David J. Penman and Brendan J. McAndrew
Institute of Aquaculture, University of Stirling, Stirling FK9 4LA, Scotland, U.K.
ABSTRACT
This paper concentrates mostly on genetic events occurring within hatcheries which have relevance to the production of fish for stocking as part of enhancement programmes. However, it should be recognised that stocking from hatcheries is not a substitute for habitat restoration or reduction in fishing intensity where the objective is to conserve natural populations. The objectives of fishery enhancement by stocking vary, for example from the conservation of genetically pristine populations in open waters at one extreme to maximisation of food production in artificial waters. Historically the major challenge facing hatcheries producing many freshwater species has been to meet the quantitative demands for fry and fingerlings: in many cases, little or no attention has been paid to genetic management of the stocks within hatcheries. It should be recognised that a lack of active genetic management of hatchery stocks does not mean that no change will take place within such stocks: on the contrary, processes such as founder effects, bottlenecks, inbreeding, domestication and negative selection may occur, with negative effects on fitness-related traits such as growth and survival. Active genetic management of hatchery stocks may be used to minimise genetic changes or to select for improved performance of such stocks. To date there have been few examples of selective breeding for improved performance of fish for fishery enhancement purposes but possibilities for this are discussed. Techniques which may be used to assist in quality control of fish destined for stocking are also discussed.
1. INTRODUCTION
“Stocking of fish for inland fishery enhancement” actually includes a broad range of different scenarios. For example, the receiving environment may be an open water body with existing stocks of the species in question, or it may be an enclosed man-made water body with no existing fish in it. The fish being introduced may be capable of forming a self-sustaining population or may be unable to breed in the receiving environment. If the latter is true, this may be due to the nature of the environment (e.g. Indian major carps being introduced into a stillwater) or due to intervention by man (e.g. triploid grass carp are used in some parts of the USA as triploid fish are incapable of breeding successfully). Table 1 shows some of the genetic considerations involved in stocking at two extremes: conservation of an indigenous species in a natural water body compared to maximising food production, perhaps from a man-made reservoir.
Table 1. A comparison of two extremes in terms of the objectives of stocking, with appropriate genetic considerations.
| Considerations | Objectives of stocking | ||
|---|---|---|---|
| Conservation | Maximising food production | ||
| Species | Native species | Native and non-native species? | |
| Stock | Local stocks | Best performing stocks | |
| Genetic techniques | No domestication or selection | Selected stocks?; other techniques where appropriate (e.g. monsoon, hybrids) | |
The population structure of indigenous freshwater species has been well studied in the case of salmonids and generally less so for other fish taxa (see Carvalho and Pitcher, 1995, and papers therein). Various forms of tagging and marking have been used for this purpose (e.g. to study migration routes), but biochemical and molecular genetics techniques have been widely and successfully applied to the analysis of natural populations, giving information about the geographical definition of breeding stocks, migration between such stocks, relatedness between populations from different parts of a species' range, etc. Variation in isozymes, mitochondrial DNA, minisatellite and microsatellite DNA have all been used to elucidate population structure. A classic example where the application of genetic techniques has revealed unexpected stock structure was the study of Ferguson and Taggart (1991), where it was shown that three genetically distinct spawning populations of brown trout (Salmo trutta) existed in Lough Melvin. These could be found together in the lake, but during the spawning season the fish separated into reproductively isolated spawning populations using different streams and areas of the lough.
Without such knowledge, allied to information about spawning sites, appropriate size and location for stocking, etc., attempts to conserve natural populations by stocking from hatcheries may well have no impact or negative impacts. For example, the fish used for stocking may not come from the same genetic stock as the fish in the local receiving environment, or fish from an appropriate source may be stocked in a location or at a life stage which does not enable them to imprint onto and subsequently follow spawning migration routes.
2. HATCHERIES AND GENETIC PROCESSES WITHIN HATCHERY STOCKS
Figure 1 illustrates the management of a hatchery which might be used to supplement fry production from a wild stock while attempting to minimise the genetic consequences of hatchery production. Broodstock are captured from the wild on each occasion when stocking is required and returned to the wild or otherwise discarded following spawning. Their offspring are reared in the hatchery to an appropriate stage and then released.
