by
T. Homb, F. Sundstol and J. Arnason
Agricultural University of Norway,
Box 25, 1432 As - NLH, Norway
| Summary | Résumé |
| The review starts with the early work in Germany which led to the formulation of the Beckmann method during World War I. Fingerling et al. found, by using this method, a considerable increase in digestibility of the organic matter when increasing amounts of NaOH were used. On the basis of their, and other, experiments a treatment with a 1.5% NaOH solution for at least 12 hours at room temperature was recommended. The technique used at that time was, however, extremely labour-intensive. This aspect was improved in Norway during World War II, where the Beckmann method has since been practised by some 10 000 farmers. Besides digestion studies showing increased figures from 42 to 68% due to the treatment, group feeding trials with dairy cows were carried out. On the basis of these an energy value of 0.73 FU/kg DM was calculated. The normal ration for milking cows included 2 FU of treated straw. Because this method can cause river pollution, much work was done to develop methods with less environmental effects. A modification being developed in Sweden includes treatment in a closed system with neutralization of the lye by phosphoric acid, without washing. Different types of dry treatment have recently been tested in vitro and to some degree in digestion studies with animals. Until now only very few of these have reached a commercial or farm level. A general feature is that in vitro digestibility can be increased by adding increasing amounts of NaOH to the straw. Several studies have disclosed that at high NaOH levels the response obtained in in vivo digestibility is less than in in vitro. In Denmark two methods of dry treatment have been developed, one of which has reached commercial level. Chopped straw is treated with NaOH in a reaction chamber and afterwards pressed into cobs or pellets in a ring dye press. A three-year pilot operation was followed in 1975 by a full-scale plant with a yearly capacity of 10 000 tons. For improving the palatability of the product it has been recommended to add some concentrates to the treated straw. Pelleted mixtures of concentrates and straw have been consumed at daily levels of 9 to 11 kg by young bulls and 20 kg by dairy cows. NH3 treatment of dry straw has reached a commercial level in Norway. Several years extensive studies have led to the recommendation to use 30 kg NH3 per ton of straw combined with six weeks reaction time. Compacted bales of straw are wrapped in a polyethylene sheet, NH3 gas is injected, and the wrapping is sealed. Daily intake in feeding trials varied from 3.5 to 6 kg for young bulls and heifers. Rations for high-yielding cows contained 5.2 kg DM of grass silage, 3 kg DM of NH3-treated straw, and concentrates according to yield. Treatment of at least 10 000 tons of straw by this method is expected in 1976. | Le document examine tout d'abord les premiers travoux qui, pendant la première Guerre mondiale, ont abouti à la mise au point de la méthode Beckmann en Allemagne. Fingerling et al. ont constaté, en utilisant cette méthode, un accroissement important de la digestibilité de la matière organique lorsque la quantité de NaOH était augmentée. Sur la base de leurs expériences et de celles d'autres chercheurs, on a recommandé un traitement par une solution de 1,5 pour cent de NaOH pendant au moins 12 heures à la température ambiante. Toutefois, la tech nique employée à cette époque était extrèmement ardue. Des améliorations ont été apportées au procédé pendant la deuxième Guerre mondiale en Norvége, où la méthode Beckmann est suivie depuis lors par quelque 10 000 exploitants. Outre des études indiquant un accroissement des taux de digestibilité de 42 à 68 pour cent à la suite du traitement, on a soumis des vaches laitiéres à des essais d'alimentation collective. Sur la base des résultats obtenus, on a calculé que la valeur énergétique atteignait 0,73 unité fourragère par kilo de matiére sèche. La ration normale pour les vaches laitiéres comportait 2 unités fourragères de paille traitée. Etant donné que ce procédé peut polluer les cours d'eau, on s'est efforcé de mettre au point des méthodes moins dommageables à l'environnement. Selon une variante élaborée en Suède, le traitement est effectué en système clos, la lessive étant neutralisée à l'acide phosphorique, sans lavage. Divers types de traitement à sec ont récemment été mis à l'épreuve lors de travaux de laboratoire et, jusqu'à un certain point, au cours d'études sur la digestion portant sur des animaux. Jusqu'à présent, quelques-uns seulement de ces procédés ont atteint le niveau commercial ou celui de l'exploitation. Leur trait commun est que la digestibilité in vitro peut étre accrue par l'adjonction à la paille de quantités croissantes de NaOH. Plusieurs études ont révélé qu' à de fortes concentrations de NaOH on obtient des niveaux de digestibilité in vivo inférieurs à ceux obtenus in vitro. Au Danemark, on a mis au point deux méthodes de traitement à sec, dont l'une est parvenue au stade de l'exploitation commerciale. La paille broyée est traitée par NaOH dans une chambre de réaction, puis pressée en pelotes ou boulettes dans une presse à disques. Une opération pilote de 3 ans a été suivie, en 1975, d'une exploitation de pleine échelle dotée d'une capacité annuelle de 10 000 tonnes. Pour améliorer les qualités gustatives du produit, il est recommandé d'ajouter quelques concentrés à la paille traitée. Des mélanges de concentrés et de paille en boulettes ont été consommés par des taurillons et des vaches laitières aux taux quotidiens respectifs de 9–11 kg et de 20 kg. Le traitement de la paille sèche à l'ammoniac a atteint un niveau d'exploitation commerciale en Norvège. Après plusieurs années d'études poussées, il a été recommandé d'utiliser 30 kg NH3 par tonne de paille avec un temps de réaction de 6 semaines. Des balles de paille fortement pressées sont enveloppées dans une feuille de polyéthyléne, et l'emballage est scellé après injection de NH3 sous forme gazeuse. La ration quotidienne distribuée lors des essais d'alimentation de taurillons et de génises variait de 3,5 à 6 kg. Les rations destinées aux fortes laitières contenaient 5,2 kg (matière sèche) d'ensilage d'herbe, 3 kg (matière sèche) de paille traitée par NH3, et une quantité de concentrés correspondant au rendement. On escompte qu'en 1976 10 000 tonnes de paille au minimum seront traitées par cette méthode. |
Resumen
Esta reseña comienza con las primeras investigaciones realizadas en Alemania en la primera guerra mundial, las que dieron origen al método de Beckmann. Empleando este método se observó que aumentó mucho la digestibilidad de la materia orgánica al aumentar la proporción de NaOH. En base a éstos y a otros experimentos, se recomendó tratar la paja con solución de NaOH al 1,5 por ciento 12 horas por lo menos, a la temperatura ambiente, pero la técnica empleada en ese entonces era demasiado laboriosa. En la segunda guerra mundial se eliminó este inconveniente en Noruega, país donde unos 10 000 agricultores aplican actualmente el método de Beckmann. Además de los estudios de digestión, que revelaron un aumento de 42 a 68 por ciento d'ebido al tratamiento, se hicieron experimentos de alimentación de grupos de vacas lecheras. En base a estos experimentos se calculó un valor energético de 0,73 unidades alimenticias por kilogramo de materia seca. La ración normal de las vacas lecheras contenía dos unidades alimenticias de paja tratada.
Como este método puede ser causa de polución de las aguas, se han hecho muchas investigaciones para encontrar métodos que produzcan poco o ningún efecto ambiental negativo. Una modificación que se está perfeccionando en Suecia contempla el tratamiento en circuito cerrado y la neutralización de la lejía con ácido fosfórico, sin ningún lavado.
Diferentes tipos de tratamiento en seco se han ensayado últimamente en investigaciones de laboratorio y en algunos estudios de digestión realizados con animales. Hasta la fecha son muy pocos los tratamientos que se aplican a nivel comercial o de explotación agrícola. En general, éstos se caracterizan porque permiten aumentar la digestibilidad “in vitro” aumentando la cantidad de NaOH que se le agrega a la paja. Varios estudios han revelado que con altos niveles de NaOH, la respuesta obtenida en digestibilidad es menor “in vivo” que “in vitro”.
En Dinamarca se han elaborado dos métodos de tratamiento en seco uno de los cuales ha alcanzado nivel comercial. La paja picada se trata con NaOH en una cámara de reacción y después se le da forma de barritas o de pellas en una prensa anular. Después de una etapa piloto de tres anos, en 1975 se instaló una fábrica de 10 000 toneladas anuales de capacidad. Para que el producto sea más apetitoso se recomienda agregarle a la paja tratada algunos concentrados. Novillos y vacas lecheras han consumido 9–11 y 20 kg diarios respectivamente de pellas de una mezcla de concentrado y paja (40:60).
El tratamiento de la paja seca con NH3 ha alcanzado nivel comercial en Noruega. Basándose en estudios extensivos de varios años de duración, se recomienda emplear 30 kg de NH3 por tonelada de paja con un plazo de reacción de seis semanas. Se envuelven en una lámina de polietilino fardos de paja bien prensados, se les inyecta NH3 gaseoso y se sella el envoltorio. En los ensayos de alimentación de novillos y vaquillas la ingesta diaria fue de 3,5 a 6 kg. Las raciones de las vacas de alto rendimiento contenian 5,2 kg de ensilaje de gramíneas (materia seca), 3 kg de paja tratada con NH3 (materia seca) y concentrados según el rendimiento. En 1976 se piensa someter a este tratamiento por lo menos 10 000 toneladas de paja.
Historical
To our knowledge the first attempts to improve the feeding value of straw by using chemical methods took place in Germany before 1900. Lehmann (1895) bolled oat straw in a 2% aqueous solution of NaOH and obtained an increase in dry matter digestibility from 37 to 63%. For wheat hulls a similar improvement in digestibility occurred. In a later paper Lehmann (1904) described another method which includes boiling under pressure (5–6 atm) for 6–8 hours. He attempted to feed the treated straw with or without washing out the lye. By varying the amounts of NaOH, water, and pressure he observed digestion coefficients from 54 to 71, while untreated straw was digested at 31–42%.
