.
Justification for selection of a diagnostic method
Routine diagnosis:
An operating budget of US$7 890 for materials would enable 6 000 tests to be made by the buffy coat technique.
A budget of US7 500 would enable the examination of 20 000 blood smears to diagnose haemoprotozoan diseases, including trypanosomiasis, although the sensitivity of this method is lower than that of examination of the buffy coat to diagnose trypanosomiasis.
Surveys and surveillance :
To illustrate the material costs of surveys and surveillance, a five year tsetse control programme can be considered. Examination of the buffy coat and of serum samples should be combined for maximum diagnostic sensitivity.
Costs of materials and transport running costs for four teams examining buffy coats in the field over the five year period would be (at fixed cost) $7 890 × 4 × 5 = $157 800.
If the same four teams collected sera, the costs of materials for the detection of antitrypanosomal antibodies in 48 000 sera per annum for five years would be $0.40 × 48 000 × 5 = $96 000.
Total material costs of approximately $250 000 are justified in the context of an overall programme cost of, say, $5 million.
DIAGNOSTIC, TRAITEMENT ET PREVENTION
DE LA TRYPANOSOMIASE ANIMALE SUR LE TERRAIN
RESUME
R.J. Connor
L'Afrique est le continent qui souffre le plus de disette. Pour nourrir la population, qui devrait doubler dans les 20 prochaines années, il faut accroître la production alimentarie. La trypanosomiase est considerée depuis longtemps comme un obstacle majeur au développement agricole et rural. Les moyens existent pour surmonter cet obstacle et doivent être employés.
Il n'y a pas de méthode idéale pour diagnostiquer la trypanosomiase. Toutefois, l'emploi accru de simples microscopes sur le terrain permettrait d'améliorer le dépistage de plusieurs maladies hémoprotozoaires, notamment la trypanosomiase. D'autres méthodes ne seraient rentables qu'appliquées par des équipes de spécialistes dont les travaux devraient être associés à des programmes de lutte.
Les trypanocides ont prouvé leur efficacité contre la trypanosomiase. Pour en généraliser l'emploi il faut améliorer le diagnostic et l'administration des médicaments. Une chimioprophylaxie judicieuse est prônée pour protéger certaines catégories de bétailanimaux de trait et femelles reproductrices - que les agriculteurs jugent les plus importantes du point de vue économique. Le coût du traitement doit pouvoir être amorti et son application doit être compatible avec les méthodes de gestion, en particulier l'alimentation et la reproduction, qui pourront nécessiter quelques modifications.
Des techniques sont nécessaires pour comprendre l'épidémiologie de la résistance aux médicaments et remédier à ce problème. Les techniques de culture “in vitro” sont très utiles pour rechercher de nouveaux trypanocides mais en attendant, il faut utiliser au mieux, sur le terrain, les connaisances, les méthodes et les médicaments existant. Pour mieux lutter contre la trypanosomiase et contribuer à améliorer la production alimentaire, il faut acheminer une part plus importante des ressources jusqu'au producteur.
Recommandations
Afin d'accroître la production alimentaire de façon durable, notamment en améliorant la lutte contre la trypanosomiase animale, il est recommandé d'adopter des politiques visant à acheminer la plus grande partie des ressources jusqu'au producteur.
L'effort de vulgarisation doit être intensifié pour convaincre les agriculteurs de la nécessité de payer les traitements que leurs animaux reçoivent et d'apporter de légères modifications à leurs méthodes de gestion pour améliorer la lutte contre la trypanosomiase et accroître la production.
Des stages de formation en cours de service, intensifs et de courte durée, doivent être organisés à l'intention du personnel de terrain afin d'améliorer: la capacité de diagnostic, l'estimation du poids vif, d'administration des trypanocides, l'établissement des registres et des rapports.
Il faut améliorer le diagnostic au niveau du village ou du sous-district, en fournissant les ressources et le matériel nécessaires à l'examen des frottis sanguins.
Il faut étudier l'incidence de la trypanosomiase, par des méthodes de diagnostic parasitologique directes, pour mettre au point des stratégies de prévention et de soin.
Le dépistage de la trypanosomiase et la surveillance avant, pendant et après les programmes de lutte contre les vecteurs doivent incorporer des méthodes de diagnostic indirectes, motamment celles qui sont mises au point actuellement.
Les systèmes d'enregistrement des données pour le diagnostic et le traitment doivent être normalisés pour permettre de dépister et de combattre la maladie et de vérifier l'efficacité des trypanocides.
Des prélèvements sanguins doivent être effectués, en vue d'un examen ultérieur, sur les animaux qui subissent un traitment trypanocide, à moins qu'il ne soit possible de procéder à un examen avant le traitement.
Une chimioprophylaxie judicieuse, abordable pour l'agriculteur et compatible avec la gestion, doit être assurée au prix coûtant pour les catégories de bétail les plus importantes économiquement; un traitement curatif doit être fourni ensuite.
La sensibilité aux trypanocides des populations locales de trypanosomes doit être déterminée par des essais “in vitro”, au cours de la sélection de nouveaux trypanocides.
Il faut encourager la mise au point de tests de terrain permettant de détecter le niveau des trypanocides dans le plasma.
Il faut allouer suffisamment de ressources pour qu'un personnel de terrain bien équipé et correctement encadré puisse surveiller la situation de la trypanosomiase et vérifier l'efficacité des trypanocides.