First draft prepared by
Dr. Raymond J. Heitzman
Compton, Newbury
Berkshire, United Kingdom
IDENTITY
Chemical name:
4-Amino-alpha-[(tert-butylamino)methyl]-3,5-dichlorobenzyl alcohol hydrochloride (IUPAC)
CAS number: 21898-19-1 (hydrochloride); 37148-27-9 (clenbuterol)
Structural formula
Molecular formula: C12H19OCl3 (as hydrochloride)
Molecular weight: 313.65
OTHER INFORMATION ON IDENTITY AND PROPERTIES
Appearance:
Colourless microcrystalline powder (Merck Index)
White or slightly yellowish substance (sponsor)
Melting point:
174-175.5°C (Merck Index)
170-176°C (sponsor)
Solubility:
Very soluble in water, methanol and ethanol, slightly soluble in chloroform, insoluble in benzene (Merck Index)Soluble in water, methanol and ethanol, very soluble in chloroform (sponsor)
RESIDUES IN FOOD AND THEIR EVALUATION CONDITIONS OF USE
General
Clenbuterol is used as a bronchodilator for horses and non-lactating cattle. The recommended treatment schedule is 0.8 m g/kg BW twice daily. The maximum duration of treatment in non-lactating cattle is 10 days. It may be administered by the oral or intravenous routes of administration. Cattle may also be injected by the intramuscular route.
Clenbuterol is also used as a tocolytic in cattle. The recommended treatment schedule is a single parenteral injection equivalent to 0.8 m g/kg BW.
METABOLISM
Pharmacokinetics
The radiolabel used in all of the studies in this monograph was 14C at the C-2 position. The radiochemical purity was > 95 %.
Plasma
Clenbuterol was well absorbed after oral administration to laboratory animals, humans and the target species. In rats (Kopitar & Zimmer, 1973), dogs, rabbits (Zimmer 1974b) and humans peak blood concentrations were achieved 1-4 hours after oral dosing (Zimmer, 1976). Absorption was slower in another study in the dog (Zimmer, 1974a) and baboon (Johnston & Jenner, 1976) with peak plasma radioactivity occurring 6-7 hours after oral administration.
Peak plasma concentrations (range 0.24-1.8 m g/l) occurred in 0.25-3 hours following i.m. administration to calves or cows except in one study in three cows where the maximum concentration occurred at 8 hours; the time to peak concentration was 6-12 hours after oral administration (see text, Table 1, LLoyd-Evans, 1994). The plasma half-life in cattle varied from 16 to 105 hours depending on the subpopulation tested. Peak plasma concentrations (range 0.37-1.59 m g/l) occurred in 1.5-3 hours following i.m. or oral administration to horses with half-lives in the range 9-21.4 hours (Hawkins et al, 1984, Johnston & Dunsire, 1993).
Excretion into Faeces and Urine
Laboratory Animals and Horses
After oral administration of 14C-Clenbuterol the radioactivity was quickly distributed throughout the tissues of rats and mice and shown to cross the placental barrier of the mouse (Kopitar 1969), the dog (0.43 % of dose in 4h) (Rominger & Schrank, 1982) and the baboon (1.5% of dose in 3.5 h) (Schmid, 1980). The excretion of 14C-Clenbuterol after oral administration is summarised in Table 1. The results indicate that the major fraction of the drug is excreted into the urine. Similar patterns of excretion were observed if the drug was administered parenterally or by inhalation (Huntingdon Res. Centre, 1978).
Table 1. Excretion of 14C-Clenbuterol after oral administration
|
Species |
Time period after dose (h) |
% dose in urine |
% dose in faeces |
Reference |
|
Rat |
0 - 72 |
62.5 |
20.8 |
Kopitar, 1970 |
|
Rabbit |
0 - 72 |
88.5 |
8.9 |
Zimmer, 1971 |
|
0 - 96 |
92 |
0.2 - 5 |
Zimmer, 1974b |
|
|
Dog |
0 - 96 |
85 - 87 |
3.5 - 9 |
Zimmer, 1974a |
|
0 - 96 |
74 |
3.7 |
Zimmer, 1974b |
|
|
Horse |
0 - 336 |
75 - 91 |
6 - 15 |
Johnston & Dunsire, 1993 |
|
Baboon |
0 - 120 |
62* |
16 |
Johnston & Jenner, 1976 |
* includes cage washings but not cage debris.
Cattle
Eight studies (Nos. 1-8 in Table 2) using cattle administered 14C-clenbuterol either orally, as an intramuscular or intravenous injection, showed that excretion as a percentage of the dose was 50 - 85 % in the urine, 5-30% in the faeces and where applicable, 0.9 - 3 % in the milk when measured both during the dosing period and for 4-15 days after dosing.
