T- toxicological evaluation; R-residue and analytical aspects
*New compound
TOXICOLOGY
Cyprodinil is the ISO approved name for (4-cyclopropyl-6-methyl-pyrimidin-2-yl)-phenyl-amine. Cyprodinil is a systemic fungicide that acts by inhibiting the biosynthesis of methionine. Cyprodinil has not been evaluated previously by JMPR.
In studies of metabolism in rats, radiolabelled cyprodinil administered by gavage as a single dose of 0.5 or 100 mg/kg bw, or as repeated doses of 0.5 mg/kg bw per day for 14 days, was rapidly absorbed from the gastrointestinal tract and excreted. Approximately 75% (range 71-85%) of an orally administered dose was absorbed over 48 h. At doses of both 0.5 and 100 mg/kg bw, two plasma level maxima of radioactivity were observed at approximately 0.5-1 h and 8-12 h, probably owing to reabsorption of material excreted in the bile. Approximately 92-97% of the administered dose was eliminated within 48 h in the urine (48-68%), faeces (29-47%), and bile (accounting for up to 35.4% of the dose in cannulated rats), with elimination being almost complete by 7 days. Seven days after single or repeated oral administration at the low dose, total tissue residues accounted for 0.15-0.60% of the administered dose. Cyprodinil was primarily metabolized by hydroxylation of the phenyl and pyrimidine rings and methyl group, and excreted mainly as glucuronide or sulfate conjugates in urine, faeces and bile. Approximately 3-8% of the parent compound was detected in the faeces. Excretion, distribution and metabolite profiles were essentially independent of dose, pre-treatment and site of radiolabel, although there were some quantitative sex-dependent differences in urinary metabolites.
Cyprodinil has low toxicity when administered by the oral, dermal or inhalation routes. LD50 values after oral administration were >2000 and >5000 mg/kg bw in rats and mice, respectively. The LD50 in rats treated dermally was >2000 mg/kg bw. The LC50 in rats treated by inhalation was >1.20 mg/l (the highest attainable concentration) after an exposure of 4 h. Clinical signs of toxicity such as piloerection, hunched posture, dyspnoea, and reduced locomotor activity were seen. Cyprodinil was not a skin or eye irritant but was a skin sensitizer.
In short-term studies in mice, rats and dogs, dosing with cyprodinil in the diet or by gavage resulted in reduced body-weight gain and reduced food consumption at higher doses. These effects were seen at doses of 6000 ppm (equal to 849 mg/kg bw per day), 1000 mg/kg bw per day, and at and above 15 000 ppm (equal to 446 mg/kg bw per day) in mice, rats and dogs, respectively. The major target organs were the liver, kidney, and thyroid in rats, and the liver in mice and dogs. Increases in liver weights were observed in mice at 6000 ppm (equal to 849 mg/kg bw per day) and in rats at or above 2000 ppm (equal to 134 mg/kg bw per day). Increases in thyroid weight were observed in rats at and above 2000 ppm (equal to 134 mg/kg bw per day). Some mild to moderate histopathological changes in the liver, such as hepatocyte hypertrophy and multifocal single cell hepatocyte necrosis, were seen in both mice and rats at and above 2000 ppm (equal to 25 mg/kg bw per day in mice and equal to 134 mg/kg bw per day). In the kidneys, adverse effects were manifested in rats as chronic tubular lesions and chronic kidney inflammation at and above 2000 ppm (equal to 134 mg/kg bw per day). The NOAEL in a 90-day study of toxicity in mice was 500 ppm (equal to 73.3 mg/kg bw per day). The NOAEL in a 28-day study in rats treated by gavage was 100 mg/kg bw per day. The NOAELs in a 90-day study of toxicity in rats was 300 ppm (equal to 19 mg/kg bw per day). The NOAELs in a 90-day and a 1-year study of toxicity in dogs were 7000 ppm (equal to 210 mg/kg bw per day) and 2500 ppm (equal to 66 mg/kg bw per day), respectively.
