

0114-B1
M.K. Hossain, R. Ahmed and M.B. Uddin[1]
A laboratory experiment was conducted to assess the growth inhibitory effect of leaf extracts (of different concentrations) of Albizia lebbeck and their possible phytotoxicity on some common agricultural crops (Brassica juncea (L.) Czern & Coss; Cucumis sativus L.; Phaseolus mungo L.; Raphanus sativus L. and Vigna unguiculata (L.) Walp.) as a bioassay material. The experiment was conducted in sterilized petridishes with a photoperiod of 24 hours at an average temperature of 30.0 0C. The effect of aqueous extracts of different concentrations was compared to distilled water (control). The aqueous extracts of Albizia lebbeck leaf caused both stimulatory and inhibitory effect on germination, root and shoot elongation and development of lateral roots of receptor plants. Bioassays indicate that the inhibitory effect was pronounced for higher concentrations, whereas the lower concentration showed stimulatory effect in some cases. The inhibitory effect was much pronounced in root and lateral root development as compared to the germination and shoot development of the receptor crops.
Albizia lebbeck, one of the important and preferable fast growing tree species in Bangladesh is distributed through out the country both in the public and private forests including homesteads. The species is incorporated in agroforestry programs as an associated species, but it seems that it has some inhibitory effect on agricultural crops and ground vegetation which might have been caused either by fallen leaves or plant leachates or root exudates. These exudates can be detrimental to other plants and play an appreciable role in the distribution of vegetation, the yield of various crops and weed interferences (Muller, 1966; del Moral and Muller 1970, Putnum and Duke, 1978; Rice, 1984). Much work on allelopathy has been conducted in agriculture and forestry (Overland, 1966; McPherson and Thompson, 1972; Chou and Patrick, 1976; Lodhi, 1975a, b, 1976). Some species currently used in agroforestry systems reportedly have allelopathic properties (del Moral and Muller 1970; Suresh and Rai, 1987; Watanabe et al., 1988). Joyakumer et al. (1978), Rao and Reddy (1984) and Melkania (1987) studied the inhibitory effect of Eucalyptus, Bamboo and Teak on germination and seedling growth of certain food crops but no research work so far carried out herein Bangladesh. King (1979) pointed out the need for allelopathic research of various tree species used in agroforestry as there is a good chance of allelochemicals produced by the intercrop trees affecting food and fodder crops. Therefore, it seems essential that the allelopathic compatibility of crops with trees should be checked before been introduces to agroforestry system. Hence, the purpose of the present study was to elucidate the allelopathic potential of different concentration of Albizia lebbeck leaf extracts on some common agricultural crops used in Bangladesh.
Albizia lebbeck was considered as the donor plant and the receptor plants selected were Indian mustard (Brassica juncea (L.) Czern & Coss), Cucumber (Cucumis sativus L.), Black gram (Phaseolus mungo L.), Radish (Raphanus sativus L.), and Falen (Vigna unguiculata (L.) Walp.).
The aqueous extracts were prepared from fresh leaf of 7 years old Albizia lebbeck trees. 100 gram of fresh senescent leaves were soaked in 500 ml of distilled water. The aqueous extract was filtered through sieve after 24 hours and then some extracts were diluted to make the concentrations of 10%, 25%, 50% and 75% and stored for seed treatment experiments. The following treatments were used in the experiment:
To = Seeds treated with distilled water only (Control),
T1 = Seeds treated with leaf extracts of 10% concentration,
T2 = Seeds treated with leaf extracts of 25% concentration,
T3 = Seeds treated with leaf extracts of 50% concentration,
T4= Seeds treated with leaf extracts of 75% concentration, and
T5= Seeds treated with leaf extracts of 100% concentration.
The germination test was carried out in sterile petridishes of 12 cm in size placing a Whatman No.3 filter paper on each petridish. The extract of each concentration was added to each petridish of respective treatment in such an amount just to wet the seeds. The control was treated with distilled water only. 20 seeds of each agricultural crop were placed in the petridish and each treatment was replicated 5 times. The petridishes were set in the analytical laboratory of the Institute and the experiment extended over a period of seven days to allow the last seed germination. The seed was considered as germinated when the radicle emerged and the germination was recorded daily. The results were determined by counting the number of germinated seeds, number of lateral roots and measuring the length of primary root and main shoot on 7th day of the experiment. The data were subjected to analysis of variance and Duncan's Multiple Range Test (DMRT). Ratio of germination and elongation were calculated as suggested by Rho and Kil (1986).