Table 2. Studies using 14C-Clenbuterol in cattle and equines
|
Study # |
Animals |
Dose regime |
Tissues |
Sampling times |
Reference |
|
|
P.U.Fc. (hA) |
M.L.K.F.IS (daysB) |
|||||
|
1 |
9 preruminant calves |
0.8 b.i.d 11 im x 4 |
P.U.Fc.M.L.K.F.IS
|
0-360 |
1, 7, 10 |
Hawkins et al, 1985b |
|
oral x 7 |
(3) |
|||||
|
2 |
9 ruminant calves |
0.8 b.i.d 21 im |
P.M.L.K.F.IS |
0-(3) |
0.25, 6, 10 |
Cameron et al, 1987 |
|
3 |
12 ruminant calves |
0.8 b.i.d 21 im |
P.M.L.K.F.IS |
|
0.25, 5, 28 |
Hawkins et al, 1993b |
|
4 |
3 cows |
0.8 b.i.d 11 im x 6 |
P.U.Fc.Mk.M.L.K.F.IS |
0-240 |
2h, 2, 5 |
Hawkins et al, 1985a |
|
oral x 5 |
||||||
|
5 |
1 cow |
1.6 single oral |
P.U.Fc.Mk. |
0-240 |
|
Schmid & Zimmer, 1977a |
|
0.8 s.i.d. oral x 3 |
||||||
|
6 |
1 cow |
0.8 single im |
P.U.Fc.Mk. |
0-238 |
|
Schmid & Zimmer, 1977b |
|
0.5 s.i.d. im x 3 |
||||||
|
7 |
3 cows |
0.8 single oral |
P.U.Fc.Mk. |
0-144 |
|
Cameron & Phillips, 1987 |
|
3 cows |
0.8 single iv |
P.U.Fc.Mk. |
0-144 |
|
||
|
3 cows |
0.8 single im |
P.U.Fc.Mk. |
0-144 |
|
||
|
9 cows |
0.8 single im |
P.U.Fc.Mk.M.L.K.F.IS |
0-144 |
0.25, 3, 6 |
||
|
8 |
1 cow |
0.6 single iv |
P.U.Fc |
0-96 |
|
Schmid, 1977 |
|
9 |
3 cows |
0.52-0.74 s.i.d. |
M.L.K.F.IS |
|
0.5h, 3, 6 |
Schmid & Zimmer, 1977c |
|
10 |
3 horses |
0.8 b.i.d 21 oral |
P.U.Fc.M.L.K.F. |
0-396 |
1,4,6 |
Hawkins et al, 1984 |
|
11 |
12 horses |
0.8 b.i.d 21 oral |
P.U.Fc.M.L.K.F. |
0-540 |
0.5,9,12,28 |
Johnston & Dunsire, 1993 |
Key; P, plasma; U, urine; Fc, faeces; Mk, milk; M, muscle; L, liver; K, kidney; F, fat; IS, injection site; s.i.d, once a day; b.i.d., twice daily; A sampling time from first dose; B sampling time from last dose; Key for dose regime: 0.8 b.i.d 11 im x 4 oral x 7 (Study 1) means 0.8 m g/kg bw twice daily im for 2 days (4 im doses then 0.8 m g/kg bw twice daily orally for 3 days + 0.8 m g/kg bw once a day for 1 day (7 oral doses) - total 11 doses of 0.8 m g/kg bw
Metabolism in laboratory animals
Clenbuterol was the major compound excreted in the urine of all the laboratory species examined. There was greater amount of metabolism in the rat compared to the other species tested. The contribution of Clenbuterol to the total residues found in urine after the administration of 14C-Clenbuterol to several species is shown in Table 3.
Table 3. Excretion of residues into the urine
|
Species. |
Dose route |
TR as % dose |
CL as % dose |
CL as % in TR |
Reference |
|
Rat |
oral |
43-58 |
32-42 |
ca. 73 |
Zimmer, 1971 |
|
Rabbit |
oral |
88 |
19 |
22 |
Zimmer, 1971 |
|
Rabbit |
oral |
89 |
34 |
38 |
Zimmer, 1974b |
|
Dog |
oral |
66-107 |
17-20 |
ca. 20 |
Zimmer, 1974a |
|
Dog |
oral |
72 |
15 |
21 |
Zimmer, 1974b |
|
Baboon |
oral |
73 |
18 |
25 |
Johnston & Jenner, 1976 |
|
Baboon |
i.v. |
70 |
25 |
36 |
Schmid, 1982 |
|
Monkey |
i.v. |
48 |
n.m |
n.m. |
HRC, 1978 |
|
Man |
oral |
67 |
35 |
52 |
Zimmer, 1974c |
|
Calf |
i.m./oral |
59-66 |
24-26 |
ca. 40 |
Hawkins et al., 1985b |
|
i.m. |
n.m. |
n.m. |
42 |
Hawkins et al., 1993b |
|
|
Cow |
i.m./oral |
47-67 |
22-49 |
n.m. |
Hawkins et al., 1985a |
|
Horse |
oral |
74 |
|
31-49 |
Hawkins et al., 1984 |
CL is Clenbuterol, TR is total residues, n.m. is not measured, i.v. is intravenous.
The metabolism of Clenbuterol was studied in more detail in the dog and the metabolic profile in the urine was determined (Schmid & Prox, 1986). The results are shown diagrammatically in Figure 1. The authors concluded that the biotransformation of Clenbuterol is slow (relative to other b -agonists), since there are no direct points of access for the enzymes, monoamine oxidase and catechol-O-methyl transferase, or for efficient sulphate conjugation. The main metabolites were formed by oxidation along the long side chain in the 1 position of the ring, while the 2-amino-3,5-dichloro moiety remains intact.
Figure 1. Metabolic Profile of Clenbuterol in Dog Urine
Metabolism in Cattle
The metabolite profile seen in cattle is qualitatively similar to that seen in laboratory animals and in humans. Clenbuterol accounts for 60-86% and 22-53% of the total radioactivity in plasma (Schmid & Bucheler, 1987) and urine (Hawkins et al., 1985b) respectively. Other metabolites quantified in urine included N-AB 930 (ca. 3%), N-AB 931 (R-CHO) (2-4%), N-AB 933 (6-40%) and NA1141 (3-34%) (see Figure 1 for key to metabolites) (Hawkins et al, 1985b, Hawkins et al., 1993a, Hawkins et al., 1985a).
Metabolism of radiolabeled Clenbuterol in bovine liver followed a similar pattern with the majority of the extractable residues being Clenbuterol. The resulting profiles from several studies are shown in Table 4. The percentage of the total residues (%TR) which are extractable from the liver was > 80% in livers collected 2-6 hours after drug administration. At longer withdrawal times (WT) the extractable fraction of the TR varied from about 50% in two studies to 87% in another study (see Table 4).
Table 4. Metabolic profiles of residues in bovine liver
|
Reference |
WT (h) |
TR extractable (%) |
as a % extractable TR |
|||||
|
NAB 365 (UD) |
NAB 930 |
NAB 931 |
NAB 933 |
NA 1141 |
Polar/base-line |
|||
|
Hawkins et al, 1985b |
24 |
50 |
64 |
ND |
ND |
ND |
ND |
26 |
|
Hawkins et al, 1985a |
2 |
89 |
65 |
ND |
ND |
ND |
ND |
35 |
|
Baillie et al., 1980 |
3 |
89 |
52* |
ND |
ND |
4 |
11 |
34 |
|
Hawkins et al, 1993b |
6 |
81 |
90 |
0.3 |
1.4 |
1.5 |
2.1 |
4 |
|
120 |
87 |
49 |
4.7 |
ND |
4.8 |
ND |
42 |
|
|
Cameron & Phillips, 1987 |
6 |
84 |
95 |
ND |
ND |
ND |
ND |
5 |
See Figure I for key to metabolites. UD is the unchanged drug, clenbuterol.* NAB 365 Cl and NAB 739 could not be further separated due to their having similar chromatographic properties.
