

0425-A1
Mustafa Akyuz[1], Aysegul Sahin, Hakk Alma, Ibrahim Bektaþ and Ayhan Usta
Because of increasing petrol prices and the high energy requirements in the production of synthetic polymers, the valuation of bark wastes or other lignocellulosics in material applications, rather than in energy production, is growing in importance. Moreover, the development of degradable polymeric products (i.e., biodegradable, photodegradable, chemidegradable, envirodegradable ones) has become increasingly important from an ecological standpoint.
In this study, some wood barks of several tree species such as Pine, Cedar, Eucalyptus, Acacia, Chestnut, Poplar, Oak, Spruce and Alder were phenolated by using sulphuric acid (H2SO4) as a catalyst at a temperature of 130°C for 1 hour. The phenolated barks obtained were cured and hardened with hexamethylentetramine (HMTA) and other materials. Then they were ground in a mortar to obtain fine molding powders. The molding compounds thus prepared were molded into test specimen in a die with a dimension of 4.0 x 10.0 x 80.0 mm at 190°C under a pressure of about 30 kgf/cm2 for 5 minutes. Then, the mechanical and biodegradation properties (static flexural strength and modules of elasticity) of the phenolated bark-based molding materials were evaluated. In addition, ash percent of barks was determined. Mechanical properties (i.e., the modulus of rupture (MOR) and modulus of elasticity (MOE)) were determined according to Japanese Industrial Standard K 7203. Ash percent of barks was determined according to ASTM Standard Designation E 1755-95. Moreover, in this study Fusarium equiseti, Aspergillus candidus, Sordaria fimicola, Penicillum freguentans and Byssochlamys, fulves fungi were used for the decomposition of molding materials in soil.
The results showed that the barks of various tree species were easily phenolated in the presence of sulphuric acid and the phenolated bark could be molded under the pressure and temperature, like commercial novalak resin. On the other hand, the reaction type of the bark phenolysis could be classified as exothermic as in the production of phenol-formaldehyde resin.
Bark constitutes approximately 10-15 % (by volume) of tree (1). This ratio changes remarkably according to species. The rising of oil prices and the high energy requirements in the production of synthetic polymers urge the future use of bio-energy sources such as bark wastes or the other ligno-cellulosics in material application rather than in energy production. Meanwhile, a big necessity to develop degradeble polymeric products has been ingreasig from ecological standpoint, so requiring that biomass wastes incorporate into biodegradable.
As a result of these, barks are an important biomass resources. These huge amounts of barks have almost completely been burning for getting energy so far, which is not reasonable way of using a material. Therefore, in this study we aimed at eva luatng the bark wastes as an important raw material in the making of composite materials of high value (2).
As is known obviously, plastics can be processed for a variety purposes by hot-melt molding, whereas barks and similar lignocellulosics cannat. This results from the lack of plasticity of these materials; lignocellulosics cannot be melted, dissolved or softeed sufficiently for making molding materials. Therefor, the methods for processing lignocellulosics are very limited. If plastic properties could be given to lignocellulosics, it would be a more useful material.
For example, in the production of resin-type molding materials, after being mixed with a curing agent, e.g., hexamethylenetetramine (HMTA), wood floor as a filler and other coventional molding agents, the resultant phnolated wood bark matrixes can be molded at a temperarure of about 190-200°C for about 2-5 min (3-4).
In this study, thr barks of several tree speciess, such as Calabrian pine, Cedar, Eucalyptus, acacia, Anatolia chestnut, Spruce, alnus barbata and Turk oak, were phenolated in the presences of sulfuric acid as a catalyst. The concentration on the phenolation parameters (e.g.,) the amonts of unreacted bark residue and the reacted phenol so-called “reacted phenol”, and the obtained pheolated barks were cured with hexamethylenetetramine (HMTA) at abput 190°C under pressure (5-6). Subsequently, the mechanical properties of the phenolated bark-based molding materials were evaluated.
