4.1 Disinfectation of waste streams
4.1.1 Ozone
4.1.2 Ultraviolet light
4.1.3 Disinfection by thermal processing
Unfortunately, there is very little information available (as discussed in Section 3.4) on the survival of pathogens of interest to the farmed salmon and shrimp industries. It is interesting to note that to date, more detailed work has been published on the destruction and survival of viral fish pathogens than on bacteria. This is perhaps surprising since work published upon the former implies that there are adequate means available to quantify survival or destruction. At the present time, the enumeration technologies for bacterial survivors are far more advanced than those available for viruses. The explanations for these observations are not obvious, however it is clear that there is a lack of published quantitative information on both bacterial and viral survival in aquaculture waste.
Insofar as the treatment of aquaculture waste and wastewater is concerned, a number of approaches have been followed in addition to the traditional processing of meal, silage and compost. Waste which has been shown to be contaminated or suspected to be contaminated with bacterial or viral pathogens has been heated; treated with formic acid, sodium hydroxide or halogens such as chlorine and iodine (Torgersen and Hastein, 1995) or calcium oxide, calcium hydroxide, ultraviolet light and/or ozone (Lotz, 1997). The latter two are most applicable to the treatment of wastewater containing only small or no suspended particulates. Although many of these methods have been tested on some types of bacterial and viral pathogens, the work has been spotty and incomplete. Dr. Peter J. Walker of the CSIRO Tropical Agriculture Department in Australia stated that "I believe there is an absence of basic data on the stability of prawn viruses under various conditions of temperature, pH and ionic strength. This is probably because of the absence of cell culture assays for virus titrations" (personal communication). Certainly, the recent Norwegian approach is to process "high risk" offal conservatively at �133�C for at least 20 minutes based upon Law 12.4.1957 No. 2 (1994). This definition of sterilization would no doubt destroy all known pathogens, even if present in high numbers. The real problem is to identify and segregate high risk from low risk waste streams. It is for this reason that the Norwegian government forbids the feeding of aquaculture by-products back to cultured salmon. In fact in Norway, by-products from the farmed salmon industry must be processed in a separate facility from the groundfish or herring offal.
It is clear that the Norwegian salmon industry has learned from experience that biosecurity in all of its forms pays substantial dividends.
In a recent article Lotz (1997) has outlined the requirements for biosecurity in the shrimp industry. Lotz (1997) defines biosecurity as:
" sets of practices that will reduce the probability of a pathogen introduction and its subsequent spread from one place to another"
He further divides biosecurity as it pertains to shrimp aquaculture into two categories:
Quantitative risk assessment of shrimp farming facilities was then categorized in three different ways as shown in Figure 4.1. The first is water status where WS-1 indicates that the source contains no wild animals (e.g. ground water) and contains no effluent from other facilities; WS-2 indicates that the source contains wild animals and/or contains effluent from other facilities, but is pre-treated (U.V. light, O3, chlorination, etc) and WS-3 which indicates that the source contains wild animals and or effluent from other facilities and is untreated.
The second is isolation status category where IS-1 means the facility is physically and operationally isolated from all other facilities; and IS-2 means the facility is not adequately isolated physically or operationally isolated from other facilities. The third is the pathogen status rating where PS-A means that the facility has been monitored for at least six months and no pathogens detected; PS-B means that the facility has been monitored for < six months and no pathogens detected; and PS-C indicates that the facility is contaminated.
Figure 4.1 also indicates the equal relative importance of adequate disinfection of feed, equipment and waste streams including mortalities and water effluent. The absolute requirement for knowledge of the dose-response curves for disinfection of waste steams is essential and an understanding of the quantitative effects of physical and chemical methods of disinfection on pertinent pathogens is implicit. Of course, it goes without saying that the biosecurity of the entire facility is also dependent upon pathogen-free broodstock and incoming water.