The data in Table 4 are used to calculate the content of Clenbuterol as a percentage of the total residues and the results are given in the last column of Table 5. There are differences in the values for the two methods. This is most noticeable for the value for 120 hours withdrawal time where the value of 14 % by the GC-MS method may be low. However the proportion of Clenbuterol in horse liver samples taken at 9 or 12 days WT was < 10% (Johnston & Dunsire, 1993; Hawkins et al, 1993c).
The metabolism of clenbuterol in bovine kidney is similar to the one described for liver (Hawkins et al, 1985a&b, 1993a). Parent compound accounts for 58-85% of the extracted radioactivity at 6 hours post dose. In muscle and milk parent compound makes up for 70-100% of the total radioactivity (Schmid, 1990a&b).
The pharmacological activity of the metabolites was determined and only compound NA1141, with an activity of 20% that of Clenbuterol hydrochloride, possessed any activity.
Selected tissues from the radiodepletion studies were analysed by a GC-MS method for the content of Clenbuterol (Schmid, 1990b). The results are shown in Table 5.
Table 5. Clenbuterol measured by GC-MS and its percentage of total residues in bovine liver
|
Animals |
WT (h) |
Total Residues (m g/kg) |
Clenbuterol (m g/kg) |
Clenbuterol as % TR |
|
|
GC-MS |
Table 4 |
||||
|
Calf |
24 |
9.2 |
5.3 |
58 |
32 |
|
Calf |
6 |
20.7 |
13.1 |
63 |
73 |
|
120 |
3.9 |
0.6 |
14 |
43 |
|
|
Cow |
2 |
29.8 |
27.2 |
91 |
58 |
|
Cow |
48 |
8.6 |
4.4 |
51 |
45 |
Metabolism in the Horse
Clenbuterol accounts for 45 % of the total radioactivity in plasma (Zimmer, 1977). In urine, 45 % of the excreted radioactivity is parent compound clenbuterol. The metabolite pattern in urine obtained during a repeat-dose residue study (Hawkins et al., 1984) showed that parent component accounted for 31-49 % of urine radioactivity, NAB 821 (R-CHOH-CH2OH) for 0-11% and NA 1141 for 10-16%. Approximately 23-30% of urine radioactivity remained at the baseline during metabolite profiling.
Investigations in equine liver tissue, the target tissue for 14C-Clenbuterol, revealed that parent compound accounts for 38-90% of total radioactivity at early sacrifice time points of 12 and 24 hours post dose (Hawkins et al., 1984, Hawkins et al., 1993c). Extraction efficiency at these time points ranges between 59% and 85%. Apart from clenbuterol, NA 1141 (10%) and NAB 821 (3-7 %) could be identified in equine liver tissue. At later sacrifice time points there was a quantitative change in the metabolite pattern observable, though not a qualitative one. The metabolism of clenbuterol in horse kidney is similar to the one described for liver. Parent compound accounts for 89% of the extracted radioactivity at 24 hours post dose, while NA 1141 makes up 11%.
TISSUE RESIDUE DEPLETION STUDIES
Radiolabeled Residue Depletion Studies
Cattle
Residue depletion studies were performed in both calves and cows using 14C-Clenbuterol administered by the i.m. and/or the oral routes. The studies used are those numbered in Table 2; 1,2 and 3 for calves and 4, 7 and 9 for cows. Depletion of total residues is rapid in all edible tissues of calves and cows (see Table 6 for references and results).
In calves, the results from GLP-certified total balance studies (No. 1,2) show that in individual calves killed at 6,7 or 10 days after last dose, total residues had fallen below limits of detection (0.06 m g/kg and 0.18 m g/kg, respectively) in muscle at all time points. Study 3, a GLP-certified study, uses adequate numbers of calves to establish that at 28 days, total residues in liver are less than 1 m g/kg and in muscle and at the last two injection sites less than 0.2 m g/kg. The calves in study 3 received the dose of clenbuterol hydrochloride for the maximum time as intended for the respiratory preparation, 10.5 days of twice-daily treatments at 0.8 m g/kg.
In cows there are three studies. Study 9 is an in-house orientation study in three cows; study 4, GLP-certified, concerns total balance following repeated doses of clenbuterol hydrochloride. These demonstrate rapid depletion of radioactivity from edible tissue. Study 7, GLP-certified, confirms this using a total of 9 lactating dairy cows which received the recommended single injection of clenbuterol hydrochloride, as intended for the tocolytic preparation. By 6 days, total residues in liver were less than 1 m g/kg, in muscle less than 0.1 m g/kg, and in samples of injection site were less than 0.5 m g/kg.
Table 6. Total residues (mean ± SD m g/kg) of radioactivity in tissues after administering 14C-Clenbuterol to calves and cows
|
Study No. |
No. cattle |
WT (days) |
Muscle |
Liver |
Kidney |
Fat |
Injection Site |
Reference Table 2. |
|
Calf |
3 |
1 |
0.86 ± 0.39 |
9.20 ± 3.33 |
9.09 ± 3.74 |
0.96 ± 0.58 |
0.98 ± 0.33 |
Hawkins et al. 1985b |
|
3 |
7 |
ND |
1.39 ± 0.19 |
0.41 ± 0.02 |
ND |
0.13 ± 0.16 |
||
|
3 |
10 |
ND |
0.85 ± 0.10 |
0.27 ± 0.07 |
ND |
0.08 ± 0.10 |
||
|
2 |
3 |
0.25 |
2.17 ± 0.27 |
36.6 ± 9.5 |
38.7 ± 8.4 |
0.82 ± 0.42 |
2.49 ± 0.70 |
Cameron et al. 1987 |
|
3 |
6 |
0.09 ± 0.10 |
7.37 ± 2.2 |
3.16 ± 0.5 |
ND |
0.32 ± 0.20 |
||
|
3 |
10 |
ND |
4.32 ± 0.5 |
2.15 ± 0.6 |
ND |
0.28 ± 0.20 |
||
|
3 |
4 |
0.25 |
0.79 ± 0.2 |
20.7 ± 4.8 |
16.1 ± 2.3 |
0.55 ± 0.1 |
1.66 ± 0.3 |
Hawkins et al. 1993b |
|
4 |
5 |
0.16 ± 0.03 |
3.9 ± 0.7 |
2.2 ± 0.5 |
0.12 ± 0.2 |
0.39 ± 0.1 |
||
|
4 |
28 |
ND |
0.89 ± 0.1 |
0.46 ± 0.2 |
ND |
0.18 ± 0.03 |
||
|
Cows |
1 |
2 hours |
1.45 |
29.8 |
14.7 |
0.58 |
4.79 |
Hawkins et al. 1985a |
|
1 |
2 |
0.34 |
8.6 |
3.9 |
0.31 |
3.24 |
||
|
1 |
5 |
0.19 |
4.4 |
1.4 |
0.35 |
2.92 |
||
|
7 |
3 |
0.25 |
0.22 ± 0.14 |
6.26 ± 0.92 |
5.11 ± 1.27 |
0.09 ± 0.13 |
5.39 ± 4.13 |
Cameron & Phillips 1987 |
|
3 |
3 |
0.01 ± 0.01 |
1.17 ± 0.47 |
0.42 ± 0.13 |
0.02 ± 0.04 |
0.14 ± 0.24 |
||
|
3 |
6 |
0.01 ± 0.01 |
0.65 ± 0.24 |
0.18 ± 0.09 |
0.02 ± 0.04 |
0.22 ± 0.32 |
||
|
9 |
1 |
0.5 hours |
0.67 |
8.19 |
8.06 |
0.23 |
130.6 |
Schmid & Zimmer 1977c |
|
1 |
3 |
0.03 |
1.96 |
0.99 |
0.07 |
0.31 |
||
|
1 |
6 |
ND |
0.84 |
0.2 |
0.08 |
2.53 |
The dosages are given for each study and referenced in Table 2. ND is not detected with LOD Study 1, 0.06 m g/kg muscle; 0.17 m g/kg; Study 2, 0.18 m g/kg; Study 3, 0.1 m g/kg.