In studies of chronic toxicity and carcinogenicity in mice and rats, there were no treatment related neoplastic findings. In mice, a slightly increased incidence of hyperplasia in acinar cells of the exocrine pancreas in males was observed at the highest dose tested, 5000 ppm (equal to 558 mg/kg bw per day). The NOAEL for systemic toxicity in mice was 2000 ppm (equal to 196 mg/kg bw per day) based on an increase in the incidence of focal and multifocal hyperplasia of the exocrine pancreas in males, reduced body weights in males and females, increased relative kidney weights in females, and increased relative liver weights in males and females, seen at the highest dose tested. In rats, histopathological changes in the liver (spongiosis hepatitis) and increased liver weights were observed at and above 1000 ppm (equal to 35.6 mg/kg bw per day). The NOAEL for systemic toxicity in rats was 75 ppm (equal to 2.7 mg/kg bw per day), based on a dose-related increase in the incidence of spongiosis hepatitis at doses of 1000 and 2000 ppm in males. There was no evidence of significant chronic toxicity in females. Cyprodinil was not carcinogenic in mice or rats.
Cyprodinil gave negative results in a battery of studies of genotoxicity in vitro in bacteria and cultured mammalian cells, and in a mouse micronucleus test in vivo.
The Meeting concluded that cyprodinil is unlikely to be genotoxic.
In view of the lack of genotoxicity and the absence of carcinogenicity in rats and mice, the Meeting concluded that cyprodinil is unlikely to pose a carcinogenic risk to humans.
In a two-generation study of reproduction in rats, reproductive parameters were not affected at the highest dose tested (4000 ppm, equal to 295 mg/kg bw per day). The NOAEL for parental systemic toxicity was 1000 ppm (equal to 74 mg/kg bw per day) based on decreased body-weight gain in F0 females at the highest dose tested, 4000 ppm (equal to 295 mg/kg bw per day). The NOAEL for offspring toxicity was 1000 ppm (equal to 74 mg/kg bw per day) based on decreased pup body weights for F1 and F2 pups at the highest dose tested. Cyprodinil was not teratogenic in rats and rabbits at doses of up to 1000 and 400 mg/kg bw per day in rats and rabbits, respectively. In the study of developmental toxicity in rats, lower fetal body weights and an increased incidence of delayed ossification at a dose of 1000 mg/kg bw per day were considered to be secondary to maternal toxicity. At the highest dose tested, a slight increase in the number of litters in which pups were born with an extra (13th) rib was observed in rabbits in the presence of maternal toxicity, an effect that was not considered to be toxicologically relevant.
In a study of an acute neurotoxicity in rats, doses of 600 and 2000 mg/kg bw caused reduced activity, hunched posture, piloerection, increased responsiveness to stimuli, and hypothermia; the NOAEL was 200 mg/kg bw. In a study of subchronic neurotoxicity, no signs of neurotoxicity were observed in a functional observation battery, on evaluation of motor activity, or on neuropathological examination, in rats receiving cyprodinil in the diet at concentrations of up to 8000 ppm (equal to 601 mg/kg bw per day), the highest dose tested. The NOAEL was 800 ppm (equal to 54.5 mg/kg bw per day) based on liver, kidney and thyroid histopathology, and reduced body-weight gain seen at the highest dose tested (8000 ppm, equal to 601 mg/kg bw per day).
Several soil and plant metabolites of cyprodinil were investigated in the Ames test and for acute oral toxicity at the limit dose (2000 mg/kg bw). The LD50 for each of these metabolites was > 2000 mg/kg bw and no mutagenic potential was detected.
The Meeting concluded that the existing data were adequate to characterize the potential hazard to foetuses, infants and children.
Toxicological evaluation
The Meeting established an ADI of 0-0.03 mg/kg bw based on a NOAEL of 2.7 mg/kg bw per day in a 24-month study in rats fed with cyprodinil, on the basis of liver effects (spongiosis hepatitis) seen in males at higher doses, and a 100-fold safety factor.
The Meeting concluded that the establishment of an acute RfD for cyprodinil was not necessary, on the basis of its low acute toxicity, the absence of development toxicity in rats and rabbits, the lack of neurotoxicity following single exposures, and absence of any other toxicological end-point that would be elicited by a single dose.
A toxicological monograph was prepared.