The barks of Calabrian pine (Pinus brutia Ten.), Cedar (Cedrus libani), Eucalyptus (Eucalyptus camaldulensis), Acacia (Robinia pseudoacacia), Anatolia Chestnut (Castenea sativa), Spruce [Picea orientalis (L). Link], Blak alder (Alnus barbata) Oak (Querqus cerris) and White poblar (populus densifora) tree speciescollected from the various forests in Turkey, were used instead of formaldehyde in making commerical novalak-type resin. Chemicals used in the study are as follows: Phenol (as sovent), sulfuric acid (as catalist), methanol (as diluting agent), hexamethylenetetramine (HMTA) (as curing agent), Zinc stearate (as lubricating agent) and calcium hydroxide (as accelerating agent). All the chemicals used without further prification. Commerical novalak resin was used as a commerical product and provided from plastic engineering.
Twenty grams of the above mentioned grained tree bark powder (20-80 mesh-dry), phenol (20,30 and 40 g) in crystal form and 96 % sulfuric acid (0.5,1, 2 and 4 % based on phenol weight) were charged into seperable four-necked glass resin vessel (500 ml) heated by using a hating mantle equipped with a temperature controller and a thermocouple inseted in the reacror at 110, mainly 130 and 150°C under atmospheric pressure for 60 min.
After the completion of each phenolation process (i.e., at end of reaction), the resulting mixture was diluted with methanol and then filtered with aglass-fiber filtre (3.1 ìm particle retainable) to seperate the residue of biomass (methanol- insoluble part) from methanol-soluple part. The resulting resudie was oven dried and weighed. Then, the amount of residue was determined in percent. The sulfuric acid remained in the methanol-soluble part was neutralized with magnesium oxide (MgO), and the neutralized part solution was once again filtered with a glass-fiber to move the salt obtained during the neutralization process. Eventually, methanol was evaporated from the solution at 50°C under vacum, and the free phenol (unreacted phenol) was distiled under a reduced pressure of 40 mm Hg T 180°C for 1 h to get condensed phenolated bark in a solid form.
The phenolated bark matrix dissolved in acetone was mixed together with HMTA, zinc stearate, and Ca(OH)2. The resulding mixtures were kept in an oven at 70°C for 1 h to remove acetone and then ground in a mortar to obtain fine molding powders. The molding compounds thus prepred were molded into test specimen in adie with a dimension of 4.0 x 10.0 x 80.0 mm at 190°C under a pressure of about 40 MPa for 5 min.
In order to determine the bark residue, mixtures obtained at end of reaction were diluted with methanol and filtered. The remaining residue was oven-dried at 103 ± 20°C in an oven and weighed. Finaly, the amount of bark residue was calculeted by the following Eq.1.
BR (%) = [(W0-Wr)/W0] X 100
1
Were, BR is the residue in percent, Wo is the starting amount of bark (g) and Wr is the amount of bark residue (g).
At end of phenolation reaction, the amount of phenol reacted with the bark compenents (e.g., cellulose, lignin and hemicellulose), i.e. “reacted phenol”, was calculated by the following Eq.2;
RPh (%) = [Pt- (Wo - Wr)/(Wo - Wr)] X 100
2
Were, RPh is the percent reacted phenol and Pt is the total amount of phenolated barks (g).
In order to understand the percentage of inorganic material in biomass used in this study. It was decided to measure the ash percents of the biomass. Ash percent was determined according to ASTMstandart designation E 1755-95. The oven-dried samples were placed in the dried percelain crucibles and furmaced at 575 ± 25°C for 4 hours. Then, the ash percent was calculated by the followimg Eq. 3;
Ash percent (%) = [(Wac-Wc)/Wo] X 100
3
Were, Wac is the total weight of ash and crucible after furnacing (g), Wc is the weight of crucible (g) and Wo the amont of starting biomass (g).
Mechanical properties (i.e., the modulus of rupture (MOR) and modulus of elasticity (moe) were determined according to JIS (Japonese Industrial Standart) K 7203 with the exception that dimensions of the specimens are 4.0 X 10.0X 80.0 mm. The crosshead speed used was 2 mm / min. The properties were determined by using a universal testing machine (Instone) equipped with a computer containing a pactage program for mechanical properties. For the measurement of the mechanical properties in dry-state, the samples were conditional at 20 ± 2°C and 60 ± 5 % RH (relative huidity) for at least 1 week. For the measurements, five samples were used and the average calculated by the following Eqs. 4 and 5 available in the standart, respectively;
MOR = [(3PD/2WH2)] (MPa)
4
MOE =[D3/(4WH3) X (F/Y)] (MPa)
5
were, MOR is being strength or “modulus of repture” (MPa), MOE is the modulus of elasticity (MPa), P is maximum breaking load (N), D is ditsnce between two stands (mm), W is the width of sample (mm), H is the thickness of sample (mm) and F/Y is the slope of stress-strain curve (N/mm).