Total Residues at the Injection Site
In several of the studies in which Clenbuterol is administered intramusculary there were both single and multiple injections. In one study in calves, 21 injections of 0.8 m g/kg BW were given at different sites over a period of 10.5 days (Cameron et al., 1987). Samples were collected from the multiple sites and analysed for total residues (radioactivity). Because the injections were given at different times data for a wide range of times after dosing is possible even in the same calf. The results are plotted in figure 2 for time after injection versus the residue. There is a wide variation in the results and there is no correlation between time after dosing and the concentration of residues at the injection sites, in fact the residues found at 246 hours were as high as those seen at 6 hours after injection. The same sampling schedule was not followed in a later study (Hawkins et al., 1993b) where only samples of injection sites were collected from the sites of the last two injections in each calf, i.e. injected on days 10 and 11.
Figure 2. Radioactive residues at intramuscular injection sites of calves
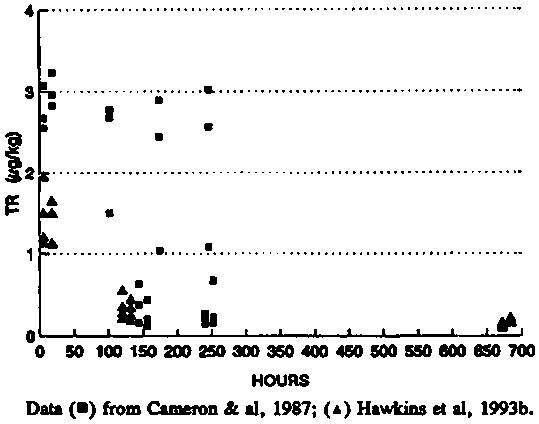
Residues in Milk
In a GLP study using 9 Friesian cows, mean body weight 538 kg, the cattle were divided into groups of 3 and given a single dose of 0.8 m g/kg BW [14C]-labelled clenbuterol hydrochloride by the oral, intravenous or intramuscular route. Samples of milk were taken for analysis (Cameron & Phillips, 1987) and the results are shown in Table 7.
Table 7. Total residues in milk after administration of 14C-Clenbuterol by different routes
|
Withdrawal Intervals (h) |
Total Residues (m g eq/l) in milk from groups of three (3) cows |
||||||||
|
Oral |
Intravenous |
Intramuscular |
|||||||
|
Pre-dosing |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
ND |
|
7 |
0.40 |
0.42 |
0.37 |
0.62 |
0.72 |
0.58 |
0.53 |
0.84 |
0.68 |
|
23 |
0.51 |
0.43 |
0.35 |
0.27 |
0.29 |
0.37 |
0.31 |
0.51 |
0.36 |
|
31 |
0.40 |
0.34 |
0.28 |
0.16 |
0.16 |
0.27 |
0.19 |
0.35 |
0.24 |
|
47 |
0.21 |
0.15 |
0.12 |
0.08 |
0.08 |
0.15 |
0.09 |
0.16 |
0.10 |
|
55 |
0.14 |
0.11 |
0.10 |
0.06 |
0.05 |
0.11 |
0.07 |
0.12 |
0.07 |
|
71 |
0.08 |
0.06 |
0.04 |
0.07 |
0.03 |
0.07 |
0.04 |
0.07 |
0.04 |
|
79 |
0.06 |
0.04 |
0.02* |
0.02* |
0.02* |
0.06 |
0.03 |
0.06 |
0.03 |
|
95 |
0.03 |
0.03 |
0.01* |
0.01* |
0.01* |
0.03 |
0.02* |
0.04 |
0.02* |
|
103 |
0.03* |
0.01* |
0.01* |
0.02* |
0.02* |
0.02* |
0.02* |
0.03 |
0.01* |
|
119 |
0.02* |
0.01* |
0.01* |
0.01* |
0.01* |
0.02* |
0.01* |
0.02* |
0.02* |
* derived from data < 30 dpm above background; ND is not detected and derived from data < 10 dpm above background
In a second study all 9 cattle were given a single dose of 0.8 m g/kg BW [14C]-labelled clenbuterol hydrochloride by the intramuscular route (Cameron & Phillips, 1987). Three cows were slaughtered for tissue residue analysis before the milk was collected and the residues in the milk for the remaining 6 cows were measured. The results are shown in Table 8.
In a non-GLP study these milk samples were analysed by GC-MS to determine the residues of clenbuterol (Schmid 1990a). The results for the total residues and clenbuterol are summarised in Table 8. For the first 2-3 days after the end of treatment, most of the residues in milk consisted of unmetabolised clenbuterol. The very low concentrations (0.015-0.050 m g/l at 79 hours post injection) of radioactivity at subsequent time points did not appear to be clenbuterol. [Note: The LOQ claimed for the method is 0.050 m g/l but this is not substantiated in Schmid, 1990a]
In a GLP study, 3 lactating cows were given twice daily i.m. doses of the combination product 14C-clenbuterol (0.8 m g/kg BW)/sulphadiazine (12.5 mg/kg BW)/trimethoprim (2.5 mg/kg BW) for 3 consecutive days (Hawkins et al 1985a). They were then given twice daily oral doses on days 4-5 and a single oral dose on day 6. The total residues in the milk reached peak values of 3.2, 3.5 and 3.9 m g/l during administration and bad declined to 0.18 m g/l by 108 hours post-dosing in the one cow kept on the study. The residues in milk of this cow collected at 12 hour intervals (twice a day milkings) after the last dose were: 1.58, 1.04, 0.77, 0.51, 0.39, 0.27, 0.21 and 0.18 m g/l. These high and persistent residues in milk clearly support the contraindication for this particular therapeutic use in lactating cows.