Levels relevant to risk assessment
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
18-month study of toxicity and carcinogenicitya |
Toxicity |
2000 ppm, equal to 196 mg/kg bw per day |
5000 ppm, equal to 558 mg/kg bw per day |
|
Carcinogenicity |
5000 ppm, equal to 558 mg/kg bw per dayc |
|
||
|
Rat |
2-year study of toxicity and carcinogenicitya |
Toxicity |
75 ppm, equal to 2.7 mg/kg bw per day |
1000 ppm, equal to 35.6 mg/kg bw per day |
|
Carcinogenicity |
2000 ppm, equal to 73.6 mg/kg bw per dayc |
|
||
|
Multi-generation study of reproductive toxicitya |
Parental toxicity/offspring toxicity |
1000 ppm, equal to 74.0 mg/kg bw per day |
4000 ppm, equal to 295 mg/kg bw per day |
|
|
Study of developmental toxicityb |
Maternal toxicity |
200 mg/kg bw per day |
1000 mg/kg bw per day |
|
|
Embryo- and fetotoxicity |
200 mg/kg bw per day |
1000 mg/kg bw per day |
||
|
Study of acute neurotoxicityb |
Neurotoxicity |
200 mg/kg bw per day |
600 mg/kg bw per day |
|
|
Rabbit |
Study of developmental toxicityb |
Maternal toxicity |
150 mg/kg bw per day |
400 mg/kg bw per day |
|
Embryo- and fetotoxicity |
400 mg/kg bw per day |
- |
||
|
Dog |
1-year study of toxicitya |
Toxicity |
2500 ppm, equal to 66.0 mg/kg bw per day |
15 000 ppm, equal to 449 mg/kg bw per day |
a Diet
b Gavage
c Highest dose tested
Estimate of acceptable daily intake for humans
0-0.03 mg/kg bw
Estimate of acute reference dose
Unnecessary
Studies that would provide information useful for the continued evaluation of the compound
Further observations in humans.
Summary of critical end-points for cyprodinil
|
Absorption, distribution, excretion, and metabolism in mammals |
|||
|
Rate and extent of oral absorption |
Rapid; maximum reached in blood by 0.15-1.0 h; about 71-85% absorbed after 48 h |
||
|
Dermal absorption |
At 6 mg/cm2, in vivo absorption in rats was 21.7% in 0-24 h; at 870 mg/cm2, in vivo absorption was 1.9% in 0-24 h |
||
|
Distribution |
Extensive; highest concentrations in liver, kidney, spleen, and blood |
||
|
Potential for accumulation |
No evidence of significant accumulation; about 0.2-0.6% of the total dose found in tissues after 168 h |
||
|
Rate and extent of excretion |
Excretion was rapid; >90% excreted in to urine (48-67%) and faeces (27-45%) within 48 h |
||
|
Metabolism in animals |
Very extensive; metabolic pathways include hydroxylation of the phenyl and pyrimidyl rings and conjugation with sulfate or glucuronic acid; limited cleavage of bond between phenyl and pyrimidyl rings; about 3-8% unchanged cyprodinil in faeces |
||
|
Toxicologically significant compounds |
Cyprodinil |
||
|
Acute toxicity |
|||
|
Mouse, LD50, oral |
> 5000 mg/kg bw |
||
|
Rat, LD50, oral |
> 2000 mg/kg bw |
||
|
Rat, LD50, dermal |
> 2000 mg/kg bw |
||
|
Rat, LC50, inhalation |
> 1.2 mg/l (maximum attainable concentration, 4-h exposure, nose only) |
||
|
Rabbit, dermal irritation |
Not an irritant |
||
|
Rabbit, eye irritation |
Not an irritant |
||
|
Skin sensitization |
Sensitizing (maximization test) |
||
|
Short-term studies of toxicity |
|||
|
Target/critical effect |
Histopathological findings in liver, kidneys and thyroid |
||
|
Lowest relevant oral NOAEL |
19 mg/kg bw per day(90-day study in rats) |
||
|
Lowest relevant dermal NOAEL |
No suitable study is available |
||
|
Lowest relevant inhalation NOAEL |
No studies are available |
||
|
Genotoxicity |
No genotoxic potential |
||
|
Long-term studies of toxicity and carcinogenicity |
|||
|
Target/critical effect |
Degenerative liver lesions (spongiosis hepatitis) in males, in rats |
