Through chromosome manipulations a new genetic status can be attributed to a given individual by altering or modifying its genome for better performance in its environment. These chromosome manipulations or chromosomal engineering techniques involve tampering with the original genomic structure of a given individual in a systematic manner and abiding by certain biological laws (Reddy, 1992). Chromosome engineering may involve addition of extra set(s) to the existing complement, resulting in triploid or tetraploid individuals, or replacement with a duplicate set of chromosomes from the same individual, the resultant products being either gynogens with maternal inheritance or androgens with paternal inheritance.
Gynogenesis is the process of embryonic development with solely the maternal genome and without paternal genetic input, a phenomenon similar to parthenogenesis. Gynogenesis occurs in nature and can be also induced.
Natural gynogenesis has been reported in some members of the family Poecilidae, e.g. Poecilia formosa and Cyprinidae e.g. Carassius auratus gibelio (Gold, 1979). The cross between certain species of Cyprinidae such as the Chinese grass carp and silver carp also resulted in gynogenetic offsprings (Andriyasheva, 1973, Mantelman, 1973, Reddy, 1989 and 1995).
Artificial induction of gynogenesis involves genetic inactivation or destruction of DNA content of milt by exposing it to either ultraviolet or gamma rays and activation of eggs with this genetically inactivated milt. However, these activated eggs develop into haploid embryos/hatchlings and do not survive unless diploidy is restored by subjecting the activated eggs to either thermal or pressure shocks at an appropriate stage after activation, just before the extrusion of the polar body (meiotic gynogenesis) or blocking the first mitotic division in the developing/activated egg (mitotic gynogenesis). The pace of development may differ from species to species and depends on the ambient temperature (Figure 6 & 7). Artificial gynogenesis has been successfully induced in many species of fish including carps (Golovinskaia, 1968; Purudom, 1969; Burlakov et al., 1973; Stanley et al., 1975; Chourrout D. 1980; John et al., 1984 and 1988; Wu et al., 1986; Reddy et al., 1993).
Meiotic gynogenesis was successfully induced in Indian major carps for the first time in L. rohita and C. catla in the year 1981 (John et al, 1984) and in Cirrhinus mrigala (John et al., 1988, later by Hussain et al., 1997). The procedure includes, genetic or DNA denaturalization or inactivation in the penetrating spermatozoa by exposure to either ultraviolet or gamma rays. For inactivating genetic component of the male gametes, ultraviolet irradiation is a simple device and very effective in fishes. To restore diploidy in the activated egg, either thermal stocks (heat or cold) or pressure shock are usually administered. However, the efficacy of these shocks, especially thermal shock, and their intensity and duration of treatment seems to vary from species to species. The effective intensity lies at some point around the sublethal levels. Thus in all the species of Indian carps, cold shocks below 10°C and heat shocks above 42°C proved to be lethal (John et al., 1984 & 1988 and Reddy et al. 1993). Effective genetic inactivation of sperm DNA was possible in Indian major carps by exposing the milt, diluted to 1:4 with cold Hank's solution, (milt 1 and Hank's 4 parts), to a 15w ultraviolet germicidal tube at a distance of 20 cm and irradiated for 17–20 min (John et al, 1984). The milt was poured into petri dishes fixed on ice blocks, keeping the column depth of milt to 2mm and placing the petri dish on a mechanical shaker for irradiation. It is preferable to use milt from a different species since improper irradiation can be detected by the presence of hybrids. In the case of Indian major carps meiotic gynogenes of rohu were produced by activating the eggs with the irradiated milt of catla and vice versa (John et al., 1984) and mrigal gynogens were produced by activating the eggs with the irradiated milt of common carp (John et al., 1988).