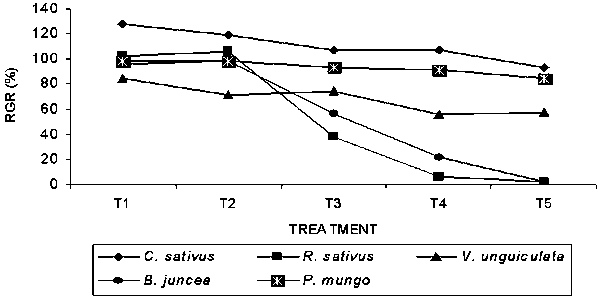
The germination percent of all the 5-receptor plants are shown in Table 1. In most cases variation of germination percent due to concentration varied evenly. With the increase of concentration, the inhibitory effect was progressively increased. In all cases maximum inhibitory effect was found in T5 treatment (100% conc.) except V. unguiculata. Maximum inhibitory effect (-98.33%) was found on B. juncea in T5 treatment followed by R. sativus (-98.0%) in the same treatment and the lowest (-1.67%) was found on B. juncea at T2 treatment. The maximum (128.57%) relative germination ratio (RGR) was found in C. sativus at T1 treatment while the minimum (1.67%) was in B. juncea at T5 treatment (Fig-1). Among the receptor crops, C. sativus was less sensitive to the application of different extracts. However, the species responded differently with the extracts. It was also observed that leaf extracts of A. lebbeck delayed the germination significantly in all the receptor corps in comparison to the control.
Table 1. Germination percent of receptor agricultural crops to distilled water (T0) and different concentrations of Albizia lebbeck extracts (T1-T5). Values in the parenthesis indicate the inhibitory (-) or stimulatory (+) effects in comparison to control (T0) treatments.
|
Treatment |
Agricultural crops |
||||
|
C. sativus |
R.sativus |
V. unguiculata |
P. mungo |
B.juncea |
|
|
T0 |
70.00 bc* |
83.33 a |
98.33 a |
98.33 a |
100.00 a |
|
T1 |
90.00 a |
85.00 a |
83.33 ab |
96.67 a |
96.67 a |
|
T2 |
83.33 ab |
88.33 a |
70.00 bc |
96.67 a |
98.33 a |
|
T3 |
75.00 abc |
31.67 b |
73.33 bc |
91.67 a |
56.67 b |
|
T4 |
75.00 abc |
5.00 c |
25.00 d |
90.00 ab |
21.67 c |
|
T5 |
65.00 c |
1.67 c |
56.67 c |
83.33 b |
1.67 d |
*values in the columns followed by the same letters(s) are not significantly different (P£0.05) according to Duncan's Multiple Range Test (DMRT)
Fig. 1. Relative germination ratio (RGR) of bioassay species grown in petridishes at different concentrations of Albizia lebbeck extracts (T1-T5).
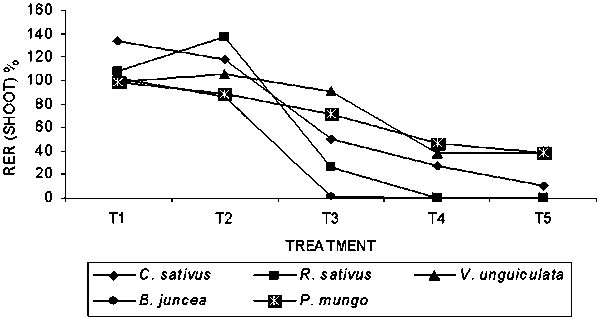
The mean shoot length (cm) of the seedlings of all the receptor agricultural crops are shown in Table 2. Stimulatory effect was found in T1 and T2 treatment in comparison to control but the inhibitory effect was progressively pronounced with the increase of leaf extract concentrations. In all cases significant inhibitory effect of shoot length was found in T5 treatment followed by T4 and T3 treatment respectively. Among the germinated seedlings, the maximum (-99.40%) inhibitory effect was found on B. juncea at T3 treatment followed by C. sativus (-90.23%) at the T5 treatment and the lowest (-0.75%) inhibitory effect was found on V. unguiculata at T1 treatment. The highest (+36.92%) stimulating effect on shoot elongation was found on R. sativus at T2 treatment followed by C. sativus (+34.11%) at T1 treatment. Maximum (17.37cm) elongation of shoot was observed in V. unguiculata followed by P. mungo (17.32cm) in control treatments. Among the survivors, maximum (134.11%) relative elongation ratio (RER) of shoot was observed in C. sativus at T1 treatment while the minimum (59%) was in B. juncea at T3 treatment (Fig-2).