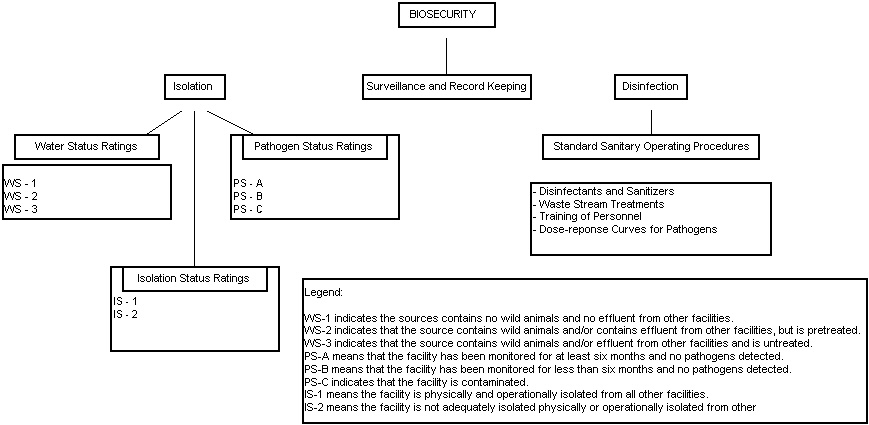
Figure 4.1 Risk Assessment in Shrimp Farming Operations (Adapted from Lotz, 1997)
Waste streams cannot be treated effectively with common chemical disinfectants since most losetheir efficacy in the presence of heavy organic loadings with the possible exception of iodine. In addition, chemical disinfectants, themselves are pollutants if released into the environment. Therefore there are only three practical well-established means of dealing with aquatic pathogens found in the waste streams and these include ozone, ultraviolet irradiation and heat treatment.
Ozone (O3) is a powerful oxidant which has proven effective in a range of aquaculture applications over the years. It reacts rapidly with pathogenic bacteria and viruses and produces primarily oxygen as an end product, thus making it more environmentally-friendly than most other chemical disinfectants. Its main drawbacks are cost of generation and because of its highly reactive nature, it is potentially hazardous to humans and cultured fish. Most commercial ozone is produced by passing oxygen between two plates separated by an electrical potential, a process which is energy-intensive and the process itself is only about 10% efficient (Summerfelt and Hochheimer, 1997).
Storage of ozone is also a problem since compression of the gas generates heat which in turn causes the breakdown of O3. Thus on-site generation is presently the only option for potential users of this disinfectant. In addition, ozone reacts and degrades some plastics such as PVC and accelerates the corrosion process for unprotected steel, making it difficult or unwise to use steel-reinforced concrete which could come in contact with ozone-treated water.
The real limitation of ozone gas is that it is highly reactive and loses its potency when in contact with organic material and particularly reactive to nitrites (Rosenthal and Kruner, 1985). Its efficacy as a disinfectant is reduced in the presence of bicarbonate and carbonate ions which scavenge OH radicals which are produced in ozonated water (Legube et al., 1986).
Ozone was shown to reduce bacterial pathogens (Aeromonas salmonicida, Vibrio anguillarum, Vibrio salmonicida, Yersinia ruckeri) by at least 4 log cycles within 60 sec (residual = 0.20 mg/L) in fresh water, but took substantially longer in a salt water environment (Liltved et al., 1995).
Infectious pancreatic necrosis virus was also reduced by 4 log units within a 60 sec exposure period. In this same study, Liltved et al. (1995) also pointed out that although effective for the disinfection of both inlet and effluents from aquaculture operations, residual O3 levels of 0.0093 mg/L were highly toxic to trout.
Owsley (1991) reported that O3 treatment of infectious haematopoietic virus in water was effective as a disinfectant at the 0.20 mg/L level within a 10 min time period. Colberg and Lingg (1978) reported that O3 was effective in reducing four fish bacterial pathogens (Aeromonas salmonicida, Aeromonas liquifaciens, Pseudomonas fluorescens, and Yersinia ruckeri) by 99% in a simulated recirculation system. There is no doubt that O3 is an effective disinfectant when used for water treatment and Theisen et al. (1998) successfully used ozone to disinfect nauplii of brine shrimp used as a starter diet for larval fish.
However, ozone must be used to disinfect waters with relatively low organic solids content and thus not really applicable to treating heavily loaded aquaculture waste streams.
Ultraviolet light has been used for many years to disinfect water in land-based (closed) aquaculture systems. U.V. light disinfects water used to depurate shellfish which have been exposed to faecal pollution. Spotte and Adams (1981) pointed out that although U.V. light may be used effectively to eliminate pathogens from inlet or outlet waters, in conventional closed recirculation systems, the generalized bacterial destruction kinetics were first order, suggesting that regardless of the size or efficacy of the U.V. system, the numbers of fish pathogens never reach zero. The same first order kinetics are often seen in various types of disinfection including both physical and chemical methods. It is important therefore that not only the slope of the inactivation curves are determined, but also that one knows initial numbers of organisms to be destroyed (see Section 4.1.3).