Although several suggestions on the feeding value of cellulose occurred early, for instance by Lehmann (1891), the scientific background for the high value of purified cellulose was published in 1900 by Kellner and Köhler. They demonstrated that rye straw, when fed to steers after a thorough treatment in a paper mill, showed very high digestibility figures, i.e. 95.8 for crude fibre and 88.3 for organic matter. Kellner (1905) also arrived at the significant conclusion that the net energy values of digestible cellulose and of digestible starch were of the same magnitude. These data, which have long been considered classical, were the first valid evidence of the ruminants' high ability to utilize cellulose when the indigestible matter normally present with cellulose has been removed.
The Oexmann method may be considered a follow-up to Keliner's results. Straw was boiled in paper mills, dried, and mixed with molasses (Schneldewind, 1919; von der Heide et al., 1916).
However, extraction of more or less pure cellulose from straw is expensive and produces much waste material. During the years up to about 1920, a number of modifications of the Lehmann method occurred in Germany. Some of them applied heating by use of steam: Colsman's method (Deutsche Landw, Presse, 43, 1916, 770–771) and the Dahlem method (Fingerling, 1924).
During the same period other types of chemicals were also tried, as a rule with less effect than NaOH; Na2CO3 (Honcamp and Baumann, 1921); CaOH (Ellenberger and Waentig, 1919; Honcamp and Pommer, 1922); HCI (Honcamp and Blanck, 1919). Also mentioned are copper oxide ammonia, chlorine and ammonia (cit. Homb, 1948).
All the modifications of the Lehmann method were too costly and laborious to be widely used in practice. For the work to follow, however, all these trials, and errors, were vital.
Beckmann's method
Beckmann introduced NaOH treatment at room temperature and recommended originally the use of a 1.5–2% concentration for 3 days (Deutsche Landw, Presse, 46, 1919, 12–13; Fingerling, 1924). Fingerling and Schmidt (1919) and Fingerling et al. (1923) carried out outstanding series of digestion studies and concluded that straw treated by this method could compete with most of the abovementioned boiling methods. The results of their experiments, with varying amounts of NaOH, are given in Table 3.1.
| Treatment | Digestion coefficient |
| Untreated | 45.7 |
| 2 kg NaOH/100 kg straw | 46.3 |
| 4 kg NaOH/100 kg straw | 50.2 |
| 6 kg NaOH/100 kg straw | 61.1 |
| 8 kg NaOH/100 kg straw | 66.1 |
| 10 kg NaOH/100 kg straw | 66.2 |
| 12 kg NaOH/100 kg straw | 71.2 |
Source: Fingerling et al., 1923
The authors concluded that 12 kg NaOH per 100 kg straw should be the recommended level of treatment. With the amounts of water supplied this was equivalent to a 1.5% solution.
In the same way, increasing treatment time, from 1 1/2 to 3 days, was tested. Though a small increase also occurred after 12 h, this period of time was recommended for practice (Fingerling and Schmidt, 1919).
The apparatus involved in Beckmann's system consisted of two asphalt-sealed wooden barrels, one containing the NaOH solution, while the washing process took place in the other. The straw had to be moved manually from one barrel to the other. No statistics are available concerning the amount of straw treated by this method during and after World War I. Our impression, gained from reading contemporary German Journals, is that the labour involved represented a limiting factor. During World War II treated straw was reintroduced in Germany, as an emergency feed. The recommended procedure was to build several low wooden barrels, placed in a system allowing the waste lye to flow from one to another for reuse (Mitt. f. d. Landw. 58, 1943, 851–853). Ehrenberg (1944) recommended feeding relatively high amounts of Beckmann-treated straw, for instance 10 to 30 kg per cow per day. Information of what actually was used in Germany during these years is lacking.
Outside Germany the Beckmann method has been tested in Switzerland (Thomann, 1921) and in Great Britain (Watson, 1941); Slade et al., 1939). In Jealotts Hill, England, digestion studies verified the German results, with about 70% digestibility of organic matter. The equipment used was two concrete basins. The lye treatment took place in the lower basin; the straw was then placed on a platform between the two basins to drain, after which it was moved to the upper basin for washing.
In Norway digestion experiments with Beckmann-treated straw was initiated in 1940, and the results given in Table 3.2 agree to a satisfactory degree with Fingerling's data.
| Untreated straw | Beckmann-treated straw | ||
| Chopped straw | Unchopped straw | ||
| Number of trials | 7 | 10 | 5 |
| Organic matter, % | 42 | 68 | 66 |
| N-free extracts, % | 37 | 61 | 62 |
| Crude fibre, % | 51 | 79 | 76 |
| Digested N/kg DM, g | 0.5 | -1.4 | -1.7 |
Source: Homb 1948, 1956
While the digestion experiments were carried out with wethers, three group feeding experiments with cattle were made during the years 1942–44, yielding energy values calculated at 0.73 feeding units (FU) per kg DM, or about the same as for good-quality silage. Using Kellner's method for net energy calculations, this would equal a value of 79 (Homb, 1948). Assuming a dry matter percentage of 18, about 8 kg of wet treated straw would be equivalent to 1 FU (1 kg of barley). The cows consumed 15 to 16 kg of treated straw per day.
In Norway, also, treated straw was initiated as an emergency feed. The farmers' experience was very good, and they insisted on its continuation after World War II. From the beginning the straw was treated on the farm. Most farmers however had an insufficient water supply, and from the 1950s about 90 000 tons of dry straw, equivalent to 45 mill. FU, were treated by year, half on the farms and half at cooperative establishments; in 1969, 10 600 farmers used treated straw for feed. For various reasons (pollution, etc.) the amount of treated straw produced by the Beckmann method has declined in recent years.
There may be several reasons why this method was more widely used in Norway than in other countries. A fortunate improvement of the technique should be mentioned first. The arrangement developed from the first consisted of two concrete, or in some cases, iron, basins connected by a system of pipes (Hesthamar, 1943; Homb, 1956), permitting the NaOH solution to be circulated by an electrically driven pump and maintaining a constant concentration. The same pump is also used to recirculate the lye solution between basins. One basin is used for the lye treatment, while the treated mass in the other is being washed. One basinful of straw is ready for feeding every day. The means for building the apparatus on the farm have been marketed, and drawings, etc. have been distributed by the equipment companies.
Another factor which may explain the rapid increase in straw treatment in Norway, is the traditional 2-storey barn, with storage rooms for hay and straw above, allowing the chopped straw to be carried to the basin by gravity.
Contrary to expectations, the treatment of straw continued to increase after 1945 on Norwegian farms. If it had not been for the lack of water, it would have grown still more. (Usually 50 l water per kg of dry straw were considered necessary, although several modifications designed to save water were recommended.
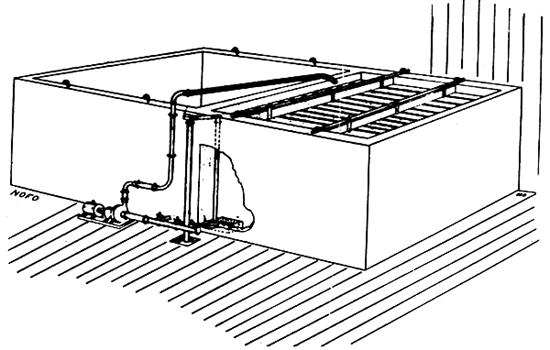
Fig. 3.1. Equipment for on-farm treatment by the Beckmann method (NOFO)
The next step therefore was to form cooperatives near streams or lakes. An attempt along these lines was made in 1945, following the same principles as is Fig. 3.1, except that several, much larger, basins were used. Because of the labour involved, this first cooperative did not last long. From the early 1950s a new technique was developed for handling the straw in cooperative establishments. Iron chains were placed around loosely compacted straw bales, 48 of which could be lifted from a lorry to the basin, the chains remaining in place during treatment. With the same crane the bales could be lifted onto the lorry after treatment. One cooperative construction usually has a capacity of 5 to 20 tons of dry straw per day. Advanced technical procedures enable the treatment to be made at a predetermined concentration of NaOH. The washing process also has been automated (Breirem and Homb, 1970), Fig. 3.2 illustrates such a construction.
The acceptability of Beckmann-treated straw has been investigated by Saue (1962, cit. Breirem and Homb, 1970). Using 2.5 kg hay, 10 kg grass silage, 20 kg roots, and concentrates according to yield, each dairy cow consumed 23 kg treated straw (4 kg DM). Compared to high-quality grass silage, the acceptability of treated straw was slightly lower. Many farmers prefer to give only 2 FU per cow per day (about 16 kg). In a 1945 survey, more than half the farmers reported a decline in the frequency of acetonemia when treated straw was included in the ration (Homb, 1948). Later experience in Norway seems to have verified that treated straw makes the feed ration more balanced and keeps the incidence of ketosis at a low level. Heifers, steers and bulls usually receive 1 to 2 FU per day, with a few extremes of 3 FU for finishing bulls. At the Agricultural University Farm, sheep have been wintered on 40 to 50% of total FU as treated straw.
NaOH treatment with limited amounts of water
The question of pollution due to waste from straw treatment has become a more topical problem than it was some years ago. Even in Norway, which is not a densely populated country, more restrictions have been enforced in order to control the pollution of streams and lakes. Cooperatives have from the beginning had to obtain a licence for treating straw. In addition to the lye waste, some 15 to 20% of the dry matter of the straw will be lost to the stream. The bulk of this material consists of protein, hemicellulose, lignin, and of such minerals as potassium, chlorine, sulphur, etc. Recycling the washing water has already been mentioned as a method of saving water, but the dry matter losses to the stream will continue.