The cows were administered an i.m. injection of 14C-Clenbuterol at a dose of 0.8 m g/kg BW and approximately 2500 dpm/kg BW (Schmid, 1990a).
Horse
Residues in the edible tissues were determined in three horses receiving oral doses of a formulation combining clenbuterol hydrochloride with two antibiotics. The animals were treated twice daily for ten days and then a final oral dose on day 11 (see Study 10 Table 2). A similar study, however, applying only clenbuterol hydrochloride, was carried out using 12 horses and administering 21 oral doses (see Study 11 Table 2). The results are shown in Table 9. The total residues were highest in liver and kidney, very low in muscle and not detectable in fat.
Table 9. Total residues of radiolabeled compounds (m g/kg clenbuterol equivalents)
|
Withdrawal time (days) |
Muscle |
Liver |
Kidney |
Fat | |
|
Study 10 |
|
|
|
| |
|
1 |
0.21 |
11.27 |
3.43 |
< 0.17* | |
|
4 |
< 0.17* |
3.09 |
0.43 |
< 0.17* | |
|
6 |
0.29 |
3.30 |
0.23 |
< 0.17* | |
|
Study 11 |
|
|
|
RF |
OF |
|
0.5 |
0.35 |
16.71 |
4.20 |
< 0.35* |
< 0.00* |
|
9 |
0.00* |
5.55 |
0.35 |
< 0.06* |
< 0.07* |
|
12 |
0.01* |
4.54 |
0.24 |
< 0.00* |
< 0.00* |
|
28 |
0.01* |
0.65 |
0.18* |
< 0.09* |
< 0.02* |
*values below limit of quantification (< 10 dpm above background); RF is renal fat; OF is omental fat.
Other Residue Depletion Studies (with unlabeled drug)
Stability of Residues
The effect of cooking on the heat stability of clenbuterol was investigated (Rose et al. 1995). The drug was stable in boiling water at 100· C. In cooking oil at 260· C losses were observed, indicating a half-life of about 5 min. The effect of a range of cooking processes (boiling, roasting, frying, microwaving) on clenbuterol residues in fortified and incurred tissue was studied. No net change in the amount of clenbuterol was observed in any of the cooking processes investigated except for deep frying using extreme conditions. There was little observed migration from the tissue into the surrounding liquid or meat juices. Clenbuterol residues were found not to be evenly distributed in the incurred raw tissue used for the investigation. The findings of this investigation show that data obtained from measurements on raw tissue are applicable for use in consumer exposure estimates and dietary intake calculations.
Depletion Studies
There were no studies submitted by the sponsor but numerous studies are reported in the open literature. These include studies using the recommended therapeutic dosage and numerous studies in which clenbuterol was administered at a dose (ca. 10 times the therapeutic dose) to enhance the growth performance of farm animals. The general conclusions were that residues of unchanged clenbuterol accumulate in the eyes, lungs, hair and feathers. The highest residues in the "basket" tissues were found in the liver and kidney. The residues of clenbuterol in tissues and body fluids were measured in cattle treated with the therapeutic dose of the drug (Elliott et al, 1995). During treatment many tissues and body fluids contained residues of clenbuterol. After a 14 day withdrawal period residues of clenbuterol were detectable only in the eyes (mean 27.1 m g/kg) and to a much lesser extent lung and kidney (mean 0.3 m g/kg). By day 28 residues were only detected in eyes (mean 6 m g/kg) and these were still present at day 42. The authors conclude that it is not possible to differentiate between the legal and illegal use of the drug solely on residue analysis. This is in contrast to the opinion of the sponsor who believes that differentiation between legal and illegal use would be possible based on liver analysis, if the analytically determined concentrations of clenbuterol were related to the withdrawal time claimed to have been observed by the farmer.
Seven female Brown Swiss calves were used to study the pharmacokinetics of clenbuterol after an effective anabolic dosage of 5 m g/kg BW was given twice daily for 3 weeks. (Meyer & Rinke, 1991). Analyses of clenbuterol concentrations in different tissues was done by enzyme immunoassay (EIA). Tissue samples were taken from three calves on the last day of administration and from two more after 3.5 or 14 d of clenbuterol withdrawal. The rate of clenbuterol elimination was dependent on time and tissue clenbuterol concentrations in the lung dropped from a mean of 76 m g/kg to a level of less than 0.8 m g/kg after 14 days whereas in the liver the clenbuterol concentrations decreased from 46 m g/kg to 0.6 m g/kg within 14 d of withdrawal. Highest levels were always found in the eye: 118 m g/kg, 57.5 m g/kg and 15.1 m g/kg after 0, 3.5 d and 14 d of withdrawal, respectively.
Bound Residues/Bioavailability
The majority of the radiolabeled residues were extractable with mild solvents. The amount of bound residues is small and insufficient to be taken into account in the calculation of MRLs.
METHODS OF ANALYSIS RESIDUES IN TISSUES AND MILK
There are more than one hundred methods, published in the open literature since 1990, for the determination of residues of clenbuterol and other similar b -agonists in biological samples (for examples and full details of selected methods used in the EU, see Heitzman 1994). The methods for screening include EIA, HPLC and GCMS. Confirmation of positives is performed using specific GCMS methods with sensitivities for edible tissues from 0.01 m g/kg upwards and limits of quantification (determination) from 0.02 m g/kg upwards. A typical example is the GC-MS method described by Girault & Fourtillan (1990) who measured clenbuterol and the internal standard [2H9]-clenbuterol as the perfluoroacyl derivatives (m/z 368 and 377). Accuracy and precision were determined for fortified bovine tissue samples:
|
theoretical concentration (m g/kg) |
no samples |
mean observed concentration (m g/kg) |
S.D. |
CV (%) |
Error (%) |
|
0.200 |
10 |
0.213 |
15.6 |
7.3 |
+6.4 |
|
0.020 |
10 |
0.019 |
1.7 |
9.0 |
-5.5 |
The LOD was 0.010 m g/kg (based on the mean signal ± SD for 10 "blank" samples being significantly different (p < 0.001) from that for 0.010 m g/kg samples). This method was also validated for bovine and equine liver by Hawkins et al (1993a, 1994) with acceptable accuracy and precision at the LOQ of 0.100 m g/kg.