Figure 1. Movement of fish through a conservation-orientated hatchery using broodstock caught from the wild in each generation.
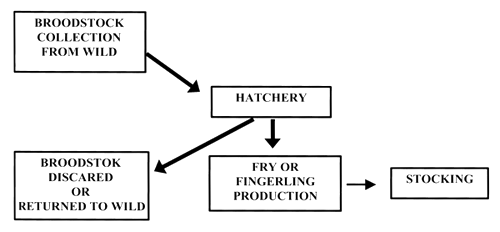
It is important in such a system to ensure that a representative sample of fish is captured from the wild population for broodstock. “Representative” means that there is no sampling bias or reduction in genetic variation in the sample compared to the wider population. In highly fecund species it might be possible to produce the target number of fry or fingerlings from relatively few parent fish. This may be sufficient to distort the patterns of genetic variation in the wild population if natural reproductive success is low, particularly if this exercise is repeated several times.
The justification for such a stocking practice is likely to be that a wild population has been significantly reduced through overfishing or habitat degradation. In severe cases it may actually be difficult to consistently obtain enough wild broodstock for the hatchery.
Figure 2 illustrates processes within a different type of hatchery, where fish obtained either directly from the wild or from another source are used to found the hatchery population but fish produced within the hatchery are used to replace broodstock in each generation. The bulk of the fry or fingerlings produced in each generation are removed from the hatchery for aquaculture or stocking purposes, while a proportion are retained to replace the current generation.
The initial hatchery broodstock are of great importance: depending on the objectives of the hatchery, these fish should come from an appropriate source and contain sufficient genetic variation to avoid creating an initial genetic bottleneck.
Figure 2. Movement of fish through a hatchery in which the broodstock are retained for several generations. 1, 2 and 3 illustrate actual or potential feedback loops involved in genetic changes to the broodstock population over time (see text further details).
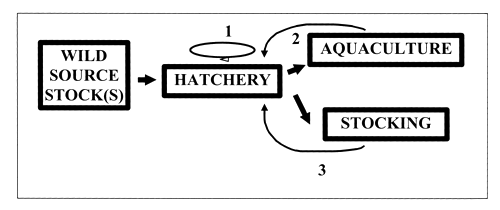
2.1 Inbreeding and unintentional selection
If little or no attention is paid to the genetic aspects of broodstock replacement in each generation within such a hatchery (feedback loop 1), inbreeding and genetic drift are likely.
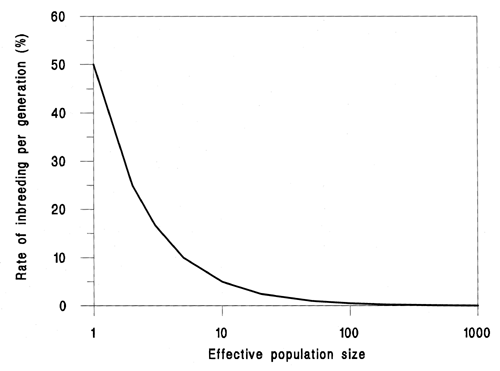
Inbreeding is the reduction of genetic variation within a population due to small population size, which generally results in negative effects on fitness-related traits such as growth rate, survival and disease resistance. The rate of inbreeding in such a closed population is determined by the management practices being used. If there is no control over the pedigree structure of the breeders then the rate of inbreeding will depend on the effective population size (Ne) of the broodstock. This will often be smaller than the actual number of broodstock held in the hatchery and depends principally on the number of fish actually contributing to the next generation, variation in the contribution to the next generation from each fish and the sex ratio of the broodstock. Occasional bottlenecks in effective population size have a disproportionate effect on the rate of inbreeding. Inbreeding is cumulative, i.e. it may increase but is impossible to reverse without the introduction of further genetic variation from outside. Genetic drift is the random change of gene frequencies due to small population size, which may result in fixation of deleterious alleles.
A degree of domestication selection, or genetic adaptation to the environment in the hatchery, is likely to occur. The extent of this will depend on factors such as the difference between the hatchery and the wild environment. Allied to this, some management practices may impose further unintentional selection: for example, if larger fingerlings are sold off and smaller ones retained for future broodstock, selection for reduced growth rate will occur.