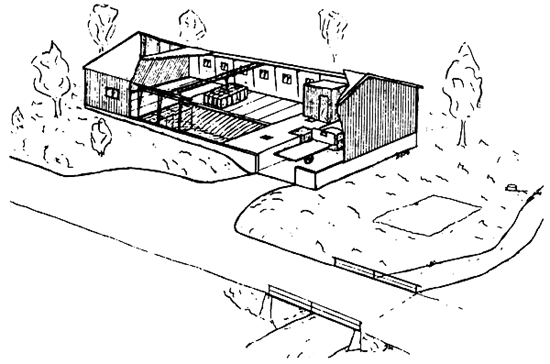
Fig. 3.2. Construction for cooperative treatment of straw in Norway (NOFO)
During the last 10 to 15 years, a number of suggestions have been made on how to save water and avoid pollution in NaOH treatment of straw. Few of them have yet come into commercial use, but it is expected that some of them will come into practical use in the near future. A short review of these investigations is therefore justified.
With a system of 3 basins and by using the washing water twice, Fyrileiv and Torgrimsby (1954) were able to reduce water consumption to 18 l per kg of dry straw.
Lampila (1963) in Finland has made smallscale tests using minute quantities of water to moisten the straw only. In this way the NaOH was used at higher concentrations than in the Beckmann method, while its use per kg of straw was less. By using 6 kg of NaOH per 100 kg of straw, he found digestion coefficients of organic matter of 62 to 63. Including washing, the water required amounted to 7 l per kg dry straw.
In the German Democratic Republic, Balduan and Piatkowski (1972) have studied the use of limited amounts of water, with and without neutralization by acetic acid.
Modified Beckmann methods in closed systems
Torgrimsby (1971) suggested a closed system in which the volume of water added to the system was equal to that removed with the treated straw. Under this method, straw is initially treated with used rinsing water containing NaOH. The method has been further developed by Wethje (1974) and is being successfully used on his farm in Sweden.
Another system has been developed by Boliden, a Swedish company. Medium-density bales of straw are sprayed with a NaOH solution, the unabsorbed solution flowing back to the supply tank. It has been recommended to allow the lye to act upon the straw for 16 to 18 hours before phosphoric acid is sprayed on the straw and circulated for 1 hour. When the surplus acid has drained off, the straw is ready for feeding. Phosphoric acid has been chosen because phosphates normally have to be incorporated as supplements in the feeds, and this should not be necessary when treated straw of this type is used.
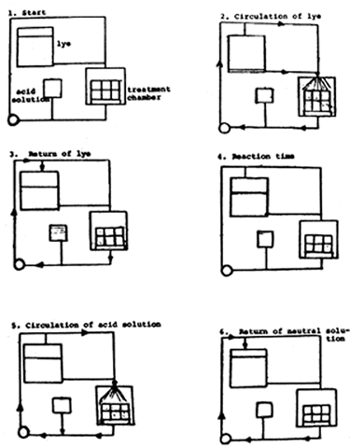
Fig. 3.3. Flow chart: wet NaOH treatment of straw with neutralization (Boliden)
No material from the straw or from the chemicals is allowed to go to waste. The system is automated and needs no inspection. The technical side of the method is at present under development. Preliminary digestion trials with sheep and feeding experiments with heifers are under way at the Agricultural University of Norway, and similar investigations are being performed in Sweden, but the results are still not clear. In vitro DMD was increased from 45% to 68%. A scheme of this modification of the Beckmann method is illustrated in Fig. 3.3.
Dry treatments
Wilson and Pigden (1964) in Canada worked with ground wheat straw and found that in vitro digestibility increased with increasing amounts of NaOH up to 9 kg per 100 kg straw. No washing took place. Feeding tests have been made with treated straw in a mixture with silage or hay, in one test after neutralization with acetic acid.
According to Raine and Owen (1976) a modification of this method has currently reached commercial application in the U.K.
Donefer (1968) mixed 100 kg oat straw with 60 l of a 13.3% NaOH solution. After 24 hours acetic acid was supplied to pH = 6. The material was dried to 90% DM and fed ad lib to sheep, with and without added 2.5% urea. Digestibility of DM increased significantly, from 45 to 61, as a result of NaOH treatment. Voluntary feed intake of treated straw increased more than three times by adding urea.
Ololade et al. (1970), also in Canada, tested the combined effects of temperature, NaOH concentration and time for treatment on in vitro digestibility of barley straw. Digestibility increased up to the highest concentration tested, 8%. By using higher temperatures it seems possible to reduce the concentrations of NaOH, and the time for treatment can be also reduced, their results show.
Data for in vitro digestibility with regard to the factors mentioned above have been reported by a number of workers (Chandra and Jackson, 1971; Singh and Jackson, 1971; Owen, 1973; Vestergaard Thomsen et al., 1973; Phoenix et al., 1974).
In vivo digestion studies have not always resulted in figures in agreement with the figures for in vitro digestibility. Vestergaard Thomsen et al, (1973) found that treatment with NaOH at 4 to 6% of straw resulted in digestibility of the same magnitude as that obtained when using the Beckmann method. At higher levels of NaOH application, in vivo digestibility increased less than in vitro digestibility. More or less similar results are reported by Mowat and Ololade (1970), Singh and Jackson (1971) and Klopfenstein et al. (1972). Mowat (1975) is therefore somewhat disappointed in in vitro studies, not supplemented with animal studies. The reason for this lack of agreement between the two methods is thought to be related to the restrictive effect of rumen microbial activity. The adversely increasing rate of passage out of the rumen, due to the increased water consumption by the animal, may also be a factor (Maeng et al., 1971; Mowat, 1975).
Raine and Owen (1976) found increasing digestibility and feed intake in sheep by using increasing amounts of NaOH from 0 to 70 g per kg straw DM, but no further improvements occurred when more NaOH was applied. No significant effect was observed as a result of partial or complete neutralization with HCl.
Young and Terry (1976) describe a simple on-farm alkali treatment of straw ensiled together with unwilted perennial ryegrass.
Methods developed at the Biotechnical Institute, Kolding, Denmark
Since the late 1960s intensive work has been done at Kolding to develop dry methods for treating straw (Rexen et al., 1975). Two different methods have been developed: a semi-dry process, and a dry process.
Although the semi-dry process has hardly come into practical use, the principles are so interesting that they are worth mentioning. Chopped straw is transported to a continuous reaction chamber. A weak NaOH solution is also pumped to the chamber, whose temperature has been raised to 60 to 80°C. The reacted straw goes to a screw press to reduce the moisture content from 85 to 60 to 70%. The solution pressed out of the mass flows back to the lye tank. In a conventional drum drier the product comes into contact with the acid gases from an oil flame and is almost fully neutralized. Some of the equipment is already in use in green-crop drying plants. However, the reaction chamber, the screw press and the lye tank must be bought for the specific purpose. Fig. 3.4 illustrates the technique used.
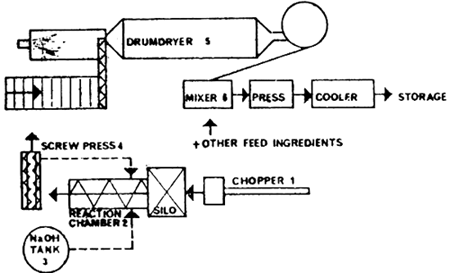
Fig. 3.4. Flow chart: semi-dry treatment of straw (Rexen et al., 1975)
The dry process implies less or no water and no drying costs, but the unreacted NaOH remains in the material, possibly limiting the amount of NaOH that can be used. The chopped straw is transported to a lye mixer, which also may be considered a reaction chamber. Thereafter the material is pressed into cobs or pellets in a ring die press, whose main function is to compress the straw and to reduce the content of unreacted NaOH in the final product. It is thought that the reaction takes place partly in the lye mixer and partly in the press (Rexen et al., 1975) (see Fig. 3.5).
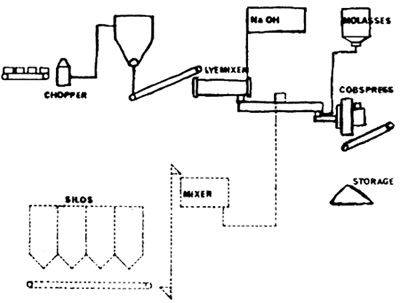
Fig. 3.5. Flow chart: dry treatment of straw (Rexen et al., 1975)
After a pilot plant had been operated for three years, a full-scale production plant, with a yearly capacity of 10 000 tons, was built in 1975.
The animal production side of this interesting project has hardly reached the same level as its technical development, but some results have been reported. The amount of NaOH used per 100 kg dry straw has been varied, and the digestion coefficients obtained are presented in Table 3.3.
There are similarities between these results and those by Mowat and others cited above. With amounts of NaOH in excess of 4%, the in vivo digestibility increases less than that obtained in vitro.
Friis Kristensen (1975) reports that the palatability of the dry process product is relatively low. Individual cows reacted very differently. Intakes of 2.6 to 8.4 kg per day were observed when they consumed 14 kg clover-grass silage, 1.3 kg dry molasses beet pulp and 4 kg concentrates with the straw. Five kg NaOH were added to 100 kg straw. In another experiment the ration consisted entirely of treated straw and concentrates. The intake of straw was, on average, 9.9 kg; when the alkalis were neutralized with HCI, it averaged 11.6 kg. He further reports that palatability seems to be high when treated straw is mixed with other feeds and pelleted. Pelleted mixtures of concentrates and treated straw (60:40) have been consumed at daily levels of 9 to 11 kg in young bulls and 20 to 21 kg in dairy cows.
| kg NaOH per 100 kg straw | in vitro digestibility | in vivo digestibility (sheep) |
| 0 | 40.8 | 48.2 |
| 2.0 | 51.8 | 51.4 |
| 3.0 | 59.6 | 59.6 |
| 4.0 | 66.4 | 62.3 |
| 5.9 | 74.0 | 66.7 |
Source: Vestergaard Thomsen et al., 1973
A group feeding experiment with Jersey cows fed mixtures with 0, 20% and 40% treated straw, showed equal milk yields among groups, indicating that the assumed value of 0.6 FU per kg of DM was correct. An experiment with young bulls of 300 to 500 kg weight resulted in slightly lower weight gain in the animals receiving treated straw than in the control group (1091 versus 1001 g/day). The difference was, however, not significant. Friis Kristensen (1975) states that more experiments are required to determine the feeding value of straw treated by the Danish dry process.