Table 2. Shoot length (cm) of receptor agricultural crops to distilled water (T0) and different concentrations of Albizia lebbeck extracts (T1-T5). Values in the parenthesis indicate the inhibitory (-) or stimulatory (+) effects in comparison to control (T0) treatments.
|
Treatment |
Agricultural crops |
||||
|
C. sativus |
R. sativus |
V. unguiculata |
P. mungo |
B.juncea |
|
|
T0 |
7.27 b* |
6.69 b |
17.37 a |
17.32 a |
3.37 a |
|
T1 |
9.75 a |
7.18 b |
17.24 a |
17.15 a |
3.43 a |
|
T2 |
8.59 ab |
9.16 a |
18.27 a |
15.36 b |
2.92 a |
|
T3 |
3.63 c |
1.75 c |
15.76 a |
12.35 c |
0.002 b |
|
T4 |
1.95 cd |
0.00 d |
6.47 b |
8.07 d |
0.00 b |
|
T5 |
0.71 d |
0.00 d |
6.77 b |
6.61 d |
0.00 b |
* values in the columns followed by the same letters(s) are not significantly different (P£0.05) according to Duncan's Multiple Range Test (DMRT).
Fig. 2. Relative elongation ratio (RER) of shoot of bioassay species grown in petridishes at different concentrations of Albizia lebbeck extracts.
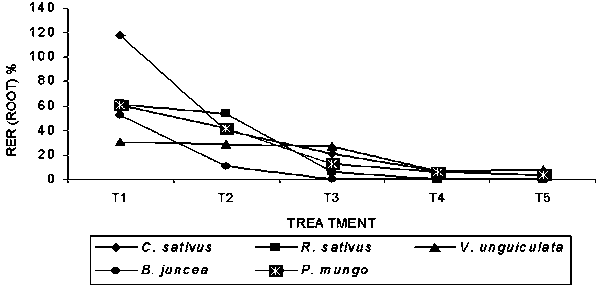
The root length of all the 5 receptor plant was greatly inhibited with the increase of extract concentration except C. sativus, where stimulating effect was observed at T1 treatment (Table-3). The inhibitory effect was pronounced at T5 treatment followed by T4, T3 and T2 treatment respectively. Among the survivors, the highest (-99.65%) inhibitory effect was found on B. juncea at T3 treatment followed by C. sativus (-96.65%) at the T5 treatment. Maximum (19.15cm) root length was observed in R. sativus followed by V. unguiculata (16.21cm) in control treatment. Maximum (117.83%) relative elongation ratio (RER) of root was observed in C. sativus at T1 treatment while the minimum (0.39%) was in B. juncea at T3 treatment (Fig-3).
Table 3. Root length (cm) of receptor agricultural crops to distilled water (T0) and different concentrations of Albizia lebbeck extracts (T1-T5). Values in the parenthesis indicate the inhibitory (-) or stimulatory (+) effects in comparison to control (T0) treatments.
|
Treatment |
Agricultural crops |
||||
|
C. sativus |
R.sativus |
V. unguiculata |
P. mungo |
B.juncea |
|
|
T0 |
7.46 a* |
19.15 a |
16.21 a |
7.57 a |
8.64 a |
|
T1 |
8.79 a |
11.87 b |
4.91 b |
4.57 b |
4.58 b |
|
T2 |
2.99 b |
10.29 b |
4.71 b |
3.19 c |
0.93 c |
|
T3 |
1.52 b |
1.09 c |
4.39 b |
0.97 a |
0.003d |
|
T4 |
0.49 b |
0.00 c |
1.06 c |
0.45 d |
0.00 d |
|
T5 |
0.25 b |
0.00 c |
1.27 c |
0.31 d |
0.00 d |
* values in the columns followed by the same letters(s) are not significantly different (P£0.05) according to Duncan's Multiple Range Test (DMRT)
Fig. 3. Relative elongation ratio (RER) of root of bioassay species grown in petridishes at different concentrations of Albizia lebbeck extracts (T1-T5).
Considering the number of lateral root development, the study revealed that root development significantly decreased with the increased concentrations. In all cases significant effect was found in T5 treatment (except C. sativus) followed by T4, T3 and T2 treatments respectively. In all cases control treatment had the highest mean lateral root number than other treatment except C. sativus on which stimulating (+48.66%) effect was found in T1 treatment. Among the survivors, the highest (-96.34%) inhibition was found on R. sativus at T3 treatment followed by C. sativus (-88.46%) in T5 and the lowest (-21.72%) in P. mungo at T1 treatment. Maximum (41.87) number of lateral roots was found in V. unguiculata followed by R. sativus (40.13) in control treatments.