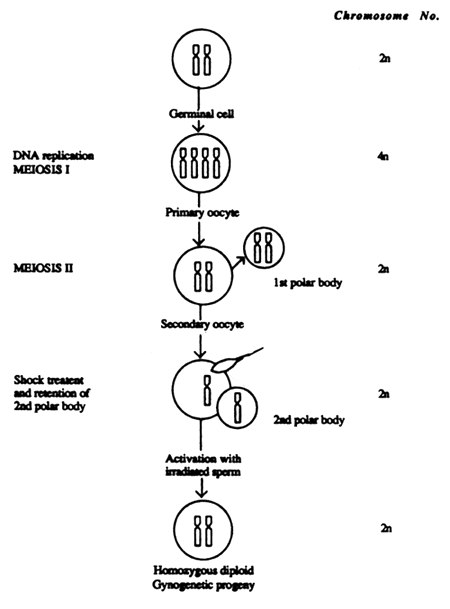
Figure 6. Illustration of events for inducing meiotic gynogenesis in Indian carps (Reddy et al., 1997)
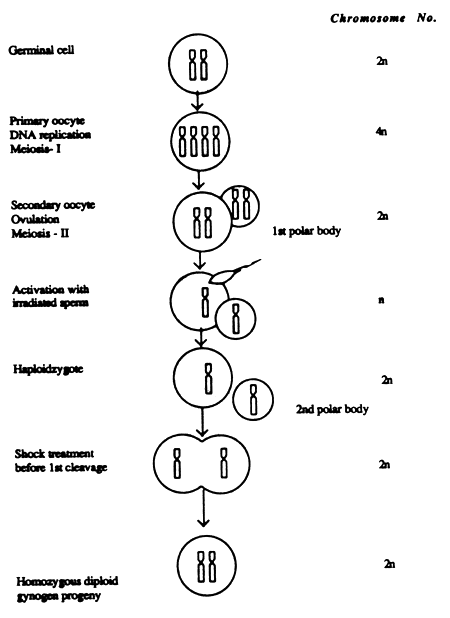
Figure 7. Illustration of events for inducing polyploidy (triploidy and tetraploidy) in Indian carps (Reddy et al., 1997)
The effective intensities of cold and heat shocks for inducing diploid meiotic gynogenesis in Indian major carps were 12°C for 10 min and 39°C for one to two minutes respectively. The appropriate time after activation for the administration of shock treatments to induce meiotic gynogenesis (retention of polar body) in the activated egg of Indian carps was reported to be 4min after activation. Heat-shock treatment is shown in Plate 8. The percentage yield of meiotic gynogens ranged from 12.6 to 18.1% (John et al., 1984). In catla, heat-shocks were more effective than cold-shocks while the reverse was true in the case of rohu (John et al., 1984).
The first report on successful induction of mitotic gynogenesis in rohu was that of Reddy et al., 1993, followed by Hussai, et al., 1994. Reddy et al., 1993 used irradiated milt of common carp, Catla catla and also Cirrhinus mrigala, to activate eggs of rohu (Labeo rohita) to produce mitotic gynogen rohu. Irradiated milt of Chinese grass carp (Ctenopharyngodon idella) was also used by Reddy et al., 1997 (unpublished) to induce mitotic gynogenesis in rohu, catla, mrigal and kalbasu. Use of milt from heterologous species, preferably having low compatibility to hybridize may be more desirable for the easy elimination of paternal genetic input with irradiation. Therefore, the milt from Chinese carps was used, as they are incompatible to produce viable hybrids with Indian carps.
While inducing mitotic gynogenesis in rohu, Reddy et al. (1993) followed the same cold shock regime as did John et al, (1984) for meiotic gynogenesis. However, the heat shock regime was raised to 40°C keeping the duration of exposure the same in both shock treatments. Mitotic gynogen rohu and, diploid and haploid hatchlings are shown in Plates 9 and 10 respectively.