Figure 3 illustrates the consequences of effective population size on the rate of inbreeding. Table 2 shows data from Kincaid (1983), who looked at the effects of various levels of inbreeding on a variety of traits in rainbow trout (Oncorhynchus mykiss). This was achieved by a series of planned crosses. Performance was compared for a number of traits against an outbred control. It can be seen that in nearly every trait there was a significant reduction in performance compared to the control in both hatchery and field conditions. In a hatchery context, it is likely that a small effective population size would lead to a gradual increase in the coefficient of inbreeding and its effects on various traits.
Table 2. The effects of inbreeding, produced by one or three generations of sib mating, on a variety of traits in rainbow trout (Oncorhynchus mykiss). Growth and survival traits were evaluated in the hatchery and during a twelve month field test in a put and take fishery (after Kincaid, 1983).
| TRAIT | Inbreeding coefficient and number of inbred-outbred half-sib family pairs | |||||
|---|---|---|---|---|---|---|
| F=25% (16 pairs) sib crosses, one generation | F=50%(13 pairs) sib crosses, three generations | |||||
| inbred mean | outbred mean | % depression* | inbred mean | outbred mean | % depression* | |
| Hatchery performance | ||||||
| Hatchability (%) | 69.1 | 83.5 | 17.2 | 84.2 | 84 | -0.2 |
| %Fry survival to 84days | 89.2 | 94.7 | 5.8 | 89.3 | 89.7 | 0.4 |
| Weight (g) 147 days | 3.4 | 3.4 | 0.0 | 3.0 | 3.6 | 16.0 |
| Feed conversion 147 | 2.3 | 2.2 | -6.7 | 2.1 | 2.0 | -5.0 |
| Weight (g) 364 days | 68.0 | 91.0 | 25.0 | 85.0 | 145.9 | 41.7 |
| Field performance | ||||||
| Weight (g) at planting | 28.6 | 31.4 | 8.8 | 36.4 | 35.1 | -3.6 |
| 6 month recovery % | 24.5 | 33.0 | 25.8 | 36.5 | 38.4 | 5.0 |
| 12 month recovery % | 7.2 | 7.2 | 0.0 | 5.9 | 7.1 | 16.9 |
| Mean weight (g) | 150.7 | 173.2 | 13.0 | 132.6 | 187.0 | 29.1 |
| Total % recovery | 31.7 | 40.2 | 21.2 | 42.4 | 45.5 | 6.9 |
| Biomass index# | 2858.8 | 3883.7 | 26.7 | 3162.1 | 4188.5 | 24.5 |
* % depression is calculated as outbred mean minus inbred mean divided by outbred mean.
# Biomass index is the total biomass recovered per 100 fish planted.
Figure 3. The consequences of different effective population sizes on the rate of inbreeding.
Two scenarios can be used to illustrate the possibilities for unintentional poor genetic management in hatcheries. In both cases the hatcheries are maintained over several generations without further introductions of broodstock from outside:
A hatchery for a large, highly fecund species such as catla (Catla catla), one of the major carps, is founded from a small number of fish obtained from another hatchery. Only the absolute minimum number of female breeders is maintained to cope with the demand for fry or fingerlings, and fewer males than females are kept since females are the limiting factor on egg supply. When broodstock need to be replaced, these are taken from the smaller fingerlings remaining from the last few batches when the bulk of the production has been sold from the hatchery. A mitigating factor for such a species is that the process of inbreeding might be slowed by the relatively long generation time: this could be about five years on average, assuming three years to firste maturation followed by three or four years of breeding. Eknath and Doyle (1990) estimated effective population sizes and rates of inbreeding for catla and other carps in Indian hatcheries, although they used a shorter estimate of generation time.
A hatchery for a smaller, less fecund species such as the Nile tilapia (Oreochromis niloticus) is founded, again from a small number of fish obtained from another hatchery. It is necessary to maintain a relatively large number of mature adults to cope with demand for fingerlings. Fry are harvested from breeding ponds by netting from the margins. Broodstock replacement is carried out by taking some of the largest fingerlings remaining in one of the breeding ponds. In fact, these are from the first few batches of fry produced in the pond and are larger because they are older than the others. The effective population size is likely to be very small as only a few families will be represented.