If great amounts of NaOH are consumed, it is reasonable to believe that alkaiosis would appear. This question has partly been answered by Stigsen (1975). The Danish work showed considerably higher amounts of net base in urine of cows on unneutralized straw than when the straw had been neutralized with HCI. The cows consumed 10 kg treated straw per day, supplemented with urea and minerals, and the amount of NaOH was 5 kg per 100 kg straw. According to this investigation a constant compensated alkalosis may occur when large amounts of unneutralized treated straw are fed. It is also reported that alkalosis is not prevented by neutralization with organic acids, as these are metabolized in the animal organism.
The author also states that under most practical conditions the daily ration of treated straw would be within limits tolerable for the cattle. This might also apply to the relatively high dosages of Na that lead to higher water intake and increased excretion of urine. Neutralization of the alkali does not counteract this effect. With regard to the long-term effect of high dosages of NaOH, it should be added that Stigsen's experiments lasted only a few weeks. Other Danish observations of a few cows fed 5 to 7 kg treated straw daily for 1 to 3 years have not indicated that there were problems due to the acid base balance (Friis Kristensen, 1975). This point is expected to be more clearly elucidated by further Danish research.
Ground straw with added NaOH, pelleted in Norway
A cooperative company, Felleskjøpet i Trondheim, has over the last 7 or 8 years developed a product consisting of pelleted straw, with or without added molasses and other concentrates. This was originally only a physical treatment. During the last two years 2.5% of a 50% NaOH solution has been sprayed on the ground straw before pelleting. The pelleting process involved increased pressure and temperature, and both experience and experiments indicate that an improvement of the feeding value takes place. Probably more important is the much higher intake of pelleted straw than whole straw. In an experiment at the Agricultural University of Norway, a ground straw made up 70% of a mixture of feedstuffs, the rest consisting of concentrates, vitamins and minerals, was tested with and without addition of the NaOH solution. The corrected daily gain for the animals on NaOH-added material was 1144 g, versus 1037 g for the control group (Unpublished data). The difference was not significant. Further experiments are under way. A side effect of NaOH in this case was to harden the pellets; this was considered advantageous.
Treating straw with ammonia (NH3)
A few preliminary tests with NH3 treatment of straw in Denmark and Norway during the 1950s showed somewhat varying results. Beginning in 1969–70, Norsk Hydro and NOFO initiated more serious research on NH3 treatment. Initially several extensive series of laboratory trials were performed with varying dry matter contents of the straw, varying temperatures, reaction times and amounts of NH3 applied. In vitro digestion figures served as criteria of the effect. The conclusions of all these trails were that amounts of NH3 and reaction times were to some degree compensatory factors. Moistening the straw seemed to have no noticeable effect. A moderate addition of NH3 (2.5 to 3.5%), combined with at least six weeks' reaction time has therefore been recommended (MO, 1975; NOFO, 1976).
Air-tight rooms were used for the laboratory trials. When adapting the method to farm practice in 1975, a closed system of polyethylene sheets around compacted bales in the field was chosen. Staff from the company travelled from farm to farm, injected the ammonia and sealed the polyethylene sheets around the stacks. This system seems to work satisfactorily.
With regard to in vivo digestibility, a few mean figures for organic matter from a series of 1973 experiments are of interest: untreated straw 56; after 10 days' treatment 64; after 20 days' treatment 69.
In a growth study with heifers in 1974, (Homb et al., 1975), 3 kg barn-dried hay were compared with 3 kg of NH3-treated straw. The rest of the ration consisted of pellets of dried grass and 0.5 kg concentrates, the same being fed to both groups. The average daily gain in 116 days was 883 g for the hay group and 861 g for the straw group. In an acceptability test with 30 heifers the intake of NH3-treated straw averaged 6 kg per day, or 1.75 kg DM per 100 kg live weight. In addition to the treated straw each heifer received 1.5 kg concentrates per day. The digestion coefficients for organic matter were determined as 66 for treated straw, 48 for untreated straw, and 59 for hay (in vivo tests).
An experiment with young bulls was carried out during the winter 1975–76. Two groups of bulls, 14 in each, received 3.5 kg barley straw per day, treated or untreated, in addition to a basic ration of hay and concentrates. The NH3-treated straw was consumed more rapidly, the daily gains were 675 and 846 g for the groups on untreated and treated straw, respectively. The difference between groups was significant. (Kvåle and Homb, 1976, unpublished).
In an experiment with high-yielding cows one half of the animals were fed roughages as 5.2 kg DM in grass silage plus 3.5 kg NH3-treated straw, while the control group received 8.5 kg DM in grass silage. Both groups were fed concentrates according to actual yield. No noticeable difference in milk yield was observed (MO, unpublished).
Nedkvitne (Unpublished) has tested NH3- treated straw in the winter rations of ewes. High-quality hay served as the control. Though the results have not been analysed, it should be mentioned that palatability for hay was better than for straw.
The NH3 treatment leads to an increased N content in the straw. The degree to which this absorption of NH3 can be utilized by the animals is not known, and research to clarify this has been initiated (MO).
The season 1976–77 is seeing a considerable increase in the use of NH3-treated straw in Norway. The organization of distribution of polyethylene sheeting and NH3 has been revamped, enabling the companies to reach more farmers.
References
Balduan, G., Piatkowski, B. (1972). Untersuchungen zum Aufschluss von Getreldestroh. 1. Mittellung. Behandlung mit Natronlauge und die anschliessende Neutralisierung. Arch. Tierernähr. 22:485.
Breirem, K. and Homb, T. (1970). Fórmidier og Fórkonservering. Forlag Buskap og Avdrått, Gjøvik.
Chandra, S., and Jackson, M.G. (1971). A study of various chemical treatments to remove lignin from coarse roughages and increase their digestibility. J. Agric. Sci. 77:11.
Donefer, E. (1968). Effect of sodium hydroxide treatment on the digestibility and voluntary intake of straw. Proc. Sec. World Conf. Anim. Prod. (Maryland), 446.
Ehrenberg, P. (1944). Beispiele für die Fütterung mit aufgeschlossenem Stroh. Mitt. f. d. Landw. 59, 312.
Ellenberger, W., and Waentig, P. (1919). über Strohaufschliessung mit Kalk ohne Anwendung von Wärme. Deutsche Landw. Presse, 46, 1.
Fingerling, G. (1924). Die Ernährung der landwirtschaftlichen Nutztiere. Verlag Paul Parey, Berlin.
Fingerling, G., and Schmidt, K. (1919). Die Strohaufschliessung nach dem Beckmannschen Verfahren. I. Einfluss der Aufschliessungszeit auf den Umfang der Nährwerterschliessung. Landw. Ver suchsst. Sonderabdruck, 115.
Fingerling, G., Schmidt, K. and Schuster, A. (1923). Strohaufschliessung nach dem Beckmannschen Verfahren. Il. Einfluss der Laugenmenge auf den Umfang der Nährwerterschliessung. Landw. Versuchsst., 100, Sonderabdruck 1.
Friis Kristensen, V. (1975). Chemical and physical treatment of straw. Paper at EEC seminar on “Improving the nutritional efficiency of beef production”, Theix, France, 14–17 Oct. 1975
Fyrileiv, E. and Torgrimsby, J. (1954). Vannforbruket ved luting av halm kan reduseres. Norsk Landbr., 20, 55.
Heide, R. von der, Steuber, M. and Zuntz, N. (1916). Untersuchungen über den Nährwert des Strohstoffs. Biochem. Z., 73, 161.
Hesthamar, T.B. (1943). Halmluting frå teoretisk og forsøksmessig synspunkt, Tidsskr. f. d. Norske Landbr., 50, 113.
Homb, T. (1956). Norwegische Erfahrungen bei der Strohaufschliessung nach dem Beckmannschen Verfahren. Futterkonservierung, 2, 129.
Homb, T., Saue, O., Matre, T. and Berg, N. (1975). Halmbehandling og fóring med halm. Husdyrforsøksmøtet 4.–5. febr., 117.
Honcamp, F. and Blanck, E. (1919). Untersuchungen über den Futterwert des nach verschiedenen Verfahren aufgeschlossenen Strohes. I. Mitteilung: Aufschluss des Strohes mit Salzsäure. Landw. Versuchsst., 93, 175.
Honcamp, F. and Pommer, E. (1922). Untersuchungen über den Futterwert des nach verschiedenen Verfahren aufgeschlossenen Strohes. V. Mitteilung: Aufschluss des Strohes mit Ätznatron und Ätzkalk in der Kälte. (Beckmann-Verfahren). Landw. Versuchsst., 99, 231.
Keliner, O. (1905). Die Ernährung der landwirtschaftlichen Nutztiere. Verlag Paul Parey, Berlin.
Keliner, O. and Köhler, A. (1900). Untersuchungen über den Stoff- und Energie-Umsatz des erwachsenen Rindes bel Erhaltungsund Produktionsfutter. Landw. Versuchsstat., 53, 1.
Klopfenstein, T.J., Krause, V.E., Jones, M. J., and Woods, W. (1972). Chemical treatment of low quality roughages. J. Anim. Sci., 35, 418.
Lampila, M. (1963). Utilization of rumen N by rumen microflora for synthesis of cellular protein with NaOH treated fibrous polysaccharides as an energy source. Ann.Agric.Fenniae, 2, 105.
Lehmann, F. (1891). Der Nährwert der Cellulose. Landw. Versuchsst. 38, 337.
Lehmann, F. (1895). Landwirtschaftliche Jahrbücher, 24, Ergänzungsband l, 118.