The application of U.V. disinfection is limited to water and contaminated surfaces. U.V. does not have the ability to penetrate into cracks and crevices as do chemical disinfectants. Also, U.V disinfection is dependent upon clarity of both the water to be disinfected as well as the irradiation sources which are often prone to the accumulation of algae and organic deposits.
Although U.V. light is capable of disinfecting both fresh and sea water sources (Liltved et al., 1995) it is far more effective at eliminating bacterial pathogens than viral pathogens and cannot be used to disinfect solid wastes. Such wastes may be decontaminated by heating and/or chemical treatments.
Perhaps the most appropriate way of dealing with solid or semi-solid aquaculture waste streams is to apply heat until a specified number of pathogens has been eliminated. In food processing of low acid foods in hermetically sealed containers, heat is applied according to a pre-determined process protocol which is scientifically determined for each product type, container and product formulation. Modern day thermal processing is based upon the thermal death time characteristics of pathogenic organisms, and in particular, conservative (safe) thermal processes are always based upon the most heat-resistant pathogens. In addition, it is important to realize that the conduction of heat through solid wastes such as fish offal, will vary according to the source of the waste, particle size and shape, bone content, degree of spoilage before processing, composition, etc. In Norway, much of the silage produced from fish processing plants is collected and shipped to strategic locations such as Reiber and Sons in Tromso where the liquified waste is put through a continuous heat exchange process to ensure destruction of all pathogens and spoilage organisms.
Figure 4.2 a illustrates a typical survivor curve where heat is applied to a pathogenic microorganism (bacteria or virus) at a constant lethal temperature. Typically, the rate of destruction follows first order kinetics where a plot of log10 survivors is linear with time of heating at a constant temperature. The thermal resistance at any given temperature may conveniently be expressed as the ‘decimal reduction time’ (D) which is defined as the time (minutes) for the survivors to be destroyed by one log cycle which represents 90% of the initial population.
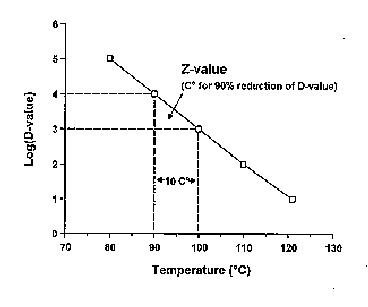
To take the problem of thermal process design one step further, consider a bacterium which is heated at different lethal temperatures for varying time periods. If the ‘D’ values determined for each heating temperature, are then plotted on semi-log paper against heating temperature, a linear relationship is typically obtained (Fig. 4.2 b) and is called the ‘thermal death time’ (TDT) curve for a specific organism. The TDT curve allows the thermal process authority to determine the lethal effects of a process in which the temperature changes with time. Such data is normally determined by incubating a known number of organisms in a thermostatically controlled bath for different time periods and then determining numbers of survivors after heating for different time periods. These data can only be reliably determined if suitable methods for survivor enumeration are available. These methods are often available for fish bacterial pathogens but many of the viral pathogens cannot be accurately enumerated. Such data are essential if reliable thermal processes are to be developed for the treatment of solid aquaculture waste. For a more complete treatment of thermal process science, see Stumbo (1973).
It is perhaps important to note that the initial pathogen load is extremely important when designing such a process. For example, in Fig. 4.2 a, the process depicted represents a 7 D reduction at 121�C. If the initial pathogen load had been 1010 organisms per gram, one could reasonably expect that on average the final load would be 103 organisms per gram waste.
The most limiting factor against the implementation of the thermal processing of aquaculture waste is the prerequisite for the ability to enumerate pathogen survival. This is possible for many of the bacterial pathogens but enumeration methods are available for only a limited number of the viral pathogens. Another consideration for this approach is cost. Certainly, the initial processing into silage on site and then the subsequent thermal treatment of silage at a central processing facility is probably the most practical approach, provided waste not decontaminated by the silage process is transported without environmental contamination.
Figure 4.2 a Typical survivor curve for the heat treatment of food borne pathogens at any single temperature
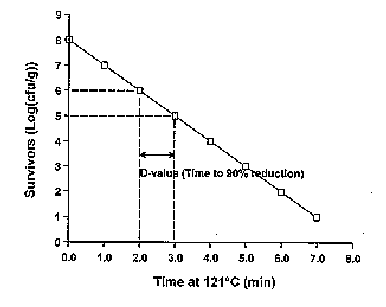
Figure 4.2 b Typical thermal death time curve showing the relationship between decimal reduction times for any particular pathogen and heating temperature