

0127-B1
P. Abasolo[1], Hiroyuki Yamamoto, Masato Yoshida and Takashi Okuyama
The forest has provided enormous benefits to man since the beginning of time. To ensure the continuity of such goods and services, forest plantations and finding alternative raw materials are essential. One important alternative material is rattan cane. The present report deals with the thermal softening of Palasan cane (Calamus merrillii Becc.) aimed at improving its processing. To accomplish this, creep compliance test at different temperature settings was evaluated using a Thermomechanical Analyzer (TMA). Structural as well chemical analyses were performed to determine the cause of such behaviour. Creep compliance of the samples increased monotonously with loading time similar to wood. Compliance rates were more or less the same for all temperature settings except for one particular setting that significantly deviated from the rest. This temperature represents the softening temperature of that particular site. Creep compliance curves of the different settings provided a good description of the heat sensitivity of the cane. Higher compliance values would mean lower thermal sensitivity and lower compliance values means higher thermal sensitivity of the material. Thermal softening was highly influenced by the cellular make-up of the stem, as well as the sensitivity of the individual chemical constituents that comprise the cell wall. Among the tissues, ground parenchyma promoted thermal softening of the cane. Within the cell wall, hemicellulose was the element that encouraged softening. As the cane is heated, hemicellulose will soften first permitting the cellulose constituents to move freely within the walls. Once the maximum softening temperature is reached, hemicellulose will regain its original solid state and retain the new configuration of the stem. This process continues as long as no permanent damage takes place within the walls due to the overheating of the material.
Since time immemorial the forest have provided people of various goods and services that enabled them to survive. Indeed from the cradle to the grave, men have depended on the forest. Now, the forest is besieged with a lot of pressures brought about by the continues increase in the demand for the finished products resulting to the over exploitation of the resource. Not to mention the unhampered conversion of forest lands into agricultural as well as residential lands to accommodate the growing population. Thus, plantation establishment and also alternative raw materials are very vital to meet future demands.
One good alternative raw material is rattan canes. With its unique characteristics, it is extensively used in the furniture industry providing employment to several hundreds of people worldwide. In fact, this resource is ranked second in importance, just next to timber, with its contribution to the Gross National Product (GNP) of developing countries such as the Philippines. Because of its significance as a forest resource, there are already a lot of studies on rattan canes aimed at improving it’s processing. However, most of them are only concentrated on its structure, physical and mechanical properties. None has yet tackled the influence of heat on cane softening. Thus, this research was conducted to evaluated the impact of heat on the thermal softening of Palasan canes. It is our aim to explain how softening occurs with in the stem in order to determine the best possible condition for the efficient conversion of rattan canes.
Sample preparation
Samples from the basal, middle and top portion of the stem of Palasan canes (Calamus merrillii Becc) were collected. These samples were dissected into peripheral, intermediate and core sections. From these sections, 0.45 x 0.5 x 15 cm sticks were prepared. Similarly, 40 - 60 mesh grounded samples were processed from the individual sections using a Wiley mill.
The sticks were further reduced to 0.5 cm match-ending blocks. The measurement involves the detection of minute changes in length which could be affected by microstructural prestresses (Spatz et al. 1999) and irreversible thermal-expansion (Sasaki and Okuyama 1983) of the samples. To reduce this possibility the blocks were boiled in water for at least two hours. To evenly distribute the load on the cross sectional surface of the blocks, 0.12 - 0.17 mm thick micro cover glass was glued on the smoothened surface of the block. Thermal expansion of the cover glass was considered negligible due to its thickness.
Tissue analysis
Thin transverse-sections were dissected out from the sample blocks using a sliding microtome. These slices were stained with safranin and fast-green and were mounted on a clean glass slide. Using a Ziess microscope with a Kontron image analyzer attachment, fiber, vascular bundle and ground parenchyma area percentage were determine. Average of 25 measurements were used in the analysis.
Chemical analysis
Extractive was first removed from the sample powders of the individual zones with an ethanol-benzene solvent (1:2) using the Soxhlet apparatus. From the extractive samples, alphacellulose, holocellulose, lignin content by weight were determined following the ASTM standards of 1975 (D1104-56, D1103 - 60, D1106-56). The average of two measurements was used in the evaluation.
Creep compliance test
Creep compliance of 11 temperature settings (from 50ºC to 100 ºC, with a 5 deg interval) was measured using a Seiko-Extra 6000 Thermomechanical Analyzer (TMA). To initiate measurement, instrument calibration was performed. Next, the tip of the probe was allowed to touch the surface of the sample and a 5 g compressive load was applied. This enabled the machine to automatically detect the height of the sample. The whole set-up was then submerged in water set at the desired temperature for 10 min. This allowed the sample and water temperature to equalize. Finally, load was elevated to 150 g, the probe height was reset to zero, then the compressive strains brought about by the load were determined for 30 min. Creep compliance was then derived using the formula:
S(t) = e(t)/d0 (1)
where S(t) is the creep compliance (MPa - 1), e(t) is the amount of strain and d0 is the amount of applied stress (MPa). Average compliance of two measurements per temperature setting was used in the analysis.