Lehmann, F. (1904). Das Aufschliessen von Stroh. Deutsche Landw. Presse, 31, 207.
Maeng, W.J., Mowat, D.N. and Bilanski, W. K. (1971). Digestibility of sodium hydroxide-treated straw fed alone or in combination with alfalfa silage. Can. J. Anim.Sci. 51, 743.
Mo, M. (1975). Halm - avfall eller fór l åra framover? Landbrukets Årbok 1976, 236. Johan Grundt Tanums forlag.
Mowat, D.N. (1975). Crop residues for cattle feed. McGinnis Conf, on Commercial Uses of Wastes a Animal Feed, April 24–25, 1975.
Mowat, D.N., and Ololade, B.G. (1970). Effect of sodium hydroxide treatment on digestibility and voluntary intake of straw. Can. Soc.Anim.Prod. 35 (Abstr.)
Nofo (1976). Ammoniakkbehandlung av halm. A/S Norsk Fórkonservering, Oslo.
Ololade, B.G., Mowat, D.N. and Winch, J. E. (1970). Effect of processing methods on the in vitro digestibility of sodium hydroxide treated roughages. Can.J.Anim.Sci. 50, 657.
Owen, E. (1973). Personal communication.
Phoenix, S.L., Bilanski, W.K. and Mowat, D.N. (1974). In vitro digestibility of barley straw treated with sodium hydroxide at elevated temperature. ASAE Transactions, 17, 780.
Raine, H.D. and Owen, E. (1976). On-farm treatment of straw with sodium hydroxide - effect of amount of alkali and post-treatment neutralization upon intake and digestibility by sheep. Paper at 27th Annual Meeting EAAP, Zürich, 23–26 Aug. 1976.
Rexen, F.P., Stigsen, P. and Friis Kristensen, V. (1975). The effect of a new alkali technique on the nutritive value of straws. Proc. 9th Nutr. Conf. Feed Manufacturers, Nottingham. Butterworth, London.
Schneidewind, M. (1919). Fütterungsversuch über die Wirkung des Strohmehls und des Oexmannschen Zellulosefutters. Deutsche Landw. Presse, 43, 91.
Singh, M. and Jackson, M.G. (1971). The effect of different levels of sodium hydroxide spray treatment of wheat straw on consumption and digestibility by cattle. J.Agric.Sci. 77, 5.
Slade, R.E., Watson, S.J. and Ferguson, W.S. (1939). Digestibility of straw. Nature 143, 942.
Stigsen, P. (1975). Kullhydratkildens og neutralisationens betydning for udnyttelse af natriumhydroxyd-behandlet halm hos malkekøer. Lic. afhandl. i kvaegets fodring. Den Kgl. Veterinaerog Landbohøjskole, København.
Thomann, W. (1921). Vergleichende Versuche über die Zusammensetzung und Verdaulichkeit von Rohstroh und aufgeschlossenem Stroh. Landw. Jahrb. d. Schweiz, 35, 667.
Torgrimsby, J. (1971). Personal communication.
Vestergaard Thomsen, K., Rexen, F. and Friis Kristensen, V. (1973). Forsøg med natriumhydroxid-behandling af halm. Ugeskr. f.agr. og hortonomer 118, 436 and 437.
Watson, S.J. (1941). Increasing the feeding value of cereal straws. J. Royal Agr.Soc. England, 101. Reprint.
Wethje, F. (1975). Patent application, Awapatent Malmö, 30.5.1975.
Wilson, R.K. and Pigden, W.J. (1964). Effect of sodium hydroxide treatment on the utilization of wheat straw and poplar wood by rumen microorganisms. Can.J.Anim.Sci. 44, 122.
Young N.E. and Terry, R.A. (1976). On-farm alkali treatment of straw and its feeding value in mixed diets for ruminants. Paper at 27th Annual Meeting EAAP Zürich, August 23–26, 1976.
(Including by-products of the wood and cellulose industry)
by
M. Linko
Technical Research Centre of Finland
Biotechnical Laboratory, Helsinki, Finland
| Summary | Résumé |
| Cellulose is a constituent of all kinds of plants. Different wood and non-wood plants or their products constitute possible raw materials for biological or enzymatic treatment. In spite of the abundance of cellulose it is not very easy to find suitable cellulosic materials, which could be collected economically from a limited area, taking into account collecting, transport, handling and storage costs. The correct choice of material depends on local conditions. Most cellulosic materials contain three major organic components: cellulose, hemicellulose and lignin. In the development of processes based on biological or enzymatic breakdown of cellulose the greatest possible number of materials should be screened. The economic feasibility of the hydrolysis of cellulosic materials depends essentially on the availability of active cellulase preparations at a reasonable price. Many cellulolytic organisms are able to grow on cellulosic materials, but Trichoderma viride is outstanding for production of cellulases. Several types of enzymes are involved in complete hydrolysis of cellulose. It can be expected that in the future two or more organisms will be used to produce all the necessary types of enzymes, including hemicellulases. Native cellulose is very resistant to enzymatic hydrolysis. The highly crystalline structure and the presence of lignin effectively prevent the attack of cellulases, making the hydrolysis slow and incomplete. For a practical hydrolysis process it is necessary to treat the cellulosic material in some way prior to the use of enzymes. This is also an important stage from the economic point of view; together with the price of the enzyme preparations, the cost of pretreatment is one of the most prohibitive factors in designing an economically feasible full-scale process. The pretreatments are aimed at loosening the highly crystalline structure of cellulose and extending the amorphous parts. The removal of lignin is also essential. A decrease of one third in the lignin content of hardwood or two thirds in that of softwood increases the digestibility of these materials to 60 percent which, for ruminants, is equivalent to a medium quality hay. The hexoses and pentoses obtained in total hydrolysis of cellulose and hemicellulose can be used for SCP production. In processes with separation of enzyme production, hydrolysis and SCP production the microorganism has a freer choice of medium as compared to direct cultivation of cellulolytic organisms on cellulosic substrates. Besides the conventionally used Candida species, Paecilomyces varioti is one of the suitable organisms for SCP production. It is cultivated industrially on sulphite waste liquor. | La cellulose est un élément constituant des végétaux de tous genres. Différentes plantes ligneuses et non ligneuses, ainsi que les produits qui en proviennent, constituent donc des matières premières qui se prêtent éventuellement au traitement biologique ou enzymatique. Malgré l'abondance de la cellulose, il n'est pas toujours facile de trouver des substances cellulosiques appropriées que l'on puisse se procurer économiquement dans une aire limitée, compte tenu des frais de ramassage, de transport, de manutention et d'entreposage. Les conditions locales détermineront le choix de celle qui
convient. La plupart des substances cellulosiques contiennent trois composantes organiques principales: cellulose, hémicellulose et lignine. En élaborant des procédés fondés sur la dégradation biologique ou enzymatique de la cellulose, it importe de passer en revue le plus grand nombre posible de substances. La rentabilité de l'hydrolyse des substances cellulosiques dépend essentiellement de la possibilité de disposer de préparations de cellulase active à un prix raisonnable. Des nombreux organismes cellulolytiques capables de proliférer sur les substances cellulosiques, Trichoderma viride est un agent hors de pair pour la production de cellulases. Plusieurs types d'enzymes participent à l'hydrolyse totale de la cellulose. A l'avenir, on utilisera très probablement plusieurs organismes pour produire tous les types nécessaires d'enzymes, y compris les hémicellulases. La cellulose naturelle est très résistante à l'hydrolyse enzymatique. Sa structure fortement cristalline et la présence de lignine s'opposent efficacement à l'action des cellulases, de sorte que l'hydrolyse est lente et incomplète. Pour que le procédé donne des résultats pratiques, il est néssaire de soumettre la substance cellulosique à quelque for me de traitement préalable à l'utilisation des enzymes. Ce traitement constitue également un stade important des opérations sous l'angle économique; le coût du prétraitement, associé au prix des préparations enzymatiques est l'un des éléments particulièrement prohibitifs lors de la conception d'un procédé en grandeur réelle réalisable du point de vue économique. Les prétraitements ont pour objet de relâcher la structure fortement cristalline de la cellulose et d'en distendre les parties amorphes. L'élimination de la lignine est également indispensable. Une réduction d'un tiers de la teneur en lignìne des bois durs ou des deux tiers de celle des bois tendres accroît la digestibilité de ces substances jusqu'à concurrence de 60 pour cent ce qui, pour des ruminants, équivaut à un foin de qualité moyenne. Les hexoses et les pentoses obtenus par hydrolyse totale de la cellulose et de l'hémicellulose peuvent être utilisés, par exemple pour la production de protéines monocellulaires. Dans les procédés où l'on sépare la production enzymatique, l'hydrolyse et la production de protéines monocellulaires, le micro-organisme a un choix plus vaste de milieux par comparaison avec la culture directe des organismes cellulolytiques sur des substrats cellulosiques. Outre l'espéce Candida utilisée traditionnellement, Paecilomyces varioti est l' un des organismes qui conviennent à la production de protéines monocellulaires. Il est cultivé à l'échelle industrielle sur la liqueur résiduaire de sulfite. |
Resumen
La celulosa es un componente de toda clase de plantas. Las diferentes plantas (leñosas o no) y sus productos son posibles materias primas para el tratamiento biológico o enzimático. A pesar de su abundancia, no es fácil encontrar materiales celulósicos idóneos que se puedan recoger en una área limitada con costos de recolección, transporte, movimiento y almacenamiento suficientemente bajos. La determinación del material más idóneo depende de las condiciones locales.
En su mayor parte los materiales celulósicos contienen tres componentes orgánicos principales: celulosa, hemicelulosa y lignina. Al elaborar procesos para la descomposición biológica o enzimática de la celulosa, conviene escoger entre el mayor número posible de materiales.