2.2 Selective breeding for aquaculture
Intentional selective breeding has been carried out to improve the per formance or properties of fish in aquaculture (e.g. Gjedrem, 1992; Gjerde, 1993; Eknath et al., 1993). Some traits of interest (e.g. red coloration in tilapias, scaling patterns in common carp) have fairly simple inheritance patterns and fish can easily be bred in the hatchery with the desired phenotypes (e.g. McAndrew et al., 1988). Others (e.g. growth rate, age at maturation) are affected by many genes (these are called polygenic traits) and are also strongly influenced by environmental factors. It is thus considered that it is generally more efficient to select the best performing fish in the intended aquaculture environment, rather than on the basis of performance in hatchery conditions which may differ in several respects. This may be done in a number of ways. Some hatcheries will be part of a farm where fish are grown to market size, in which case the selected fish can be taken from the production cycle. Tagging may be used to mark families which are then split, with a proportion being transferred to ongrowing sites while some are retained within the hatchery. Information from the performance of the marked fish during ongrowing can then be used as the basis of the selection of broodstock from the marked fish retained in the hatchery. Recently, microsatellite DNA loci have been used to identify fish to family groups as a valuable tool in selective breeding (O'Reilly and Wright, 1995).
Selective breeding can become more complicated if it is aimed at producing fish for diverse aquaculture environments: for example a variety of aquaculture systems such as cages, ponds or raceways, high or low input levels (feed or pond fertilisation) or environmental variation (rainfall, temperature, pH). Where some strains or genoytpes of a species perform better in certain conditions and others do better in another set of conditions (known as genotype-environment interaction), it may be necessary to select for strains for specific conditions.
This is in fact an extension of the argument for selecting on the basis of performance in aquaculture rather than performance in the hatchery: selection for performance in one type of environment may not guarantee good performance in another. In fact, the evidence is mixed. Some studies have suggested little or no genotype-environment interaction under normal aquaculture conditions, e.g. Norwegian salmon (Gjerde, 1993) and tilapia (Eknath et al., 1993) when wild fish were the source populations, while others have shown strong effects using different farmed stocks, e.g. European common carp versus Chinese common carp (Wohlfarth et al., 1983).
2.3 Selective breeding for stocking?
While it has been demonstrated for several species that selective breeding can be used to produce better performing fish for aquaculture, few breeding programmes have specifically set out to produce fish which have been improved for fisheries enhancement by selective breeding. It has been shown that the return rate of Atlantic salmon in ranching operations can be increased by selection (Jónasson, 1995).
It is not necessarily the case that fish which have been selected for faster growth or other traits in an aquaculture environment will show similar improvements when used in enhancement programmes. They will generally be placed into environments which have lower food levels than in aquaculture. Wohlfarth et al. (1983) found that while European common carp strains grew faster than Chinese strains in productive ponds, this difference was eliminated in less productive ponds. Domestication may also lead to altered behaviour: Einum and Fleming (1997) found that juvenile farmed Atlantic salmon were more aggressive and grew faster than wild fish but they showed reduced levels of predator avoidance. Hybrids between the wild and farmed strain appeared to be intermediate for these traits suggesting that interbreeding between farmed and wild stocks could change the characteristics of the wild population if there were sufficient introductions or escapes.
Obtaining information on performance of stocked fish for a selective breeding programme would be very difficult in most cases, due to the nature of many of the water bodies being stocked.
2.4 Choices of strains and production systems for stocking
From the above considerations, there are several options for stocking, including:
Offspring of wild fish from the same water body, produced in a single generation within a hatchery (Fig. 1).
“Zero effect” hatchery (breeding population maintained within a hatchery but with efforts made to minimise inbreeding, selection, etc.), using local wild stock as origin of hatchery stock.
“Zero effect” hatchery, with hatchery population originating from the wild but chosen from several tested to establish which is most productive in the water body(ies) being stocked.
“Aquaculture selected” stock.