The method proposed by the sponsor is based on GC-MS. Samples of muscle and liver were prepared by maceration and digestion with enzymes (subtilisin) followed by extraction with reversed phase material (C-18 Sep-Pack), cleanup by solvent distribution and derivatisation (silylation). The ion m/z 351 was used for quantification (Schmid & Bucheler, 1987). A very similar method is described for milk (Schmid, 1990a). Specificity was demonstrated against matrix "blanks". It was shown that trimethoprim and sulfadiazine, which may be co-administered with clenbuterol, did not interfere with the assay. Clenbuterol metabolites have different lipophilicity, and molecular weights and so would not be expected to interfere. The LOQ was stated to be 0.100 m g/kg. However acceptable accuracy and precision were not demonstrated at this concentration and linearity was shown only over the range 0.250-2 m g/kg. The method was adapted for measuring residues in milk (Schmid, 1990a). Linearity for clenbuterol was achieved over the range 0.125-1 m g/l with recoveries of 14C-clenbuterol of 77-106%. The claimed LOQ is 0.050 m g/l but no data is presented to support this.
APPRAISAL
Clenbuterol is manufactured as a 50:50 racemic mixture. Most of the pharmacological activity is associated with the levo form. It is a direct-acting b 2-sympathomimetic agent used to treat respiratory diseases in cattle and horses and is administered as multiple oral or parenteral doses. For non-lactating cattle the maximum duration of treatment is restricted to 10 days. It is also used as a tocolytic in cattle when the recommended treatment schedule is a single parenteral injection equivalent to 0.8 m g/kg body weight. Although unapproved for such purposes it is used at doses many fold higher than the recommended therapeutic uses acting as a repartitioning agent in many farm species.
All the residue studies submitted by the sponsor were carried out using the 14C-radiolabeled racemic (chiral) mixture and were compliant with GLP requirements. Clenbuterol was well absorbed after oral administration to laboratory animals, humans and the target species. In most species peak blood concentrations were achieved 2-3 hours after oral dosing. The plasma half-life in cattle varied from 16 to 105 hours depending on the sub-population tested. The substance was widely distributed in the tissues and was shown to cross the placenta in pregnant rats, dogs, baboons and cows. In all species, excretion was predominantly via the urine as unmetabolised clenbuterol.
Eight studies using cattle that were administered 14C-clenbuterol either orally, or an intramuscular or intravenous injection, showed that excretion as a percentage of the dose was 50-85 % in the urine, 5-30 % in the faeces and where applicable, 0.9-3 % in the milk when measured between administration and 4-15 days after dosing. After oral administration of radiolabeled drug to horses, 75-91 % and 6-15 % of the dose was excreted in the urine and faeces, respectively, over a 14 day period. The metabolic pathways were similar in all the species studied though there were quantitative differences in the amounts of metabolites formed.
The metabolite profile seen in cattle is qualitatively similar to that seen in laboratory animals and in humans. Metabolism of radiolabeled Clenbuterol in bovine liver followed a similar pattern with the majority of the extractable residues (> 50%) being Clenbuterol. There were 4 minor metabolites and some unidentified polar metabolites.
Investigations in equine liver tissue using 14C-Clenbuterol revealed that parent compound accounts for 38-90% of total radioactivity at early sacrifice time points of 12 and 24 hours post dose. Apart from clenbuterol one metabolite formed by hydroxylation of a tertiary methyl group (NA 1141) (10%) and NAB 821 (R-CHOH-CH2OH) (3-7 %) could be identified in equine liver tissue. At later sacrifice time points (> 24 h) there was a quantitative change in the metabolite pattern observable, though not a qualitative one. The metabolism of clenbuterol in horse kidney is similar to the one described for liver. Parent compound in kidney accounts for 89% of the extracted radioactivity at 24 hours post dose, while NA 1141 makes up 11%.
In cattle the total residues were much higher in those receiving multiple daily doses compared with those administered a single injection. In both type of treatments the highest residues were observed in liver and kidney and very low residues were present in muscle and fat. The total residues were < 0.3 m g/kg in muscle and fat from about 6 days after treatment with multiple doses but were at levels between 9.85 and 0.35 m g/kg in liver and kidney for 6 to 28 days post dosing. Residues in muscle and fat consisted mostly of clenbuterol shortly after administration but in liver and kidney the percentage declined with increasing withdrawal time. The relationship between residues of clenbuterol and the total residues was determined 6 hours, 3 days and 6 days after treatment. Residues in muscle (including injection site muscle) consisted mostly of clenbuterol. At the 6-hour time point, residues in liver consisted almost entirely of clenbuterol; after 6 days the percentage of clenbuterol had declined to less than 50%.
Residues at the injection sites in muscle varied and there was no correlation between the concentration and time during the first eleven days after dosing. However, in one study the residues were low (< 0.25 m g/kg) at 28 days after dosing.
The use of the drug as a tocolytic may result in residues in milk in the period following parturition. Potential levels of residues in milk as a result of this treatment were investigated by administering lactating cows with a single intramuscular (im) injection of radiolabeled clenbuterol. The residues in milk over a three day sampling period consisted almost entirely of unmetabolised clenbuterol. The very low concentrations, 0.015-0.050 m g/l at 79 hours post injection, of radioactivity at subsequent time points did not appear to be clenbuterol. In consideration of whether the drug could be administered to lactating cows in a multiple dosing formulation containing clenbuterol with two antibiotics, three cows were given i.m. injections followed by twice daily oral doses of radiolabeled Clenbuterol. The total residues in the milk reached peak values of 3.2, 3.5 and 3.9 m g/l during administration and had declined to 0.18 m g/l by 108 hours post-dosing in the one cow kept on the study. Because of these unacceptably high levels of residues this combination product is not recommended for use in lactating cattle.