Plate 8. Heat-shock treatment to activated/fertilized eggs of Indian major carps to induce diploid gynogenesis/polyploidy respectively

Plate 9. Mitotic gynogen rohu (L. rohita)
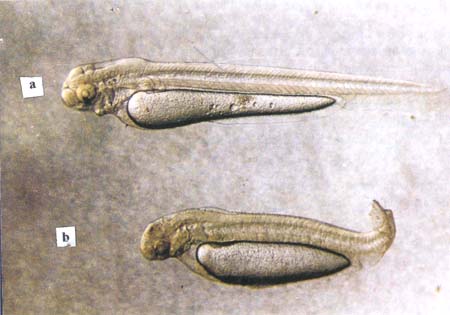
Plate 10. a. Diploid (normal (embryo)) b. haploid embryo (developed from an egg, activated by a genetically inactivated/denaturated sperm)
The yield of mitotic gynogens as reported by Reddy et al. (1993) was 15–30% up to fry stage and 0.66–10% up to fingerling stage with heat shocks and 30 and 4.3% up to fry and fingerling stages, respectively with cold shock treatments. The average survival rates ranged from 1.13% with heat shocks to 4.3% with cold shocks. Regarding the response of gynogens to treatment, John et al. (1984) and Reddy et al. (1993) showed that rohu responds better to cold shocks than heat-shocks, while inducing mitotic gynogenesis, as mentioned earlier, cold shocks yielded higher percentage of gynogens (4.3%) as compared to heat shocks (1.13%). However, the yield of meiotic gynogens in Indian carps was observed to be higher than that of mitotic gynogens as observed by John et al., 1984 and Reddy et al., 1993, respectively.
This trend appears similar in other species also. Purdom et al., 1985 reported 1–7% survival rates in mitotic gynogen rainbow trout. The low yields in mitotic gynogenesis are attributed to the probable expression of deleterious or lethal genes in the highly homozygous state. Variations in the quality and performance of eggs from different females or from different batches of eggs of the same female have been also reported (Mair et al, 1986). However, the experience of different workers indicates that late shock treatments (mitotic gynogenesis) usually results in lower yields than those of early shocks (meiotic gynogenesis).
The qualitative difference between these two types of gynogenesis is that, it requires about 4–5 generations through meiotic gynogenesis to achieve complete homozygosity (Nagy et al., 1984), whereas, the same result can be achieved with one generation by mitotic gynogenesis, as blocking of first mitotic division results in retention of two identical replicated mitotic products (Mair et al., 1986).
Whether meiotic or mitotic, gynogenesis is an effective tool for producing inbred lines of fish in a much shorter time when compared to the conventional method of sib-mating.
The aim of developing inbred homozygous lines in Indian major carps especially L. rohita is to test the heterosis effect that may result by intraspecific hybridization. Nagy et al. (1984) reported heterosis effect in gynogen hybrids.
Another use of gynogens is to produce gynogenetic male lines through sex reversal of the gynogen females and use these functional males to produce mono sex (female) populations. Females of Indian carps grow larger than their male counterparts in a given time which may contribute to higher production.
The progeny resulting from gynogenesis will be usually all females where female homogamety exists. In the case where the females are heterogamous then the progeny should be 50% females and 50% males. A comparative account of the process of inducing meiotic and mitotic gynogenesis in Indian carps and the resultant products is given in Table 9.
Among the members of the family pleuronectidae, gynogenesis (haploid) occurs when plaice or flounder eggs are fertilized by milt from halibut. Diploidy can be induced by the application of cold shock to those haploid eggs (Purdom, 1983).
Table 9. Details of induced gynogenesis of carps at CIFA
| Species | Gynogenesis | Shock treatment C.S. = cold shock H.S. = heat shock | Results | |||
|---|---|---|---|---|---|---|
| Nature | Intensity | Duration | ||||
| C.catla | Meiotic | Mitotic | C.S. | 12°C | 10 min | Diploid gynogenesis |
| C.catla | Meiotic | Mitotic | H.S. | 39°C | 1 min | Diploid gynogenesis |
| L.rohita | Meiotic | Mitotic | C.S. | 12°C | 10 min | Diploid gynogenesis |
| L.rohita | Meiotic | Mitotic | H.S. | 39°C | 1 min | Diploid gynogenesis |
| C.mrigala | Meiotic | Mitotic | C.S. | 12°C | 10 min | Diploid gynogenesis |
| C.mrigala | Meiotic | Mitotic | H.S. | 39°C | 1 min | Diploid gynogenesis |
| L.calbasu | Meiotic | Mitotic | H.S. | 40°C | 1 min | Diploid gynogenesis |
| L.calbasu | Meiotic | Mitotic | C.S. | 12°C | 10 min | Diploid gynogenesis |
N.B. Shock treatments were to 4 min Id inseminated eggs in all the cases.