La factibilidad económica de la hidrólisis de materiales celulósicos depende esencialmente de la disponibilidad de preparados de celulasas activas a precios módicos. Hay muchos organismos celulolíticos que crecen en materiales celulósicos. Trichoderma viride se destaca por su producción de celulasas. Para la hidrólisis completa de la celulosa se necesitan enzimas de varios tipos. Cabe prever que en el futuro se emplearán dos o más organismos para producir todos los tipos de enzimas necesarios y especialmente hemicelulasas.
La celulosa nativa es muy resistente a la hidrólisis enzimática. Por su estructura sumamente cristalina y por la presencia de lignina es muy resistente al ataque de las celulasas y, por tal razón, su hidrólisis es lenta e incompleta. Para que un proceso de hidrólisis sea práctico, es preciso someter el material celulósico a algun tratamiento, antes de emplear las enzimas. Desde el punto de vista económico ésta es también una etapa importante porque, al costo de los preparados de enzimas, se suma el del pretratamiento que constituye uno de los factores más prohibitivos en un proyecto de proceso económicamente factible a escala normal.
El pretratamiento tiene por objeto aflojar la estructura sumamente cristalina de la celulosa y dilatar las partes amorfas. Es también indispensable eliminar la lignina. Quitándole un tercio de la lignina a la madera de frondosas y dos tercios a la de coníferas, se logra un aumento de la digestibilidad de 60 por ciento y un producto que, para los rumiantes, es semejante al pasto seco de calidad media.
Las hexosas y pentosas obtenidas por hidrólisis total de la celulosa y de la hemicelulosa se pueden emplear, por ejemplo, para la producción de proteínas unicelulares. En procesos en los que la producción de enzimas, la hidrólisis y la producción de proteínas unicelulares se realizan por separado, el microrganismo tiene más libertad para elegir el medio que cuando se procede a su cultivo directo en substratos celulósicos. Además de la clásica Candida spp., Paecilomyces varioti es uno de los organismos útiles para la producción de proteínas unicelulares. Se cultiva industrialmente en licor al sulfito consumido.
Introduction
Biological or enzymatic treatment of lignocellulosic materials has been the subject of intensive research during recent years, but progress has not yet been very convincing in terms of full-scale applications. The most severe problems encountered are economic. Every full-scale process applied should, of course, be economically feasible, not necessarily on a general basis but at least locally, taking into account such factors as the individual country's balance of payments, employment levels, local resources and long-term environmental situation.
Several processes for the treatment of lignocellulosic materials are technologically possible. Most cellulosic materials contain three major organic components: cellulose, hemicellulose and lignin. The economy of processes based on biological or enzymatic breakdown of cellulose requires consideration of all of these materials.
Possible processes
Biological treatments of lignocellulosic materials include the following basic types of procedures:
enzymatic hydrolysis of cellulose and hemicellulose to fermentable sugars, followed by cultivation of organisms suitable for single-cell protein (SCP) production, such as the species Candida or Paecilomyces;
direct cultivation of cellulolytic organisms such as the species Cellulomonas on cellulosic materials;
production of SCP from process wastes already in a hydrolyzed form, such as sulfite waste liquor;
partial hydrolysis of cellulosic feed materials to improve their digestibility.
The only one of these processes so far applied on a full industrial scale is the production of SCP from sulfite waste liquor. The so-called Torula yeast process has been applied in several countries. An interesting new process is the so-called Pekilo process developed in Finland. Special advantages of this process are efficient utilization of organic acids as well as hexoses and pentoses, easy filtration and high protein content of the biomass (Romantschuk, 1975).
A method for direct cultivation of cellulolytic organisms on cellulosic materials has been worked out at Louisiana State University (Srinivasan, 1975). Enzymatic hydrolysis of cellulosic materials, including production of cellulases, has been investigated in several countries, with the United States Army Natick group as one of the main contributors (Nystrom, 1975). The economic feasibility of both of these processes is still unclear, although the technology is available.
The partial hydrolysis of cellulosic feed materials to improve their digestibility would be valuable. In fact, this may prove to be a major breakthrough for full-scale application. Cellulose is very resistant to enzymatic hydrolysis. Slow and incomplete reaction is a major problem. The enzyme complex must be very efficient, and even then the cellulosic material should be pretreated in some way prior to hydrolysis.
Cellulosic materials and their use
There are huge quantities of cellulosic materials in the world and - what is increasingly important - these resources are renewable. Cellulose is a constituent of all plants. Possible raw materials for enzymatic hydrolysis are different wood and non-wood materials or their products. The nature of cellulose and wood has led to their conventional use in the manufacture of paper, textiles and building materials, where fibre strength or rigid structure is essential, and these will no doubt remain the main uses of many valuable cellulosic materials. However, there are also huge quantities of cellulosic materials which have not been utilized at all so far. Some materials have been used for fuel. Even pollution problems are related to certain cellulosic wastes.
About 22% of the land area of the globe is covered by forests, the area of hardwood forests being somewhat greater than that of softwood forests. The areas richest in forests are the U.S.S.R., South America and North America, all of which are forested by more than 20%. In western Europe the figure is only 4%. Total world forest resources exceed 300 000 million cubic metres (Pringle, 1974).
With present harvesting methods as much as 40% of the organic substance is left in the forests. For this reason harvesting of whole trees and utilization of branches, stumps and roots is being investigated (Virkola, 1975). Woodcutting residues, bushes and small rapidly-growing trees are potential raw materials for enzymatic hydrolysis.
The principal consumers of forest resources are the pulp and paper industries and the mechanical industries producing sawn timber and plywood. World consumption of paper and board is expected to rise from 128 million tons in 1970 to 218 million tons in 1980. This will be possible only by exploitation of currently unutilized forest resources, including those of many tropical and subtropical regions (Virkola, 1975). This would probably also render available an increasing amount of different types of wood residues, potential raw material for enzymatic hydrolysis.
Waste paper could also be used for hydrolysis. For example, the U.S.A. generates 44.3 million tons of waste paper per year, of which 33.5 million tons are available (Rydholm, 1965). However, this material will probably be mainly reused for paper production.
Non-wood plants and fibres include agricultural residues such as sugarcane bagasse and cereal straw, natural plants such as bamboo, papyrus and various grasses and non-wood crop fibres such as jute, hemp, manila hemp, sisal and cotton, primarily grown for their fibre content (Atchison, 1974). The availability of some non-wood plant fibrous material is summarized in Table 4.1. The total amount of these materials in the world is very high. The most important are bagasse, bamboo, cotton and some straws.
| Raw material | Potential worldwide availability with present collection methods (thousand metric tons) | |
| Sugarcane bagasse | 55 000 | |
| Straws (wheat, rice, etc.) | 88 500 | |
| Bast fibres (jute, kenaf, etc.) | 6 099 | |
| Leaf fibres (sisal, abaca, etc.) | 904 | |
| Reeds | 30 000 | |
| Bamboo | 30 000 | |
| Papyrus | 5 000 | |
| Esparto grass | 500 | |
| Sabal grass | 200 | |
| Cotton fibre | 13 500 | |
| Estimated total | 1 027 203 |
Source: Virkola, 1975.
There may be a severe drawback in connection with the possible utilization of straws and other agricultural by-products or wastes: the complete removal of all this organic material from the fields may adversely affect the physical and chemical properties of the soil (Sloneker, in press). Similar effects may even occur in the forests if the trees are collected down to the last branch and root.
The present crops have not been optimized for production of digestible cellulose. In future, an alternative crop to trees or cane for producing cellulose may be developed.
Other materials rich in cellulose are urban waste and animal manure; the latter may be a suitable raw material for example in certain areas of the U.S.A. (Griffin et al., 1975). Some by-products or wastes of the food industry could also be utilized. Short seasons, often only 1 to 2 months, could create problems.
Peat is also an interesting raw material of plant origin. Its chemical composition varies greatly, depending on its origin and age, and except as a fuel, it has been poorly utilized. However, the surface layers are relatively rich in cellulose and hemicellulose and could be used for enzymatic hydrolysis. Peat resources are enormous, especially in the U.S.S.R. (total peat area: 73 million ha), and also in some other countries such as Finland and Canada (more than 8 million ha) (Fogarty et al., 1973).
In spite of the abundance of cellulose it is not easy to find suitable cellulosic materials which could be economically collected from a limited area, taking into account collection, transport, handling and storage costs. The choice of material depends on local conditions. In most cases there is competition for the material, if only for use as fuel. Consequently, the cost of the material used for hydrolysis would be at least its fuel value. It would be optimistic to presume a negative or zero value for the material, even if some environmental problems may be involved. A realistic example is sulfite waste liquor, which has to be treated in some way to avoid water pollution. Here the typical choices are production of SCP, baker's yeast or ethanol or concentration and burning.
An example of cost calculation for excess bagasse in the United States is presented below. The same type of calculation is applicable to any cellulosic waste (Rockwell, 1976).
| Cost of material at point of origin | $ 3.00/ton |
| Moisture | 50% |
| Cellulose (on dry matter) | 57% |
| Collection | $ 0 |
| Transportation | $ 0.10/ton |
| Storage | $ 1.35/ton |
| Replacement fuel | $ 0.90/ton |
Cost of material in dollars per ton of dry cellulose:
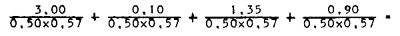 $ 10.53 + 0.35 + 4.74 + 3.16 =
$ 18.78/ton dry cellulose of SCP plant.
$ 10.53 + 0.35 + 4.74 + 3.16 =
$ 18.78/ton dry cellulose of SCP plant.
Cellulases and hemicellulases
At present only a few quite expensive cellulase and hemicellulase preparations are commercially available. The economic feasibility of the hydrolysis of cellulosic materials is essentially dependent on the availability of active enzyme preparations at a reasonable price. Production of cellulolytic enzymes has recently been reviewed (Enarl and Markkanen, 1976).