In this study Fusarium equiseti, Aspergillus candidus, Sordaria fimicola, Penicillm frequentans Byssochlamys fulves fungi were used. The fungi used in this study were provided from the collektion of Biology department of Science & Literature Faculty of Uludað University in Bursa/Turkey. The fungi were kept in Malt-Extract Agar (MAE) medium at 4°C.
The fungi have been incubated for 7-14 days having been injected into (MAE) medium at 25°C (7). Then, 10 ml sterilized physiologic water was dropped to the culture, and spore solution was prepared by being mixed with injection needle slowly (6-7).
0.2 ml of this solution was injected into 100 ml of the modified Czapek-Dox Broth (mCDB) (i.e., Glycose, 10; extract, 1.0; pepton, 1.0; NaNO3, 2.0; K2HPO4, 1.0; KCl, 0.25; MgSO4.7H2O, 0.5; FeSO4.7H2O, 0.001, ZnSO4.7H2O, 0.01 g/l) flask of 250 ml, in which tree baks-based molding materials dried and sterlized, was incubated at 25°C for 6 monts. After the termination of process, the fungi were inactivated in an oven at 80°C for 30 min (8-9). The obtained mycell mass was washd away, oven dried at for 105°C for 3 days, determined the weight loss (in percent) weighing the oven-dried samples.
In order to investigate the decomposition of the molding materials in the soil, sample (500 g) was taken and put into beaker of 250 ml. The so-prepared medium was sterilized at 121°C for 1 h. The fungi species developed by injecting them into the MEA were added to the soil. The fungi were homogeneously mixed with soil under the sterilized conditions and incubated at 25°C for 6 mounts. So as to keep the soil in moist, the sterilized physiologic water (10 ml) was added to each soil sample every 7 days. Eventualy, the molding material samples were removed from the soil and mycell particular on them, oven-dried at 105°C and weighed. By determining the weight before and after incubation, the weight losses (W1) in percent due to decomposition were calculated as follows 6;
WL (%) = [(Wo - W1)/Wo] X 100
6
Were, Wo is the weight of the sample before incubation (g) and W1 is the weight of the sample after incubation (g).
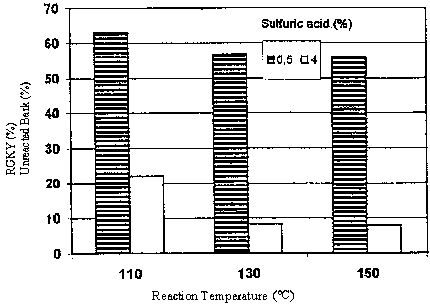
However, in this study, the phenolysis of bark was carried out by using a system with electrically heating mantle equipped with internaly temperature control. Therefore, it is very important to find out the most sufficient and reasonable temperature for the latter system. Picture 1 shows the relationship between reaction temperature and bark residue as a function of sulfiric acid concentration. As shown in this figure, the effective temperature for getting the minimal amount of residue in the phenolysis of bark is 130°C for acid concentrations of 0.5 and 4%, whereas for the oil-bath the best externally controlled temperature was 150°C (3-4). Therefor, in this study, a reaction temperature of 130°C was selected as the main reaction temperature.
Picture 1.The Effect of Reaction Temperature on the Bark Residue Percent as a Function of Sulfuric Acid Catalyst Concentration
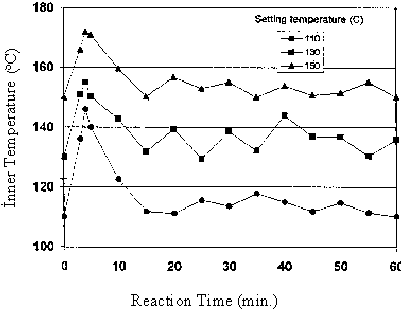
Picture 2 demonstrates the inner temperature of the phenolysis of the bark vs. reaction time as a functoin of various reaction temperature. The initial reaction of biomass with phenol in the presence of acid is strongly exothermic as already reported for the reaction between phenol and formaldehid (8).