“Enhancement selected” stock, developed from a base population specifically for enhancement purposes.
The above list is not exclusive, but planned management of the production of fry or fingerlings for fishery enhancement is likely to give better results than simply obtaining fish from whichever hatchery is capable of producing sufficient fry or fingerlings, whether the objectives are conservation or maximisation of food production.
3. MONITORING AND QUALITY CONTROL IN HATCHERY MANAGEMENT
Some aspects of hatchery management do not lend themselves easily to direct analysis, e.g. although inbreeding is frequently cited as a cause of poor performance it is very difficult to rapidly assess if a particular hatchery population is inbred. Assessment may require examination of hatchery records (which are frequently inadequate for this purpose), the use of molecular genetics techniques to compare the population with others, or comparative performance trials with other populations.
However, there are measures which can be taken to monitor hatchery management and/or to monitor or certify the products of hatcheries for specific characteristics of the fry or fingerlings produced. The latter are important to guarantee quality to the purchaser or end user of the fish from a hatchery. These measures include:
Improved record keeping. Most hatcheries keep some kind of records but these may be fairly limited and associated with production targets, e.g. number of fish produced per season, number of fish spawned. To monitor effective population size, other types of data need to be recorded, principally in relation to the replacement of broodstock, e.g. how many of the current generation contribute to the next generation.
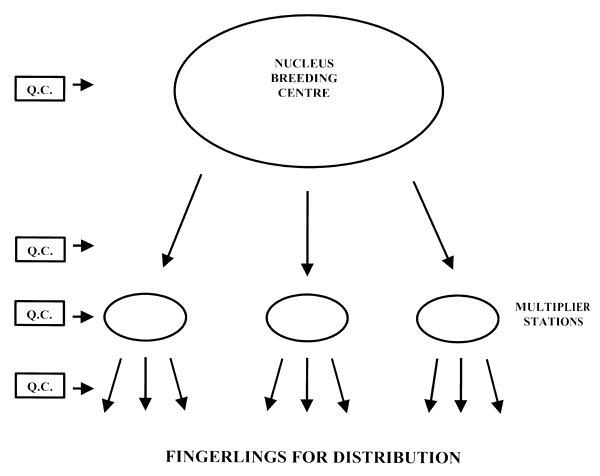
Reliable tagging techniques (e.g. PIT tags) offer the possibility of close monitoring of broodstock within hatcheries and where broodstock are transferred between hatcheries, e.g. in a large breeding programme with a nucleus breeding centre and multiplier stations (Fig. 4).
Monitoring of hybridisation where closely related species are kept in the same hatchery or where a closely related species exists in the surrounding waterways. Depending on the species and the situation hybridisation may occur in one generation only (F1 hybrids) or over several generations (introgression). Detection may be possible on the basis of morphological differences between the species in many cases but where fry are sold at a very small size or where the two species are very similar, species-specific genetic markers may be vital. The Nile tilapia (Oreochromis niloticus) is an important cultured species in S.E. Asia but feral populations of O. mossambicus also exist in these countries. Introgression between the two species has been detected using allozyme electrophoresis (Macaranas et al., 1995). In Bangladesh, introgression between the silver carp (Hypophthalmichthys molitrix) and the bighead carp (Hypophthalmichthys nobilis) has been cited as a problem (C. Price, pers. comm.). Electrophoresis of species-specific allozyme and/or DNA markers could be used to monitor this.
Triploid grass carp are stocked into waters in the USA for weed control. In some states, only triploid fish can be stocked to ensure that this species cannot breed following release (Thorgaard and Allen, 1987). Simple tests for triploidy can be used to guarantee sterility before stocking.
Monosex male tilapia offer advantages in aquaculture. These are generally produced by treatment of fry with 17α-methyltestosterone (McAndrew, 1993), or more recently by crossing YY males with XX females (Mair et al., 1995). The gonads of small fingerlings can be examined by a simple squash technique using a low power microscope (Guerrero and Shelton, 1974), offering an opportunity to determine the sex ratio in a sample of fish before sale or stocking.