Many organisms are able to grow on cellulosic materials. However, Trichoderma viride has so far been outstanding for the production of cellulases. Since several types of enzymes are involved in the complete hydrolysis of cellulose, it can be expected that in the future two or more organisms will be used to produce all the necessary types. Recent results indicate that Sporotrichum thermophile is an efficient producer of cellulases. Activity levels comparable to those obtained with Trichoderma viride have been reached in a shorter time (Coutts and Smith, 1976).
At least three types of cellulase are involved in the complete hydrolysis of cellulose: endo-β-glucanases, exo-β-glucanases and β-glucosidase (Cellobiase). In the current state of processing technology the best strains of Trichoderma viride should already enable production of cellulases at a relatively low price in full-scale submerged fermentation. One price estimate for a filtered cellulase solution without any concentration or purification is $ 0.011 per litre (Millett et al., 1975).
Cellulase preparations often contain hemicellulases (xylanases and glucomannanases) as secondary activities, but for their efficient production a separate process would probably be necessary.
Hydrolysis of cellulose
The highly crystalline structure of native cellulose and the presence of lignin effectively prevent the attack by cellulases, making hydrolysis slow and incomplete. For a practical hydrolysis process, the cellulosic material must be treated in some way prior to the use of enzymes. This is also an important stage from the economic point of view. The pretreatments are aimed at loosening the highly crystalline structure of cellulose and extending the amorphous areas.
The removal of lignin is also essential. A decrease of one third in the lignin content of hardwood or two thirds in that of softwood increases the digestibility of these materials to 60%, which is equivalent to that of hay in ruminants (Millett et al., 1975; Dekker and Richards, 1973). Biological delignification is an interesting possibility (Henningsson et al., 1973; Eriksson and Goodell, 1974; Eriksson, 1975; Hartley et al., 1975). It has been found that treatment of wood with white rot fungi capable of breaking down lignin leads to more efficient hydrolysis by cellulases or rumen fluid (Kirk and Moore, 1972).
Pretreatments with alkali are efficient but not inexpensive. NaOH swells the cellulose fibres and causes depolymerization. However, there may be some loss of material, and heating of xylose in an alkaline solution may cause formation of compounds inhibiting the growth of microorganisms. This is a serious drawback when the ultimate goal is production of biomass (Kato and Shibasaki, 1974).
The increase in cellulose digestibility resulting from an alkaline-oxidation treatment is presented in Table 4.2. The biodegradability of the cellulose fractions was determined by the in vitro rumen fluid method of Baumgardt et al, (1962). The treatment increased cellulose digestibilities on average by 85%.
| Material | % dry cellulose Untreated | digested Treated |
| Bagasse | 15.1 | 57.0 |
| Rice straw | 7.3 | 54.1 |
| Johnson grass | 66.5 | 88.0 |
| Prairie grass | 16.2 | 45.7 |
| Corn cobs | 19.3 | 44.0 |
| Oat straw | 35.5 | 66.0 |
| Wheat straw | 25.4 | 44.0 |
| Sorghum bagasse | 30.0 | 61.5 |
Source: Baumgardt et al., 1962.
Several physical treatments have also been studied. Milling to a very fine powder is one of the most efficient pretreatments tested. Ball-milling gives the best results, but the cost is high (Mandels et al., 1974). Ball-milling after alkali treatment is even more efficient than alkali treatment alone.
Several other pretreatment methods have been described (Linko, 1976). An ideal case would be the situation in which pretreatment does not involve any extra cost at all. This is possible if the material has already been used for some process prior to the hydrolysis of cellulose, e.g. for the manufacture of furfural or xylose (Markkanen and Eklund, 1975). If the use of hemicellulose for the manufacture of furfural is economical per se, this would in effect eliminate the cost of pretreatment of cellulose. The conditions of high temperature (176°C), acid environment and sudden release of pressure during the manufacture of furfural constitute an exceptionally efficient pretreatment. On the other hand, even if the furfural production process is economically feasible, the market for furfural is limited. The same is also true for the production of xylitol. Consequently, these processes can be thought of as a plausible first stage in a few special cases only.
A certain natural pretreatment is also achieved during the formation of peat, whose slow natural decomposition makes its cellulose and hemicellulose contents relatively susceptible to enzymatic hydrolysis. Here the economic optimum is a compromise between two opposing factors; decomposition of peat makes the hydrolysis essentially easier, but also leads to loss of cellulose and hemicellulose. Simple steam treatment in an autoclave significantly improves the results of peat hydrolysis (Technical Research Centre of Finland, unpublished data).
Factors affecting the hydrolysis of cellulosic materials include type of substrate, pretreatment, characteristics of the enzyme preparation, temperature, time, pH, substrate concentration, reuse of enzyme and type of reactor. Many of these factors are interdependent, making the whole process rather complicated. In an ideal case the reaction would be rapid and complete, leading to a high glucose concentration without any substantial loss of enzyme, which could therefore be reused. The present state of the technology does not yet approach this ideal situation, but the results achieved are at least promising enough to encourage further developement.
The optimum temperature of hydrolysis is between 45 and 55°C depending on the reaction time, degree of hydrolysis and reuse of enzyme (Wilke and Yang, 1975). At a temperature of 45°C, the usual reaction time has exceeded 24 hours. The optimum pH range is relatively narrow, below 5 but above 4. Consequently, pH control during hydrolysis may be necessary, since the pH tends to decrease during the hydrolysis (Markkanen and Eklund, 1975). With Trichoderma viride cellulase it has been possible to hydrolize a 10% suspension of waste from the furfural process almost completely in 20 hours at pH 5.0 and 45°C (Technical Research Centre of Finland, unpublished data).
The hydrolysis product, glucose, is inhibitory even at low concentrations (Toyama and Ogawa, 1975). The strong inhibitory effect of lactose should also be mentioned, since commercial Japanese cellulase preparations contain lactose.
Acid hydrolysis of cellulosic materials should also be kept in mind as an alternative. Some essential features for comparison have been collected in Table 4.3. The most important advantage of acid hydrolysis is perhaps reaction rate: the time needed is typically 20 minutes, whereas enzymatic hydrolysis takes several hours. The extremely corrosive conditions of acid hydrolysis cause technical difficulties and high investment costs. On the other hand, the long time needed for enzymatic hydrolysis means a bulky plant, but simple constructions and inexpensive materials can be used, since the temperature is low, the pressure atmospheric and the pH near neutral.
One serious drawback of acid hydrolysis may be the formation of growth-inhibiting compounds, for example decomposition products from sugars. Even a small quantity of compounds toxic to microbes may seriously inhibit SCP production.
| Acid hydrolysis | Enzymatic hydrolysis | |
| Pretreatment | may be necessary | necessary |
| Rate of hydrolysis | rapid (minutes) | slow (hours) |
| Temperature | high (200°C) | low (45°C) |
| Pressure | high | atmospheric |
| Yield | varies depending on material and process details | varies depending on material and process details |
| Formation of interfering by-products | probable | unlikely |
| Industrial processes in use | yes (in U.S.S.R.) | no (pilot plant only) |
| Economical feasibility | ? | ? |
| Source: Linko, 1975. | ||
Acid hydrolysis of cellulosic materials is practised industrially in the U.S.S.R., but calculations of economical feasibility have not been presented. Many figures for the feasibility of enzymatic hydrolysis have been suggested, but for lack of a full-scale plant these figures are uncertain.
Cultivation of micro-organisms on cellulosic materials
If the hydrolysis of cellulose and hemicellulose to fermentable sugars is technologically and economically feasible, the subsequent SCP production is almost routine. There is a free choice of a suitable SCP organism, for example Candida or Paecilomyces, and the process may resemble existing procedures such as those using sulfite waste liquor.
A process based on direct cultivation of micro-organisms on cellulosic materials is bound to use one of a few efficient cellulolytic organisms such as Cellulomonas. Symbiotic growth of Cellulomonas and some β-gluco idase-producing organism such as Candida guillermondii and Trichosporon cutaneum has yielded promising results (Srinivasan, 1975). An essential increase in the biomass yield has been claimed. The raw material was sugarcane bagasse. A mixed culture of Trichoderma viride and Saccharomyces cerevisiae has been cultivated on alkali-treated straw (Peitersen, 1975).
A suitable pretreatment is also necessary prior to direct cultivation of cellulolytic organisms on cellulosic materials. Here again, alkali treatments are efficient but relatively costly.
The Louisiana State University procedure involves first treating the material with a 2 to 4% caustic solution and then subjecting this composite to a temperature of 110 to 130°C for a period of 30 to 60 minutes (Rockwell, 1976). The liquid/solid ratio during treatment is about 2 to 3:1. This relatively severe treatment not only renders the cellulose biodegradable in a subsequent fermentation step but also kills all contaminating organisms.
After alkali treatment the product can be washed free of solubilized lignin, hemicelluloses, xylose and other materials with a yield that depends on the original α-cellulose content of the waste. On urban cellulosics the yields are about 65% while on agricultural wastes, such as bagasse, they are about 50%. However, the entire mass can be fed directly to the fermenter without washing. No further caustic will then be required to hold the pH between 6.6 and 6.8. Furthermore, in addition to the insoluble portion, approximately 15% of the carbohydrates solubilized by the alkali treatment can be consumed by the organisms. On the other hand, the presence of soluble carbohydrates inhibits the cellulases.
Fermentation is normally carried out using a mixed culture of two organisms. A yeast grown symbiotically with Cellulomonas bacteria makes harvesting the protein much easier, because of the large cell size.
The material balance from bagasse to protein is presented in Fig. 4.1.
Production of SCP on soluble process wastes
Production of SCP on sulfite waste liquor has been practised in several countries for a long time. While the future of these processes is uncertain because the sulfite cellulose industry is decreasing, a considerable industry still exists and the waste liquors have to be treated in some way. An alternative to the SCP processes is the production of ethanol. Because the pentoses áre not fermented to ethanol, the remainder could at least theoretically be used for SCP production, but the sugar concentration after ethanol fermentation is low. The chemical composition of sulfite waste liquor is presented in Table 4.4.