Two studies were carried out in the horse in which the concentrations of total residues in tissues were compared with residues of unmetabolised clenbuterol. In both studies the pattern of residue depletion was similar to that of cattle. Three horses were dosed orally and the residues measured at 6 days withdrawal time were all less than 0.3 m g/kg in muscle, kidney and fat and greater than 3 m g/kg in liver. In a second study 12 ponies were dosed orally at the recommended dose level with radiolabeled drug and total residues were measured at 0.5, 3, 12, 28 days post dosing. At all times the total residues were < 1 m g/kg in liver, < 0.2 m g/kg in kidney and < 0.1 m g/kg in muscle and fat.
There are more than one hundred methods published in the open literature since 1990 for the determination of residues of clenbuterol and other similar b -agonists in biological samples. The methods for screening include EIA, HPLC and GCMS. Confirmation of positives is performed using specific GCMS methods with limits of detection (LOD) for edible tissues from 0.01 m g/kg upwards and limits of quantification (LOQ) from 0.02 m g/kg upwards. The sponsor's proposed routine analytical method was based on GC-MS. The LOQ was stated to be 0.10 m g/kg for tissues and 0.05 m g/l for milk but acceptable accuracy and precision had not been demonstrated at these concentrations. Another well validated method in the dossier, also based on GC-MS, had been shown to have a LOQ of 0.020 m g/kg and a LOD of 0.010 m g/kg for bovine tissues.
Maximum Residue Limits
The ADI of 0-0.004 m g/kg of body weight established by the Committee is equivalent to 0.240 m g per day for a 60 kg person. In recommending MRLs the Committee took account of the following factors:
- Muscle and liver are the target tissues;- The marker compound parent drug is the only residue of public health concern. Because the metabolites and bound residues are not of toxicological concern they may be discarded from the calculation of the MRL's;
- 100 % of the total residues in muscle, fat and milk are unchanged drug and 60 % of the total residues in bovine liver and kidney and 6 % of the total residues in equine liver and kidney are unchanged drug;
- There are analytical methods suitable for regulatory use; and
- The sponsors are not proposing to make the drug available for multiple use in lactating cows.
The Committee recommends MRLs for cattle and horses of 0.2 m g/kg in muscle and fat, 0.6 m g/kg in liver and kidney, and of 0.05 m g/l for cattle milk, expressed as parent drug. Using these values for the MRLs then the maximum theoretical intake for the food basket would be 0.235 m g (see Table 10).
Table 10. Intake of Clenbuterol at level of MRLs
|
Tissue |
kg in basket |
MRL (m g/kg) |
m g |
|
Muscle |
0.300 |
0.2 |
0.060 |
|
Liver |
0.100 |
0.6 |
0.060 |
|
Kidney |
0.050 |
0.6 |
0.030 |
|
Fat |
0.050 |
0.2 |
0.010 |
|
Milk |
1.500 |
0.05 m g/kl |
0.075 |
|
|
Total |
0.235 | |
The Committee noted that the maximum residues observed at the recommended withdrawal times for single or multiple dose formulations when applied to the calculation of possible daily intake gives residues which are less than 0.130 m g in both cattle and horses (see Table 11).
Table 11. Estimation of residues of clenbuterol at practical withdrawal times for cattle and horses
REFERENCES
Baillie, H.W., Cameron, B.D., Draffan, G.H., Schmid, J., (1980). 13.11.80. Investigations of the placental transfer of 14C-N-AB 365 CL in the cow. Report no. 111674 from Inveresk Research International ADME 21/80 - U-Venti 38, Plani 30, Venti TMP/S 30 - U80-0230. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Cameron, B.D., Phillips, M.W.A., Mikhail, M., (1987). 06.87. The residue kinetics of 14C-N-AB 365 CL in the calf. IRI Project No. 135009 IRI Report No. 4371 Summary table on local tolerance*, 04.89 - U-Venti 83. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Cameron, B.D., Phillips, M.W A., (1987). 09.09.87. The residue kinetics of 14C-N-AB 365 CL in the lactating cow. IRI Project No. 135014/IRI. Report No. 4445. Amendment to IRI Report No. 4445 of Hall, B.E. of 24.10.88 - U-Venti 90, Plani 56. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Elliott, C.T., McCaughey, W.J., Crooks, S.R.H., McEvoy, J.D.G. and Kennedy, D.G., (1995). Residues of clenbuterol in cattle receiving therapeutic doses: Implications for differentiating between legal and illegal use. Vet. Quarterly, 17, 100-102.
Girault, J., Fourtillan, J.B., (1990). Determination of clenbuterol in bovine plasma and tissues by gas chromatography-negative-ion chemical ionisation mass spectrometry. Biomed. Environ. Mass Spectrometry, 19, 80-88.
Hawkins, D.R., Cheng, K.N., Major, R.M., (1993a). 05.04.1993, Validation of a GC-MS method for the measurement of 14C-Clenbuterol in calf liver and measurement of samples from calves administered with 14C-Clenbuterol. (Study No. BOI/140) BOI 142/921659 - U-Venti 126, Plani 78-. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Hawkins, D.R., Cheng, K.N., Major, R.M., (1994). 14.02.1994. Bioanalytical report. Validation of a GC-MS method for the measurement of 14C-Clenbuterol in horse liver and measurement of samples from horses administered with 14C-Clenbuterol. Study No. IRI/150797 Sponsor's Study No. 6820 VEN 9212 BOI 150/942011 + Report Amendment dated 22.02.1994 - U-Venti 133, Venti TMP/S 109. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Hawkins, D.R., Elsom, L.F., de-Salis, C.M., Morris, G.R., Roberts, N.L., Cameron, D.M., Pugh, K.E., (1985a). 21.08.85. The disposition of the combination product 14-C-N-AB 365 CL/Trimethoprim/Sulfadiazine in dairy cows. Comment on the HRC report HRC/BOI lll/84680A - U-Venti 76, Plani 47, Venti TMP/S 10. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Hawkins, D.R., Elsom, L.F., de-Salis, C.M., Morris, G.R., Roberts, N.L., Cameron, D.M., Offer, J., Fish, C., (1985b). 16.2.85. The disposition of the combination product 14-C-N-AB 365 CL Trimethoprim/Sulfadiazine in calves - U-Venti 70, Plani 42, Venti TMP/S 8-. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Hawkins, D.R., Elsom, L.F., de-Salis, C.M., Roberts, N.L., Cameron, D.M., (1984). 13.11.84. The disposition of the combination product 14C-N-AB 365 CL/Trimethoprim/Sulfadiazine after oral administration to horses. With addenda: letter of K. E. Pugh to Dr. Hawkins of 22.08.85 and letter of Dr. Hawkins to K. E. Pugh of 09.09.85 - U-Venti 65, Plani 39, Venti TMP/S 7. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Hawkins, D.R., Elsom, L.F., Dighton, M.H., Kaur A., Cameron, D.M., (1993b). 29.03.1993. The pharmacokinetics, metabolism and residues of 14C-Clenbuterol (14C-N-AB 365 CL) following intramuscular administration to calves. HRC/BOI 140/921418 - U-Venti 125. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Hawkins, D.R., Elsom, L.F. Dighton, M.H., (1993c). 15.10.1993. 14C-N-AB 365 CL. Investigation of the metabolic profiles in the livers of the horses following multiple oral administration BOI 149/931490 - U-Venti 129, Venti TMP/S 105-. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Heitzman, R.J., (1994). Veterinary Drug Residues. Residues in Food Producing Animals: Reference Materials and Methods. 2nd Edition; EC Report EUR 15127. ECSC-EEC-EAEC, Brussels & Luxembourg, Publ. Blackwells Scientific. ISBN 0-632-03786-5.