Although historically many governments or international agencies have concentrated on development of techniques for seed production, with hatcheries given targets in terms of quantities of fry or fingerlings produced, most of the technical aspects of seed production have been solved for most important freshwater fish species. This should allow such agencies to focus efforts in relation to seed production on supporting and improving the quality of the seed produced. Both hatcheries and users (nurseries, fishers, etc.) should benefit from clearly focused genetic management of hatchery broodstock, with hatcheries receiving training, technical assistance, quality broodstock, etc. and users being assured of the quality of the fish received.
Figure 4. Diagrammatic illustration of the flow of fish through a breeding programme involving a nucleus breeding programme and multiplier stations supplying fish for stocking. Q.C. = quentity control (see text further details).
4. RECOMMENDATIONS
REFERENCES
Carvalho, G.R. and T.J. Pitcher. 1995. Molecular Genetics in Fisheries. Chapman and Hall, London. 139p.
Einum, S. and I.A. Fleming. 1997. Genetic divergence and interactions in the wild among native farmed and hybrid Atlantic salmon. J.Fish Biol. 50(3):463–690.
Eknath A.E. and R.W. Doyle. 1990. Effective population size and rate of inbreeding in aquaculture of Indian major carps. Aquaculture 85:293–305.
Eknath, A.E., M.M. Tayamen, M.S. Palada-de Vera, J.C. Danting, R.A. Reyes, E.E. Diosiso, J.B. Capili, H.L. Bolivar, T.A. Abella, A.V. Circa, H.B. Bentsen, B. Gjedre, T. Gjedem and R.S.V. Pullin. 1993. Genetic improvement of farmed tilapia: The growth performance of eight strains of Oreochromis niloticus tested in different farm environments. Aquaculture 111:171–188.
Ferguson, A and J.B. Taggart. 1991. Genetic differences among the sympatric brown trout (Salmo trutta) populations of Lough Melvin, Ireland. Biol. Jour. Linn. Soc. 43:221–237.
Gjedrem. T. 1992. Breeding plans for rainbow trout. Aquaculture. 57:77–80.
Gjerde, B. 1993. Breeding and selection. In: Salmon. Aquaculture. Fishing News Books. 278p.
Jónasson, J. 1995. Salmon ranching - possibilities for selective breeding. NORD 95:4. Nordic Council of Ministers, Copenhagen. 125.
Kincaid, H. 1983. Inbreeding in fish populations used for aquaculture. Aquaculture 33:215–227.
McAndrew, B.J. 1993. Sex control in tilapiines. Proceedings of the Satellite Symposium on Applications of Comparative Endocrinology to Fish Culture. Almenucar, Granada, Spain, May 22–23 1989. In: Recent Advances in Aquaculture vol. IV. Muir, J.F. and R.J. Roberts, eds.: Blackwell Scientific.
McAndrew, B.J., F.R. Roubal, R.J. Roberts, A.M. Bullock and I.M. McEwan. 1988. The genetics and histology of red, blond and associated colour variants in Oreochromis niloticus. Genetica 76:127–137.
Macaranas, J. M., N. Taniguchi, M.J.R. Pante, J.B. Capili and R.S.V. Pullin. 1986. Electrophoretic evidence for extensive hybrid gene introgression into commercial Oreochromis niloticus (L.) stocks in the Philippines. Aquaculture and Fisheries Management 17:249–258.
Mair G.C., J.S. Abucay, J.A. Beardmore and D.O.F. Skibinski. 1995. Growth performance trials of genetically male tilapia (GMT) derived from YY-males in Oreochromis niloticus L.: on station comparisons with mixed sex and sex-reversed male populations. Aquaculture 137:313–322.
O'Reilly, P. and J.M. Wright. 1995. The evolving technology of DNA fingerprinting and its application to fisheries and aquaculture. J. Fish Biol. 47(A):29–55.
Thorgaard G H. and S.K. Allen. 1987. Chromosome manipulation and makers in fishery management. In: Population Genetics and Fishery Management. Ryman, N. and Utter, F. (eds.): 319–332. University of Washington Press, Seattle.
Wohlfarth, G., R. Moav. and G. Hulata. 1983. A genotype-environment interaction for growth rate in the common carp, growing in intensively manured ponds. Aquaculture 33:187–195.