A similar line of SCP production may later find a new raw material source in cellulosic waste materials hydrolyzed by cellulolytic enzymes. On this basis a variety of processes with worldwide potential may be developed, provided that the economics of the production of cellulases is further improved.
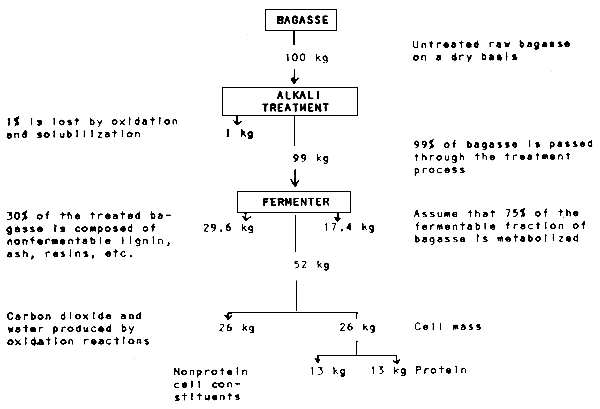
Fig. 4.1. Material balance from bagasse to protein
Source: Rockwell, 1976.
| Lignosulfonic acids | 43% | |
| Hemilignin compounds | 12% | |
| Incompletely hydrolyzed hemi-cellulose compounds and uronic acids | 7% | |
| Monosaccharides | ||
D-glucose | 2.6% | |
D-xylose | 4.6% | |
D-mannose | 11.0% | |
D-galactose | 2.6% | |
L-arabinose | 0.9% | 22% |
| Acetic acid | 6% | |
| Aldonic acids and substances | ||
| not investigated | 10% |
Source: Romantschuk, 1975.
Economic feasibility
Many factors enter into the economics of microbial protein production. Some are typical chemical engineering economics encountered in many kinds of processes, while others are less conventional and require special consideration. Some factors important to the economics of SCP production are set out below (Rockwell, 1976):
1. Raw material
2. Sterility requirements
microbial encroachment
3. Fermentation
4. Cell harvesting techniques
high-speed centrifuges vs. thickeners
5. Washing and purification techniques for removal of:
6. Product value
Factors such as drying costs, bagging and handling costs, etc. are not included in the list, since these are about the same regardless of the type of product. However, they must be included in the final cost analysis.
Esso Research and Engineering has indicated an approximate selling price of 17 U.S. cents per pound of SCP (50% protein) grown on n-paraffin. British Petroleum has indicated a price of 10 to 20 cents per pound for SCP grown on gasoil. Soybean proteins are quoted at a price of 6 to 7 cents per pound on the same basis, while fish flour sells at 15 to 20 cents per pound. It is obvious that if the market for SCP is to be animal feed supplement, then the price to be competed with is the 6 to 7 cents per pound of soybean flour (Rockwell, 1976).
For processes based on the use of cellulases produced in a separate process the enzyme cost is decisive. If an efficient continuous process for production of cellulases in submerged culture can be developed, then many applications become attractive. A real breakthrough would be the development of a constitutive mutant, preferably a thermophile, capable of producing high concentrations of cellulases in a short time without any inducer.
Present technology already enables commercial preparation of cellulases at a cost low enough to make some applications economically promising. Especially useful may be some simple procedures with a long time of hydrolysis, reducing the amount of enzyme needed. These procedures include treatments of silage.
References
Atchison, J.E., Present and potential use of sugar cane bagasse and other nonwood fibers for the manufacture of pulp and paper. A worldwide review. EUCEPA International Symposium, New forest resources for the paper industry and their application. Madrid, May 6–8, 1974, reprint No. 25.
Baumgardt, B.R., Taylor, M.W. and Cason, J.L., Evaluation of forages in the laboratory. II. Simplified artificial rumen procedure for obtaining repeatable estimates of forage nutritive value. J.Dairy Sci.45 (1962) 62.
Coutts, A.D. and Smith, R.E., Factors influencing the production of cellulases by Sporotrichum thermophile, Appl. Environ. Microbiol. 31 (1976) 819.
Dekker, R.F.H. and Richards, G.N., Effect of delignification on the in vitro diqestion of polysaccharides of bagasse. Sci. Food Agric. 24 (1973) 375.
Enarl, T-M. and Markkanen, P., Production of cellulolytic enzymes by fungi. Advances in Biochemical Engineering. Vol. 5. Springer-Verlag, Heidelberg 1977, in press.
Eriksson, K.-E., Enzyme mechanisms involved in the degradation of wood components. Symposium on enzymatic hydrolysis of cellulose. Aulanko, Finland 12–14 March 1975. SITRA, Helsinki 1975, p. 263.
Eriksson, K.-E., and Goodell, E.W., Pleiotropic mutants of the wood-rotting fungus Polyporus adustus lacking cellulase, mannanase and xylanase. Can. J. Microbiol. 70 (1974) 371.
Fogarty, W.M. Griffin, P.J. and Ward, J.A., Microbial fermentation - peat as a nutrient source. Technol. Ireland 1973 April 4, p. 21.
Griffin, H.L., Kaneshiro, T., Kelson, B.F. and Sloneker, J.H., Fermentation of whole feedlot waste and isolated feedlot waste fiber with Trichoderma viride in submerged culture. Symposium on enzymatic hydrolysis of cellulose. Aulanko, Finland 12–14 March 1975. SITRA, Helsinki 1975, p. 419.
Hartley, R.D., Jones, E.C., King, N.J. and Smith, G.A., Modified wood waste and straw as potential components of animal feeds. J. Sci. Food Agric. 25 (1974) 433.
Heikonen, M., Moisio, T. and Kreula, M., Säilörehun sokerit. Karjatalous 52 (1976) Nr. 8, p. 4.
Henningsson, B., Henningsson, M. and Nilsson, T., Defibration of wood using a white-rot fungus. Res. Note R. 78, Department of Forest Products, Royal College of Forestry, Stockholm, 1972, 26 p.
Kato, N. and Shibasaki, I., Production of antimicrobial substances by heat treatment. J. Ferment. Technol. 52 (1974) 177.
Kirk, T.K. and Moore, W.E., Removing lignin from wood with white-rot fungi and digestibility of resulting wood. Wood Fiber 4 (1972) 72.
Linko, M., Energiaa ja protelinia selluloosasta. Kemia-Kemi 2 (1975) 602.
Linko, M., Enzymatic hydrolysis of cellulose material. Advances in Biochemical Engineering. Vol. 5. Springer-Verlag, Heïdelberg 1977.
Mandels, M., Hontz, L. and Nystrom, J., Enzymatic hydrolysis of waste cellulose. Biotechnol. Bioeng. 16 (1974) 1471.
Markkanen, P. and Eklund, E., Enzymatic hydrolysis of furfural process waste. Symposium on enzymatic hydrolysis of cellulose. Aulanko, Finland 12–14 March 1975. SITRA, Helsinki 1975, p. 337.
Millett, M.A., Baker, A.J. and Satter, L. D., Pretreatments to enhance chemical enzymatic and microbiological attack of cellulosic materials. Cellulose as a chemical and energy resource. Biotechnol. Bioeng. Symp. No. 5, John Wiley & Sons, Inc., New York 1975, p. 193.
Nystrom, J.M. and Kornuta, K.A., Pilot plant investigations of Trichoderma viride cellulase production. Symposium on enzymatic hydrolysis of cellulose. Aulanko, Finland 12–14 March 1975. SITRA, Helsinki 1975, p. 181.
Peitersen, N., Single cell protein from straw. Symposium on enzymatic hydrolysis of cellulose. Aulanko, Finland 12–14 March 1975. SITRA, Helsinki 1975, p. 407.
Pringle, S.L., Present and prospective wood resources for expanding pulp wood requirements. EUCEPA International Symposium, New forest resources for the paper industry and their application. Madrid May 6–8, 1974, reprint No. 13.
Rockwell, P.J., Single cell proteins from cellulose and hydrocarbons. Noyes Data Corporation, Park Ridge, New Jersey, U.S.A. 1976, 337 p.
Romantschuk, H., The Pekilo process: protein from spent sulfite liquor. Single-Cell Protein II. S.R. Tannenbaum and D.I.C. Wang, Eds., The MIT Press, Cambridge, Mass. 1975, p. 344.
Rydholm, S., Pulping processes. John Wiley & Sons, Inc., New York 1965, 1269 p.
Sloneker, J.H., Agricultural residues, including feedlot wastes. Symposium on enzymatic conversion of cellulosic materials: technology and applications. Biotechnol. Bioeng. Symp. No. 6, John Wiley & Sons, Inc., New York 1976, p. 235.
Söderhjelm, L. and Lampila, M., The use of waste fibers as absorption material in grass silage. Paperi ja Puu58 (1976) 41.
Srinivasan, V.R., Production of bio-proteins, from cellulose. Symposium on enzymatic hydrolysis of cellulose. Aulanko, Finland 12–14 March 1975. SITRA, Helsinki 1975, p. 393.
Technical Research Centre of Finland, Biotechnical Laboratory, unpublished results.
Toyama, N. and Ogawa, K., Saccharification and agricultural cellulosic wastes with Trichoderma viride cellulase. Symbosium on enzymatic hydrolysis of cellulose. Aulanko, Finland 12–14 March 1975. SITRA, Helsinki 1975, p. 375.
Virkola, N-E., Available cellulose materials. Symposium on enzymatic hydrolysis of cellulose. Aulanko, Finland 12–14 March 1975. SITRA, Helsinki 1975, p. 23.
Wilke, C.R. and Yang, R.D., Process development and design studies for the enzymatic hydrolysis of cellulose. Symposium on enzymatic hydrolysis of cellulose. Aulanko, Finland 12–14 March 1975. SITRA, Helsinki 1975, p. 485.