Androgenesis, results in progeny with only paternal inheritance. Like gynogenesis, androgenesis also occurs in nature and can be induced, though not as efficiently or easily as inducing the former.
Natural or spontaneous androgenesis has been observed in certain hybrid crosses produced either between remotely related individuals or those with disproportionate or not so compatible genomes as in the cross between Cyprinus carpio female and grass carp male or A. nobilis x Cyprinus carpio, though, with a low incidence. Indian carps being highly compatible, among themselves, no such instances were ever reported in any of the hybrid crosses.
The process of inducing androgenesis involves the genetic inactivation of an egg and its fertilization with normal sperm of the corresponding species. The egg however, may develop into a haploid embryo and either thermal or pressure shocks have to be administered to restore diploidy as is done in the case of artificial diploid gynogenesis. The mechanism of restoring diploidy in androgenesis is quite different from that of gynogenesis. The process probably involves dispermy or other mechanisms.
Attempts of inducing androgenesis in Indian major carps have been made earlier, but were unsuccessful mainly due to the difficulties in the effective irradiation of the egg. The egg consists of a huge mass of cytoplasm and yolk that prevent the proper exposure of the chromosomes to UV rays in the nucleus which especially in carps, is oriented towards one side of the cytoplasm and usually facing downwards, covered by the cytoplasm and yolk. UV irradiation of eggs may need a longer duration which may be lethal. Irradiation under gamma rays may be effective in genetic denaturing of eggs in carps due to stronger intensity and lesser duration of exposure.
An increase in the level of ploidy of an individual by the addition of one or more set(s) of chromosomes refers to polyploidy resulting usually either in triploidy or tetraploidy. Sometimes it may also result in penta or hexaploidy. Figure 8.
The phenomenon of polyploidy has been observed to take place in nature as in the case of gynogenesis and androgenesis. It can be also induced artificially.
Polyploidy has been observed to occur in nature in some species of fish like the common carp (Cyprinus carpio) and trout mainly due to chromosomal translocation and when two distantly related fish species are crossed. The crosses between grass carp and bighead carp had produced triploid hybrids (Marian and Krasznai, 1978). However, none of the interspecific nor intergeneric hybrid crosses among Indian carps have been reported to produce such allotriploids.
Some preliminary attempts have been made to induce artificial induction of triploidy and tetraploidy in Indian major carps with varied degrees of success (Reddy et al., 1987; Reddy et al., 1990).
Reddy et al. (1987), made the first attempt of inducing polyploidy in one of the Indian major carps, Labeo rohita by using colchicine. They could induce only tetraploid and diploid mosaics. However, Reddy et al. (1990a) successfully induced triploidy in rohu, L.rohita, and tetraploidy in L.rohita and Catla catla by using thermal shocks. Similarly successful induction of tetraploidy was also reported in the case of Cirrhinus mrigala and L.rohita (Zhang, 1990). Triploidy was induced in rohu by administering heat shocks to zygotes, seven minutes after fertilization at 42C +0.5 C for a duration of 1–2 minutes. However, the incidence of triploidy was only 12% (Reddy et al., 1990). Most of the eggs died probably due to lethal temperature level. These authors also reported that rohu zygotes exposed to heat shock, prior to first cleavage at 39C+0.5C for two minutes yielded 70% tetraploids and those exposed to cold shocks of 10–15C for 10 min. yielded only 30–55% tetraploids. In the case of catla, heat shocks to zygotes at 40C exposed for two minutes prior to first cleavage yielded 30–65% tetraploids, but cold shocks were not effective.
Zhang (1990) induced tetraploidy in C.mrigala for the first time and also in L.rohita. He reported that heat shocks administered at 39–40C for two minutes in embroys 22–25 min post-fertilization could induce tetraploidy in mrigal and rohu. However, the percentage of polyploids ranged from only 10–40. Islam et al. (1994) reported induction of triploidy in L. rohita by applying heat shock at 40C for 2 min starting 4 min after fertilization. The rate of triploidy induction was found to be 60%. The survival in the heat shock groups was recorded 15% and 25% in the control groups. The growth rate of triploid individuals was found to be significantly higher than in diploids for weight and length, P<0.01 and P<0.05 respectively. Details of induced triploidy/tetraploidy in major carps are given in Table 10.