Table 4. Number of lateral roots developed in receptor agricultural crops to distilled water (T0) and different concentrations of Albizia lebbeck extracts (T1-T5). Values in the parenthesis indicate the inhibitory (-) or stimulatory (+) effects in comparison to control (T0) treatments.
|
Treatment |
Agricultural crops |
||||
|
C. sativus |
R.sativus |
V. unguiculata |
P. mungo |
B juncea |
|
|
T0 |
16.73 ab* |
40.13 a |
41.87 a |
14.73 a |
7.20 a |
|
T1 |
24.87 a |
22.53 b |
18.47 b |
11.53 b |
4.87 b |
|
T2 |
11.33 bc |
20.53 b |
15.60 b |
8.20 c |
1.87 c |
|
T3 |
8.73 bc |
1.47 c |
16.20 b |
6.47 cd |
0. 00d |
|
T4 |
4.40 c |
0.00 c |
5.60 c |
5.20 cd |
.00 d |
|
T5 |
1.93 c |
0.00 b |
9.07 c |
4.60 d |
0.00d |
* values in the columns followed by the same letters(s) are not significantly different (P£0.05) according to Duncan's Multiple Range Test (DMRT)
Response of the bioassay species to aqueous extracts varied among the 5 crops and differed significantly between concentrations for each bioassay species. It was observed that crops with small seed viz. B. juncea and R. sativus were affected with the increased extract concentration. This agreed with the findings of Lucena and Doll (1976) who found that seed size is an important factor and species with small seeds are more adversely affected. The results revealed that the leaf extracts inhibit germination of crop seeds to a certain extent and even in some cases complete inhibition was occurred. Concentrations from 25% and above significantly reduced the germination, growth and development of root, shoot and lateral root and also caused some mortality. The effect was more pronounced at higher concentrations, where the effect was so severe that T4 and T5 treatments completely inhibited the growth of some test crops. The survivors exhibited varying degree of necrosis and chlorosis, thin and grayish in color. Many seedlings lost their ability to develop normally as a result of reduced radicle elongation and root necrosis. Overall growth of seedlings was also reduced in almost all the treatments compared to control. It was concluded that the inhibition of seed germination and seedling growth was concentration dependent, i.e. inhibition was more in higher concentrations. These findings agreed with the report of Rai and Triputhi (1984) and Rizvi and Rizvi (1987) who reported that allelopathy includes both promoting and inhibitory activities and is a concentration-dependent phenomenon. Mortality of the seedlings and reduced vigor under laboratory conditions indicate the accumulation of toxic substances (allelopathic potential) of the donor plant, which is harmful to the growth of seedlings of receptor plants. These findings also correlated with the report of Rice (1984), Chou and Kuo (1986), Waller (1987) and Chou (1992), who also found that many species within the Leguminosae family contain secondary plant products that have allelopathic potential. This result also correlated with the result of Bora et al. (1999) the allelopathic effect of Acacia auriculiformis leaf extracts on seed germination of some agricultural crops. Rao and Reddy (1984) also found the inhibitory effect of Eucalyptus (hybrid) leaf extracts on germination of certain food crops. Eyini et al., (1989) reported the allelopathic effect of Bambusa arundinacea on Arachis hypogaea.
Marked reduction in root length was noticed in most of the seedlings compared to shoot length and germination. The results are in conformity with the earlier findings of Zackrisson and Nilsson (1992) in which root growth is more sensitive and responds more strongly to small variation in toxin concentration.
So, it may be concluded that the water soluble leachates from the senescent leaves of Albizia lebbeck has the allelopathic potential to reduce the germination as well as suppress the growth and development of agricultural crops. Allelopathics are often due to synergistic activity of allelochemicals rather than to single compounds. Under field conditions, additive or synergistic effects become significant even at low concentrations (Einhelliing and Rasmussen, 1978) Though laboratory bioassays in allelopathic research are of great importance, long-term field studies must be recommended to carry out before using of Albizia lebbeck in any agroforestry systems of the country.
Bora, I. P., Singh, J., Borthakur, R., and Bora, E. 1999. Allelopathic effect of leaf extracts of Acacia auriculiformis on seed germination of some agricultural crops. Ann. For. 7 (1): 143-146.
Chou, C. H. 1992. Allelopathy in relation to agricultural productivity in Taiwan: Problems and Prospects. In Allelopathy: Basic and Applied Aspects. ed. S.J.H. Rizvi, V. Rizvi, pp. 179-204, Chapman and Hall, London.
Chou, C. H., and Kuo, Y. L. 1986. Allelopathic exclusion of understory by Leucaena leucocephala (Lam.) de wit. J. Chem. Ecol. 12: 1431-48.