Tissue analysis
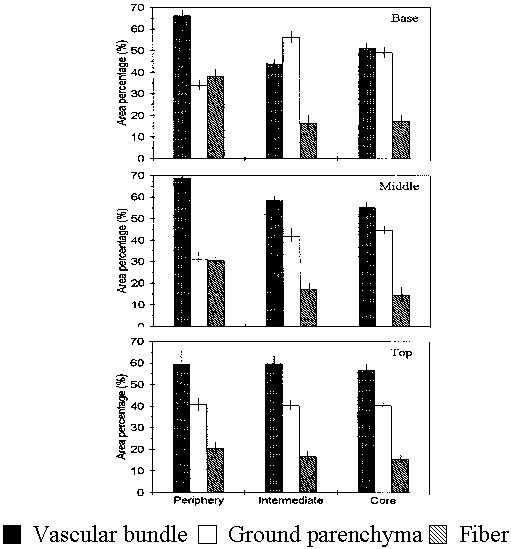
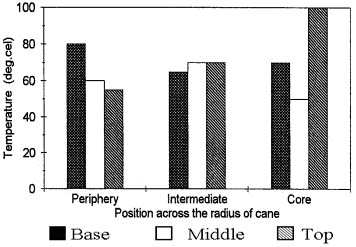
Vascular bundle area percentage was highest at the periphery and gradually decreases towards the core (Fig. 1). Similar trend was observed for fiber area percentage. Ground parenchyma area percentage showed a inverse trend. Parenchyma was highest at the core and tends to decrease towards the peripheral region.
Fig. 1 Tissue are percentage across the radius of the cane
Chemical analysis
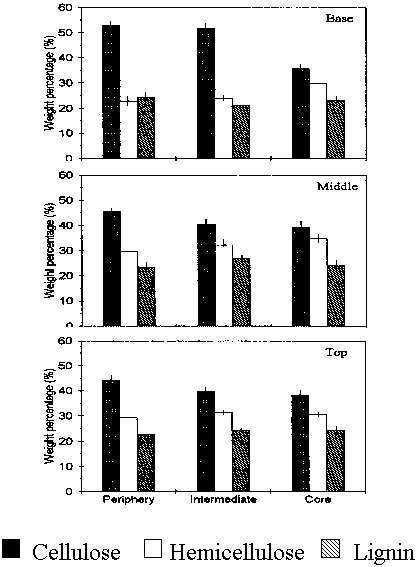
Figure 2 represents the proportion of the individual chemical constituents of the rattan cellwall of the different zones. Cellulose content ranged from 36 - 53%, hemicellulose was from 22 - 35% while lignin was from 21 - 27%. Cellulose tend to decrease from periphery to core and from base to top, while hemicellulose exhibited a consistent increase from periphery to core. Lignin, on the other hand, did not show any precise trend. This result was comparable to the chemical composition of bamboos (PROSEA 1995) and wood (Haygreen and Bowyer 1989).
Fig. 2 Proportion of the major chemical constituents of the cell wall
Creep compliance
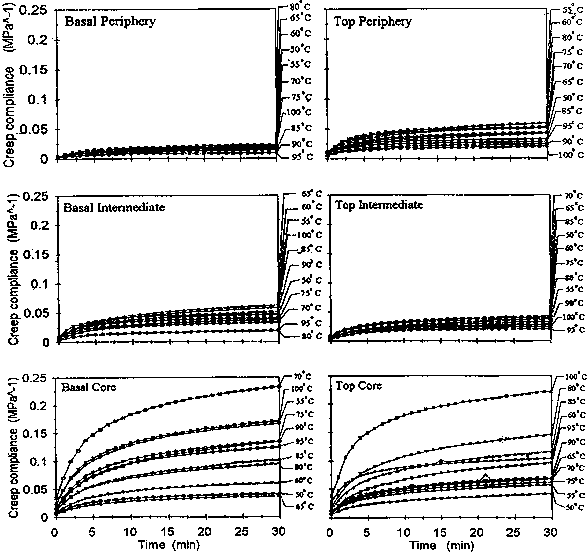
Similar to the observation of Dwianto et al. (2000) on sugi, creep compliance curves of the individual temperature setting tend to increase monotonously with time (Fig. 3). It was highest at the core and lowest at the periphery. This shows that the reaction of the match-ending blocks to heat was more or less the same. The only difference was the amount of change per temperature setting. Nevertheless, this difference was relatively small except for one particular setting that significantly deviated from the rest. For example, this was observed at 70°C for the basal-core and 100°C for the top core region.