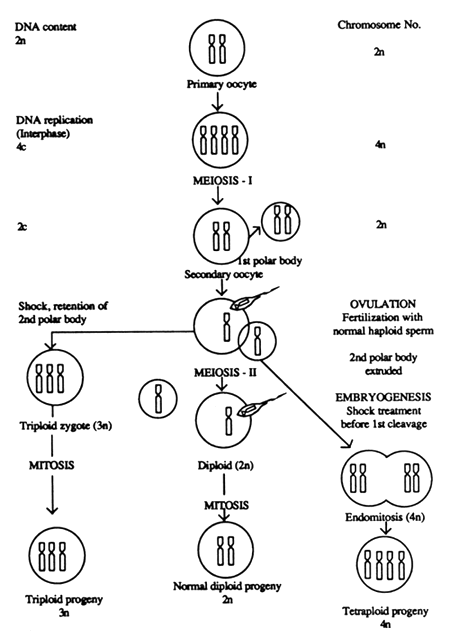
Figure 8. Illustrations of events for inducing polyploidy (triploidy & tetraploidy) in Indian carps (Reddy et al., 1997)
Table 10. Details of induced triploidy/tetraploidy in major carps at CIFA
| Species | Ploidy | Shock treatment | Percentage of tetraploids & triploids obtained | |||
|---|---|---|---|---|---|---|
| Nature | Intensity | Duration | ||||
| C.catla | - | Tretraploid | Heat shock | 40°C | 2 min | 30–55% |
| L.rohita | Triploid | Heat shock | 42°C | 1–2 min | 12% | |
| L.rohita | Tretraploid | Heat shock | 30°C | 2 min | 70% | |
| C.mrigala | Tretraploid | Heat shock | 39–40°C | 2 min | 10–40% | |
Shock treatments were administered to 4 min old inseminated eggs in all cases, except to induce triploidy in rohu where the shock was administered to 7 min old inseminated eggs.
In some species, triploids were observed to grow faster than the normal diploids (Kraznai and Marian, 1986; Wu et al., 1986 and Reddy et al., 1998 in press). Triploidy is also expected to cause sterility due to the reduced or suppressed development of the gonads. Sterile fish can be put to varied use in aquaculture depending on the species. Sterility in species like Cyprinus carpio and tilapia may help in controlling unwanted reproduction. In species like Chinese grass carp if made sterile, it can be used effectively to control weeds in the irrigation canal networks, lakes and other open waters without any fear of its establishment in such waters. Fertile individuals may establish by propagation which very often may be undesirable and sometimes even detrimental to the indigenous fish fauna.
However, observations of some workers have indicated that the sterile nature of a triploid fish depends on its ploidy status, in the sense that if an individual receives three sets of chromosomes, i.e. In from the egg proper, one In from the sperm and another In from the polar body of the egg in the complete form then it may be fertile. But where the ploidy consists of an uneven, number of chromosomes, in other words loss or addition of one or more chromosomes, leading to an aneuploid state of the genome, such individuals usually become sterile (Wu et al., 1986 and Reddy et al., 1990).
Coming to the utility aspects of ploidy manipulations and sterility, in the case of Indian major carps, sterility aiming at faster growth, related to enhancement of production may not be profitable. Males of Indian major carps mature after one year of age while females when approaching two years. By this time the fish farmer starts harvesting the fish for selling because, rearing beyond this period may not be profitable. Growth differences among sterile and fertile fish usually show up only during or after maturity.
Sterility showing faster growth may be useful in species having shorter maturity cycle. However, there are also reports that triploid sterile fish did not show better growth in species like Cyprinus carpio and tilapia (Cherfas et al., 1994; Hussain et al., 1995). Contrary to this are reports Cyprinus carpio that triploid sterile individuals have shown better growth than their diploid counter parts. (Wu et al., 1986, Malison et al., 1993 and Reddy et al., 1998).