Picture 2. Variation in Inner Temperature Versus Different Reaction Times
On the other hand, because of the new heating system applied in this study and new biomass (tree bark), whose density is much lower than the wood. It was necessary to find out the effective marginal bark to phenol ratio for the phenolysis of bimass. For this purpose, pine bark was once again selected as a reference biomass. The results are listed table 1. By increasing bark to phenol ratio from 1 to 2 (by weight), the residue fraction is found for bark to phenol ratio of 2. Therefor, abark to phenol ratio of 2 was cosidered as an effective level.
Table 1. Relationship Between Bark to Phenol Ratio and Unreacted Bark Percent
|
Bark-phenol ratio (W/W1) |
Bark - residue percent (%) |
|
1.0 |
71.2 |
|
1.5 |
55.7 |
|
2.0 |
42.7 |
Acid concentration, 1%; reaction temperature, 130°C reaction time, 1 h
Picture 3 shows the influence of sulfuric acid concentration on the residue percent of bark remained after the phenolysis process. The acid concentration has an important effect on the resiude fraction, namely, as the acid concentration increase from 0.5 to 4 %, the resiude fraction decreases significantly for all the bark species including poplar wood species used as a comparion material. In addition, the residue fraction found for the bark phenolysis at the catalyst concentrations of 1to 2 % is found to be quite similar to those for poplarwood. Thus the pine barks can also be phenolated successfully the same as wood for making bakelite-like rigid plastics.
Picture 3. The Unreacted Percents of the Several Phenolated Bark Samples As a Function of Various Sulfuric Acid Concentration
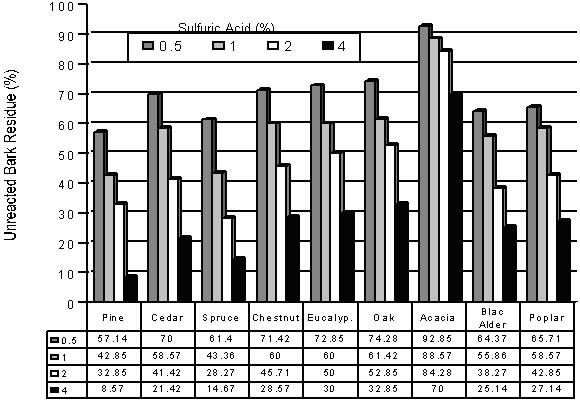
The effect of acid concentration on the acacia species is found to be less significant tan the others. This can surely be explained by a much higher ash content (7.89 %), as demonstrated in table 2, which neutralizes the sulfuric acid used as catalyst.In future studies, ash content of the whole barks species will be analyzed.
Table 2. The Ash Percents of Bark Species and Values of pH Before and After Phenolysis
|
Bark Species |
Ash Percent (%) |
pH Acid Concentration (%) |
|
|
1 |
2 |
||
|
Pine |
1.79 |
1.72 |
1.35 |
|
Cedar |
4.71 |
1.80 |
1.38 |
|
Eucalyptus |
4.46 |
2.40 |
2.25 |
|
Oak |
5.24 |
2.10 |
1.94 |
|
Chestnut |
5.73 |
2.51 |
2.36 |
|
Acacia |
7.89 |
4.25 |
3.38 |
|
Poplar |
0.24 |
0.72 |
0.53 |
|
Spruce |
2.86 |
1.75 |
1.29 |
|
Black Alder |
3.1 |
2.80 |
2.50 |
When the acid concentration reaches 4 %, the residue can be reduced to a level as 8 % for pine bark. As can also be seen from the same figure, the most effective bark species reacted with phenol in the presence of the acid is pine bark, followed by cedar, chestnut, eucalyptus, oak and acacia bark species. This can be attributed to its higher tanning and much lower ash percent (1.89 %) (table 2) in comparison to the other materials. Thus longer ash contents results in larger pH values, which decrease the reaction efficiency.
Picture 4 depicts the influence of acid concentration on the amount of reacted phenol. As shovn, when the acid concentration increases from 0.5 to 2 %, the amount of reacted phenol distinctively increase, however, it slows down obviously beyond a level of 2 %, shwing that 2 % acid concentration seems to be better in order to obtain a satisfactory level of reacted phenol.