Chou, C. H., and Patrick, Z. A. 1976. Identification and phytotoxic activity of compounds produced during decomposition of corn and rye residues in soil. J. Chem. Ecol. 2: 369-387.
delMoral, R. and Muller, C.H. 1970. Allelopathic effect of Eucalyptus camaldulensis. The American Midland Naturalist. 83:254-282
Einhellig, F.A., and Rusmussen, J.A. 1978. Synergistic inhibitory effects of vanilic and p-hydroxybenzoic acids on radish and grain sorghum. J. Chem. Ecol. 4: 425-436.
Eyini, M., Joyakumer,M., and Panniselvam, S. 1989. Allelopathic effect of Bamboo leaf extract on the seedling of groundnut. Trop. Ecol. 30(1): 138-141.
Joyakumer, M., Eyini, M., and Panniselvam, S. 1978. Allelopathic effect of Bamboo root extract on the seedling of groundnut and corn. Geobios. 14: 221-224.
King, K.F.S. 1979. Agroforestry and the utilization of fragile ecosystems. For. Ecol. Management. 2:161-168.
Lodhi, M.A.K. 1975a. Allelopathic effects of hackberry in a bottomland forest community. J. Chem. Ecol. 1: 171-182.
Lodhi, M.A.K. 1975b. Soil- plant phytotoxicity and its possible significance in patterning of herbaceous vegetation in a bottomland forest. Amer. J. Bot. 62: 618-622.
Lodhi, M.A.K. 1976. Role of allelopathy as expressed by dominating trees in lowland forest in controlling productivity and pattern of herbaceous growth. Amer. J. Bot. 63: 1-8.
Lucena, H. M., and Doll, J. 1976. Growth- inhibiting affects of Cyperus rotundus L. on sorghum and soybeans. Revista Comalfi 3: 241-256. Plant Growth Regular Abstr. 3: 1192.
McPherson, J.K., and Thompson, G. L. 1972. Competitive and allelopathic suppression of understory by Oklahoma oak forests. Bull. Torrey Bot. Club. 99: 293-300.
Melkania, N. P. 1987. Allelopathy and its significance on the production of agroforestry plant associations. In: Agroforestry for rural needs, Khosla, P. K. and Khurana, D. K. (eds.) ISTS, Solan. 221-224.
Muller, C.H. 1966. The role of chemical inhibition (allelopathy) in vegetational composition. Bull.Torry Bot. Club. 93:332-351.
Overland, L. 1966. The role of allelopathic substances in the " smother crop" barley. Amer. J. Bot. 53: 423-432.
Putnum, A. R. and Duke, W. B. 1978. Allelopathy in agroecosystem. Annual Rev. Phytopathol. 16: 431-451.
Rai, J.P.N., and Tripathi, R.S. 1984. Allelopathic effects of Eupatorium riparium on population regulation of two species of Galinsoga and soil microbes. Plant and Soil. 80:105-117.
Rao, N.S. and Reddy, P.C. 1984. Studies on inhibitory effect of Eucalyptus (hybrid) leaf extracts on the germination of certain food crops. Ind. Forester. 110 (2): 218-222.
Rho, B.J. and B.S. Kil. 1986. Influence of phytotoxin from Pinus rigida on the selected plants. J. Nat. Sci., Wonkwang Univ., 5:19-27.
Rice, E L. 1984. Allelopathy. Second Edition. Orlando, Florida: Academic Press. 422pp.
Rizvi, S.J.H., and Rizvi, V. 1987. Improving crop productivity in India. Role of Allelochemicals. In Allelochemicals: Role in Agriculture and Forestry. ed. G.R.Waller, pp. 69-75, ACS Symposium Series 330.
Suresh, K.K. and Rai, R.S.V. 1987. Studies on the allelopathic effects of some agroforestry tree crops. Intern. Tree Crops. J. 4: 109-15.
Waller, G.R. (ed.). 1987. Allelochemicals: Role in Agriculture and Forestry. ACS Symposium Series 330. American Chemical Society, Washington, D.C. 606 pp.
Watanabe, H. Sahunalu, P., and Khenmark, C. 1988. Combinations of trees and crops in the taungya method as applied in Thailand. Agroforestry Systems. 6: 169 -177.
Zackrisson, O. and Nilsson, M.C. 1992. Allelopathic effects by Empetrum hermaphroditum on seed germination of two boreal tree species. Can. J. For. Res. Vol. 22.
| [1] Institute of Forestry and
Environmental Sciences, Chittagong University, Chittagong-4331, Bangladesh.
Email: [email protected] |