Influence of moisture content
Water is a good plasticizer or softener. The intake of water or other low molecular diluents could loosen the bonds between elements, e.g., lignin, hemicellulose, even before the application of heat (Hillis 1984; Salmen 1984). This will allow the cellulose constituents to move freely within the walls, reducing the mechanical strength of the cane. For this reason, water was used as the heating medium in all the experimental runs. In so doing, moisture content was maintained all throughout the experiment.
Fig. 3 Creep compliance curves for the different temperature settings
Thermal softening of palasan
To further clarify the influence of heat on creep compliance, the compliance values of every point at 20 min in Figure 3 were extracted and were plotted against temperature (Fig 4). Result showed that regardless of the position along the length of the stem, mild changes was noticed at the peripheral region in contrast to the very abrupt changes in the core. This would mean that the periphery of the stem is more stable to changes in strength caused by heat than the core. Therefore, the sensitivity of the individual site to heat is different from one another.
To better describe this sensitivity, the temperatures where maximum compliance was observed in figure 4 were extracted and plotted in Figure 5. Considering only the basal position, it was deduced that peripheral region was less sensitive to heat because of its high softening temperature (80°C) than the intermediate (65°C) and core sections (70°C). This would imply that the different parts of the stem would behave differently to the application of heat.
Fig. 4 Influence of heat on the maximum creep compliance
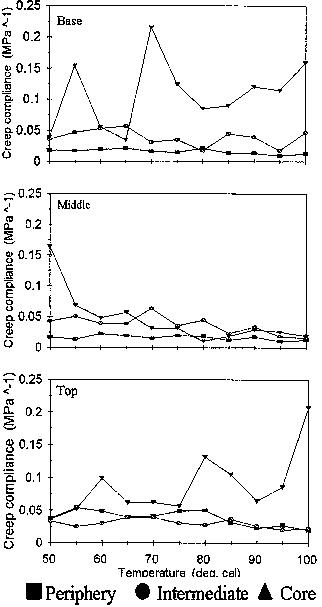
Fig. 5 Temperature where maximum creep compliance was observed
Inflluence of structure on the thermomechanical properties of palasan
Figure 6 shows the relationship between vascular bundle percentage (a) and ground parenchyma percentage (b) on the maximum creep compliance of the individual sites. As observed, increase in bundle percentage resulted to the reduction in maximum compliance. This would mean that the bundle inhibited the change in the length of the samples caused by the compressive load.
Fig. 6 Influence of tissue area percentage on maximum creep compliance
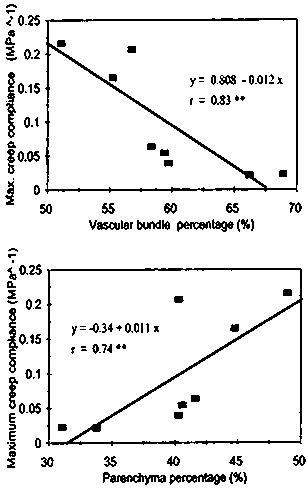
Ground parenchyma cells, on the other hand, promoted this change in length brought about by the load. As the quantity of the parenchyma cells in a particular site increases, the larger the amount of compliance. This further proved the cushioning effect of the parenchyma cells to the structure as discussed in an early report (Abasolo et al. 1999). To prevent the vascular bundle from being damaged by the compressive load, the bubble-like nature of the parenchyma cells will absorb such load and will enable the bundles to slightly bend without breaking.
Influence of chemical composition on the thermomechanical properties of palasan
Cellulose provides the skeletal framework for the cell wall responsible for its high tensile strength. Hemicellulose, on the other hand, serves as the matrix substance while lignin imparts rigidity to the structure (Kollmann and Côté 1968). Among the three, cellulose is the most stable to heat changes because of its crystalline nature. Hemicellulose and lignin, on the other hand, are more pliable and is more likely to be affected by heat due to their amorphous character.
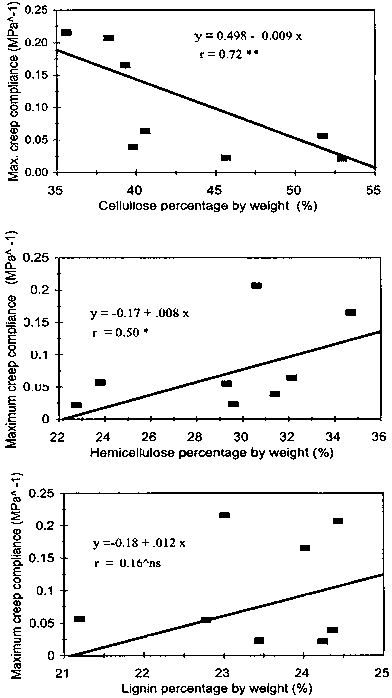
Softening temperature of these chemical constituents differ from one another. This is mainly due to their differences in bonds e.g., H-bond, Carbon-carbon bond etc; and differences in their chemical configuration. Reports showed that cellulose softens at 230ºC (Fengel and Wegener 1984) while lignin and hemicellulose at 100ºC and 80ºC (Hillisand Rozsa 1978), respectively. Figure 7 provides the relationship between the individual chemical constituents and the maximum creep compliance. Cellulose was negatively correlated with maximum creep compliance while hemicellulose showed a positive correlation. This revealed how significant the impact of hemicellulose to the softening of the cane. In the case of lignin, no significant relationship was observed.