Huntingdon Research Centre; (1978). 18.08.78. Pharmacokinetics of 14C-N-AB 365 during chronic inhalation toxicity studies in cynomolgus monkeys - U79-0212 -. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Johnston, A.M., Dunsire, J.P., (1993). 29.04.1993. Plasma kinetics, metabolism, excretion and residue kinetics of N-AB 365 CL following multiple oral administration to the horse. IRI Project No. 150797, IRI Report No. 8346 - U-Venti 127, Venti TMP/S 104. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Johnston, A.M., Jenner, W.N., (1976). 20.12.76. Study of the metabolism of 14C-labelled N-AB 365 in the male baboon Project No. 102214 - U76-0165 -. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Kopitar, Z., (1969). 21.10.69. N-AB 365-CL Autoradiographic investigations on the distribution of [14C]-N-AB 365 CL in rats and pregnant mice (ADME I B) - U69-0108. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Kopitar Z., (1970). 26.02.70. N-AB 365CL. Pharmacokinetic investigations with [14C]-N-AB 365 CL in rats (ADME I B) - U69-0109 -. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Kopitar, Z., Zimmer, A., (1973). 18.12.73. Effect of repeated doses of NAB 365 CL on the pharmacokinetic profile in rats (ADME ID)- U73-0158 -. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
LLoyd-Evans, L.P.M., (1994). Application for establishment of maximum residue limits under council regulation (EEC) 2377/90. Clenbuterol hydrochloride; expert report on the residues file - U-Venti 138, U-Planipart 80, U-Venti TMP/S 111-. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Meyer, H.H., Rinke, L.M., (1991). The pharmacokinetics and residues of clenbuterol in veal calves. J Anim Sci 69, 4538-4544
Rominger, K.L., Schrank, H., (1982). 28.09.82. Placental transfer of 14C-clenbuterol in the dog - U82-0291. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Rose, M.D., Shearer, G., Farrington W.H.H., (1995). The effect of cooking on veterinary drug residues in food: clenbuterol. Food Addit Contain, 12, 67-76
Schmid, J., (1977). 15.12.77. Pilot pharmacokinetic investigations after intravenous administration of N-AB 365 CL in a cow - U-Venti 13, Plani 9, Venti TMP/S 18 - U77-0187 -. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Schmid, J., (1980). 13.11.80. Investigations of the placental transfer of 14C N-AB 365 CL in the baboon - U80-0229. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Schmid, J., (1982). Pharmacokinetics, metabolism and tissue distribution of 14C N-AB 365 CL in the baboon Report no. 2220 from Inveresk Research International - U82-0286. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Schmid, J., (1990a). 10.01.1990. Report: N-AB 365 CL. N-AB 365 CL in the cow's milk after oral and intramuscular administration (P 64189) - U-Venti 105, Plani 63, Venti TMP/S 88 - U90-0099. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Schmid, J., (1990b). 10.01.1990. Report: N-AB 365 CL. Plasma levels and residue analysis in cows and calves (P 62/89) - U-Venti 106, Plani 64, Venti TMP/S 89. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Schmid, J., Bücheler A., (1987). 07.10.87. Report: NAB 365 CL (Clenbuterol). Quantitative determination of Clenbuterol in tissues by automated gas chromatography/mass spectrometry ADME 65/87 - U-Venti 85, Plani 51, Venti TMP/S 66 - U87-0984 -. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Schmid, J., Prox, A., (1986). 24.10.86. Isolation and structural elucidation of NAB 365-CL metabolites from the dog urine - U86-0932 -. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Schmid, J., Zimmer, A., (1977a). 17.10.77. Pilot pharmacokinetic studies following oral administration (single and multiple dosing) of 14C-N-AB 365 CL to a cow - U-Venti 12, Plani 7, Venti TMP/S 17-U77-0185-. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Schmid, J., Zimmer, A., (1977b). 15.12.77. Pilot pharmacokinetic investigations after intramuscular administration (single and multiple doses) of N-AB 365 CL in the cow - U-Venti 14, Plani 8, Venti TMP/S 19-U77-0188 -. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Schmid, J., Zimmer A., (1977c). 22.12.77. N-AB 365 CL. ADME V. Residue studies in the cow following multiple i.m. dosing with N-AB 365 CL - U-Venti 16, Plani 11, Venti TMP/S 21 - U77-0190 -. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Zimmer, A., (1971). 13.10.71. N-AB 365-CL Metabolism and species comparison in the rat, rabbit and dog (ADME IV) - U71-0091 -. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Zimmer, A., (1974a). 15.08.74. Comparison of the pharmacokinetic profile in the dog with single and repeated dosage (ADME I D) - U73-0161 -. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Zimmer, A., (1974b). 10.12.74. Pharmacokinetics and metabolite pattern in the rabbit and dog (ADME II)-U74-0116 -. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Zimmer, A., (1974c). 13.12.74. Metabolism in Man; comparison with dog and rabbit, using radioactive substance (ADME IV) - U74-0117 -. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Zimmer, A., (1976). 20.04.76. Administration of Clenbuterol in man. Single doses, multiple doses, and metabolite samples (ADME V) - U76-0159 -. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.
Zimmer, A., (1977). 20.12.77. N-AB 365 CI ADME II, V. Preliminary investigations of pharmacokinetics in a horse - U-Venti 15, Plani 10, Venti TMP/S 20 - U77-0186. Submitted to FAO by Boehringer Ingelheim Vetmedica GmbH, Ingelheim, Germany.