The highest reacted phenol fracton is found to be for pine bark, followed by cedur, chestnut, eucalyptus, oak and acacia bark species.
Picture 4. Reacted Phenol Amounts of Various The Phenolated Bark Samples at Various Sulfuric Acid Concentrations
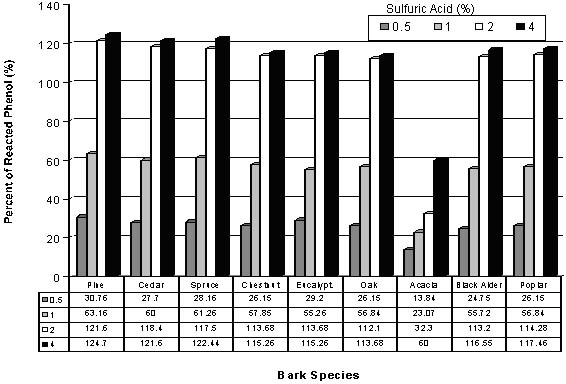
Table 3 shows the MOR values of the phenolated bark-based molding materials increase with further increase in catalyst concentration from 0.5 to 2 % and then bcome constant with further increase in catalyst concentration for the whole bark species used in this study. The molding materal prepared with phenolated pine bark has the lowest MOR values for all the catalyst levels. The molding materals made from the phenolated cedur, chestnut, eucalyptus and barks have almost the same MOR values. On the other hand, the MOR values of the phenolated bark-based molding materials obtained at the catalyst concentration of 2 % are found to be comparable to those of phenolated wood, prepared by using a catalys concentration 2 %, and commercal novalak resin-based materials.
Tablo 3. Bending Strength Values of Various Phenolated Bark-Based Molding Materials as a Function of Catalyst Concentration
|
Bark Species |
Bending Strength (Mpa) |
|||
|
Concentration of H2SO4 |
||||
|
0.5 |
1.0 |
2.0 |
4.0 |
|
|
Pine |
74.83 |
80.52 |
93.79 |
93.92 |
|
Cedar |
72.81 |
78.93 |
94.64 |
93.81 |
|
Eucalyptus |
71.45 |
79.85 |
92.79 |
92.54 |
|
Oak |
72.79 |
78.65 |
92.90 |
93.87 |
|
Chestnut |
73.75 |
79.66 |
93.63 |
94.78 |
|
Acacia |
64.50 |
76.95 |
78.85 |
81.82 |
|
Poplar |
70.50 |
75.54 |
80.24 |
81.40 |
|
Spruce |
73.21 |
79.25 |
92.45 |
93.20 |
|
Black Alder |
62.15 |
72.35 |
76.19 |
80.37 |
Table 4 shows the MOE values of the phenolated bark samples as a function of catalyst concentration. The MOE values of the phenolated bark-based molding materals increase with increasing the catlyst concentration from 0.5 to 2 % and then level of with frther increase in thecatalyst concentration for all the bark speciess. The molding material prepared with phenolated pine bark has the highest MOE values On the other hand, the MOE values of the phenolated bark-based molding materials prepared at the catalyst concentration of % 2 are comparable to those of the phenolated wood and commercal novalak resin-based molding materials, showing that they have almost the same stiffness.
Table 4. Values of Modulous of Elasticity of Various Phenolated Bark-Based Molding Materials as a Function of Catalyst Concentration
|
Bark Species |
Modulous of Elasticity (MOE x 10-²MPa) |
|||
|
Concentration of H2SO4 |
||||
|
0.5 |
1.0 |
2.0 |
4.0 |
|
|
Pine |
73.76 |
78.52 |
84.28 |
85.92 |
|
Cedar |
72.89 |
75.93 |
80.63 |
81.93 |
|
Eucalyptus |
70.65 |
74.85 |
81.79 |
81.54 |
|
Oak |
71.09 |
75.65 |
80.90 |
79.36 |
|
Chestnut |
70.75 |
75.86 |
81.67 |
82.83 |
|
Acacia |
63.53 |
68.95 |
74.85 |
74.39 |
|
Poplar |
64.75 |
67.62 |
73.86 |
73.42 |
|
Spruce |
72.75 |
78.10 |
83.30 |
84.50 |
|
Black Alder |
69.40 |
74.15 |
80.18 |
81,40 |
Table 5 shows the inital and final weights and loss of the phenolated tree barks molding materials due to biodeterioration in Broth system containin various fungi species.