Fig.7 Influence of the individual chemical constituents of the wall on the maximum creep compliance
It was hypothesized that as the rattan cane is heated up, hemicellulose will soften first allowing the movement of the cellulose within the walls. This movement will permit the cane from being shaped into different configuration. Once the maximum softening temperature of hemicellulose is attained, it will gradually re-harden, retaining the new configuration of the cane. This process can be repeated for several times as long as no permanent damage will take place on the chemical constituents of the walls due to overheating.
The behavior of the stem of Palasan (Calamus merrilli Becc.) under varying temperatures for a particular period of time was more or less similar to that of wood. Generally, different temperature settings produced more or less the same compliance rates except for one particular temperature, which deviated greatly from the rest. This temperature represents the softening temperature of that particular portion.
Creep compliance curves at the different temperatures provided a good description of the heat sensitivity of the cane. Higher compliance values would mean that the material is thermally sensitive. Such sensitivity was highly influence by the structure of the materials as well as its chemical constituents. It was therefore deduced that, among the tissues, ground parenchyma promoted thermal softening of the cane. Within the cellwall, hemicellulose was the element that encouraged softening. As the cane is heated, hemicellulose will soften first permitting the cellulose constituents to move freely within the walls. Once the maximum softening temperature is reached, hemicellulose will regain its original solid state and retaining the new configuration of the stem. Such process will continue as long as no permanent damage will take place within the walls due to overheating of the material.
Abasolo, W., M. Yoshida, H. Yamamoto and T. Okuyama. 1999. Internal stress generation in rattan canes. IAWA Journal. 20(1): 45 - 58.
ASTM. 1975. Standard test methods for alphacellulose in wood. ASTM standard D1103-60 (reaffirmed in 1968). Philadelphia, PA: Americal Society for Testing and Materials. 336-338 pp.
ASTM. 1975. Standard test methods for alphacellulose in wood. ASTM standard D1104-56 (reaffirmed in 1972). Philadelphia, PA: Americal Society for Testing and Materials. 339 p.
ASTM. 1975. Standard test methods for alphacellulose in wood. ASTM standard D1106-56 (reaffirmed in 1966). Philadelphia, PA: Americal Society for Testing and Materials. 342-343 pp.
Dwianto, W., Morooka, T and M. Norito, 2000. Compressive creep of wood under high temperature steam. Holzforschung. 54(1): 104 - 108.
Fengel, D. and G. Wegener, 1983. Wood. Chemistry, Ultrastructure, Reactions. Walter de Gruyter and Co., Berlin, 66 - 181 pp.
Haygreen,J.L. and J.L. Bowyer. 1989. Forest Products and Wood Science. An introduction. Second edition. Iowa State University Press, Iowa. 42 p.
Hillis, W.E., 1984. High temperature and chemical effects on wood stability. Part 1: General considerations. Wood Science and Technology, 18: 281 - 293.
Hillis, W.E and A.N. Rozsa. 1978. The softening temperature of wood. Holzforschung, 32(2): 68 - 73.
Kollmann, F.F.P. and W.A. Côté Jr., 1968. Principles of Wood Science and Technology. Solid Wood 1. Springer-Verlag. Berlin, 55 - 56 pp.
PROSEA, 1995. Plant Resources of South East Asia. No. 7. Bamboos. Eds. S. Dransfield and A. Widjaja. Backhuys Publishers, Lieden. 24 - 25.
Salmen, L., 1984. Viscoelastic properties of in situ lignin under water-saturated conditions. Journal of Material Science. 19: 3090 - 3096.
Sasaki, Y. and T. Okuyama, 1983. Residual stress and dimensional changes on heating green wood. Mokuzai Gakkaishi. 29(4): 302 - 307.
Spatz, H., L. Koehler and K.J. Niklas, 1999. Mechanical behavior of plant tissues: Composite materials or structures? Journal of Experimental Biology, 202(23): 3269 - 3272.
| [1] Department of Forest Products
and Paper Science, College of Forestry and Natural Resources, University
of the Philippines Los Baños, Laguna, Philippines, 4031. Tel: 63-49-536-3494;
Email: [email protected]
|