The current research in the area of chromosomal engineering in Indian major carps in India is to produce allotriploids of carp hybrids and evaluate them for resistance to parasite infection. Two of the Indian carps, Catla catla and Labeo calbasu are very resistant to parasite infections, especially the most commonly noticed ectoparasite Argulus sp. Labeo rohita, followed by Cirrhinus mrigala, are usually the first victims to be attacked by the parasite and also other diseases like the Epizootic Ulcerative Syndrome (EUS). Thus C.catla and L.calbasu are gifted. Again, between catla and kalbasu, the latter is more hardy. Kalbasu does not easily develop bruises due to frequent or excessive handling during controlled spawning activities, while catla to some extent develops them though not as severe as in L.rohita. Bruises thus developed may form easy site for secondary infection.
Because of the above mentioned traits of catla and kalbasu, it is planned to produce allotriploids with these carp species by developing hybrids with males of the weaker species like the rohu and increasing the genome input from the maternal species with better resistance and then study the nature of the allotriploids. Moreover, Mishra (1991) reported that the rohu-catla and the reciprocal hybrids are generally prone to parasite infection, unlike catla. This may be due to the reduced genomic content of catla in the hybrid.
Since the hybrids of rohu and catla are reported to have better traits (Chaudhuri, 1973, Bhowmick et al., 1981 and Konda Reddy and Verghese 1980 a, 1980 b), it is worthwhile to develop a resistant variety of these hybrids through allotriploidization. The hybrids between L. rohita and L. calbasu may also be similar. However, the incidence of parasitic infection is relatively less in these hybrids when compared to the former hybrids. i.e. the rohu-catla and the reciprocal hybrids.
Gene transfer or genetic engineering in Indian major carps was first initiated by introducing growth hormone gene in the embryos of L.rohita (Alok et al., 1995). This is the earliest work initiated on Indian major carp. These workers microinjected about 150,000 to 200,000 copies of a fusion gene containing marine metallothionine promoter (Mu MT) and the coding sequence of human growth hormone (hGH) into the fertilized cone of the embryos of L. rohita.
In total, about 434 embryos were microinjected, of which only 50 embryos survived. Ultimately, only 5 of these 50 embryos survived up to 90 days. Analysis of DNA from gill tissues of these surviving embryos has shown that only two of them contained transgene in their genome, carrying 1–2 copies of the human growth hormone gene in their genome. However, these results indicate that it is possible to introduce foreign DNA into the embryos of L. rohita (Alok et al., 1995).
Reddy et al., 1991 worked out some techniques to prolong the developmental events (cleavage) during the embryonic developments in major carps in order to facilitate gene transfer. Depending on the ambient temperature (28–30°C), one celled stage (formation of blastodisc) appears in the fertilized eggs of major carps, 20–25 min after fertilization/ insemination and lasts only for 8–10 min. after which the first cleavage commences (2-celled stage).
Reddy et al. (1991) developed techniques for facilitating the implantation of foreign gene (DNA) directly into fertilized carp egg through microinjection. The usual time of development for the formation of one celled stage in the fertilized eggs could be prolonged for 20–30 min by incubating the eggs in water 20C rather than the normal tap or pond water at 29–30C.
Secondly, by keeping the female and male gametes separately in cold Hank's solution with proper dilution (gametes to Hank's solution 1:4, John et al., 1984), it was possible to maintain the gametes viable for 2–3 hrs under refrigeration at 7–8° C or lower temperature than normal. This method allows researchers to freshly fertilize the eggs in batches at half an hour intervals, so that when one batch is injected, another batch can be made ready. Yet another method suggested was to inject female brood fish at different time intervals, so that when the fertilized eggs of one fish are injected, another fish will be ready to ovulate (Reddy et al., 1991).
At present some more laboratories in India, such as the Centre for Cellular and Molecular Biology (CCMB), and Madurai Kamaraj University are engaged in genetic engineering studies, particularly in fishes including major carps. Isolation of growth hormone genes in L. rohita and C. catla has been also successfully done (Majumdar, 1997, Personal communication).