Table.5. The Weight and weight Loss Percents of Pine Bark-BasedMolding Materials Subjected to Various Fungi Species
|
Fungi Species |
Reaction Conditions (1) |
Reaction Conditions (2) |
Reaction Conditions (3) |
||||||
|
Agirlik Weight |
Yüzde Percent |
Agirlik Weight |
Yüzde Percent |
Agirlik Weight |
Yüzde Percent |
||||
|
W0 (gr) |
W1 (gr) |
W2 (%) |
W0 (gr) |
W1 (gr) |
W2 (%) |
W0 (gr) |
W1 (gr) |
W2 (%) |
|
|
Fusarium equiseti |
0.793 |
0.681 |
14.1 |
1.174 |
0.966 |
17.7 |
0.816 |
0.787 |
3.5 |
|
Byssochlamys fulves |
0.899 |
0.612 |
31.9 |
1.007 |
0.891 |
11.5 |
1.063 |
1.003 |
5.6 |
|
Asperqillus candidus |
0.894 |
0.820 |
8.3 |
0.768 |
0.696 |
9.3 |
0.921 |
0.912 |
0.9 |
|
Sordaria fimicola |
0.984 |
0.633 |
27.1 |
0.739 |
0.602 |
18.5 |
0.986 |
0.957 |
0.3 |
|
Pencillium frequentans |
0.868 |
0.671 |
10.6 |
1.218 |
1.103 |
9.4 |
0.949 |
0.921 |
2.9 |
Reacton Condition (1): Temperature, 150°C; Time, 30 min; Phenol-to-tree baks- ratio, 2; Sulfuric acid concentration, 1 Reaction condition (2): Temperature, 150°C; Time, 60 min; Phenol-to-tree baks- ratio, 2; Sulfuric acid concentration, 1 Commerical novalak resin-based molding material (3)
1. It was found that the phenolysis of barks of various tree species in the presence of sulfuric acid, used as a catlyst, was feasible.
2. The amount of ash in the bark became very effective on the phenolysis parameters, the contents of uncreacted bark residue and reacted phenol, i. E., the higher ash, the higher content of unreacted bark residue and lower content of reacted phenol
3. It was proven that the reaction type occurring between bark compenents, such as cellulose, lignin, hemicellulose, etc., was exothermic as in commerical phenol-formaldehyde resin
4. The temperature (130°C) applied in the internally eating system used in this study was fond to be lower than that (150°C) applied in externally heating system, which saves the energy in the resin industry
5. Furthermore, the flexurel properties of all the phenolated bark-based molding materials studied, excluding phenolated acacia bark, were quite comparable to those of phenolated wood- and commerical novalak molding ones
1. Akyüz, M., 1997. Studies on the Physical and Mechanical Properties of Picea orientalis (L.) Link. Wood, Eastern Black Sea Forestry Research Institute, Technical Bulletin, 3, pp. 4-11. Trabzon, Turkey.
2. Alma, M.H., 1996. Ph.D. Thesis, Kyoto University, pp. 1-5, Kyoto, Japan.
3. Alma, M. H., Yoshioka, M., Yao, Y., and Shiraishi. N., 1996. Wood Science Thecnology, pp. 30, 39-47.
4. Alma, M.H., and Shiraishi, N., 1998. Journal of Polymer Engineering, 18, pp. 179-198.
5. Shiraishi, N., Kishi, H., 1986. Journal Applied Polymer Science, 32, pp. 3189-3209.
6. Alma, M.H., 1997. Journal of Polymer Engineering, 17, pp. 311-322.
7. Alma, M.H., Yoshioka, M., Yao, Y. And Shiraishi, 1996. Holzforschung, 50, pp. 85-90, Germany.
8. Whitehouse, A.A.K., Pritchett, E.G.K., Barnett, G., 1967. Phenolic Resin, Iliffe Books Ltd., pp. 115, London, England.
| [1] Eastern Black Sea Region
Forestry Research Institute, P.K., 90, 61040, Trabzon, Turkey. Email: [email protected] |