||
|
Lowest relevant NOAEL |
2.7 mg/kg bw per day (2-year study in rats) |
||
|
Carcinogenicity |
No carcinogenicity in mice and rats |
||
|
Reproductive toxicity |
|||
|
Reproduction target/critical effect |
Reduced pup body weight |
||
|
Lowest relevant reproductive NOAEL |
74 mg/kg per day (rats) |
||
|
Developmental target/critical effect |
No toxicologically relevant effects were observed |
||
|
Lowest relevant developmental NOAEL |
400 mg/kg per day (rabbits) |
||
|
Neurotoxicity/delayed neurotoxicity |
|||
|
Acute neurotoxicity |
No evidence of neuropathology at doses of up to 2000 mg/kg bw in rats; NOAEL was 200 mg/kg bw based on clinical signs |
||
|
90-day study of neurotoxicity |
No evidence of neurotoxicity or neuropathology; NOAEL was 54.5 mg/kg bw per day based on liver, kidney and thyroid histopathology |
||
|
Other toxicological studies |
|||
|
Metabolites: study of acute toxicity |
LD50 of > 2000 mg/kg bw for four metabolites a |
||
|
Metabolite: 90-day study, in diet |
NOAEL of 79.5 mg/kg bw per day for CGA 249287 |
||
|
Metabolites: genotoxicity |
No genotoxic potential for four metabolites a |
||
|
Medical data |
Limited data; slight eye irritation and sensitization reported in workers |
||
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
0-0.03 mg/kg bw |
2-year study of toxicity and carcinogenicity |
100 |
|
Acute RfD |
Not allocated (unnecessary) |
Not applicable |
Not applicable |
|
|
a CGA 249287: |
(4-cyclopropyl-6-methyl-pyrimidine-2-ylamine) |
|
|
CGA 275535: |
3-(4-cyclopropyl-6-methyl-pyrimidine-2-ylamino)-phenol |
|
|
NOA 422054: |
(4-cyclopropyl-6-hydroxymethyl-pyrimidine-2-ylamine) |
|
|
CGA 321915: |
4-cyclopropyl-6-methyl-pyrimidin-2-ol |
RESIDUE AND ANALYTICAL ASPECTS
Residue and analytical aspects of cyprodinil were considered for the first time by the present Meeting.
Cyprodinil, a member of the anilinopyrimidine group, is a systemic foliar and seed dressing fungicide that acts as an inhibitor of methionine biosynthesis. It has registered uses in many countries on horticultural and cereal crops.
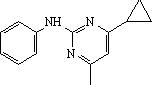
|
IUPAC name: |
4-cyclopropyl-6-methyl-N-phenylpyrimidin-2-amine |
|
Chemical Abstracts name: |
4-cyclopropyl-6-methyl-N-phenyl-2-pyrimidinamine |
The Meeting received information on cyprodinil metabolism and environmental fate, methods of residue analysis, freezer storage stability, national registered use patterns, supervised residue trials, farm animal feeding studies, fate of residues in processing and national MRLs.
Cyprodinil, labelled uniformly with 14C in the phenyl ring or at C-2 of the pyrimidine ring was used in all the metabolism studies.
Animal metabolism
The Meeting received reports of animal metabolism studies on rats, lactating goats and laying hens. The most common metabolic pathways in animals begin with hydroxylation of the methyl group or at position 5 on the pyrimidine ring or position 4 on the phenyl ring. Typically the hydroxy compounds form sulfate or glucuronic acid conjugates ready for elimination. Parent cyprodinil is a minor part of the residue and was identified in goat liver, fat and muscle and in eggs. Cleavage at the amino bridge was a minor route. The metabolism of cyprodinil in rats and farm animals is similar.
Rats. When rats were dosed orally with labelled cyprodinil, almost all (92-97%) of the radiolabel was excreted within 48 h. Most of the excretion was in the urine (48-68%) with 29-47% in the faeces. A main metabolite in faeces was identified as (6-cyclopropyl-2-phenylaminopyrimidin-4-yl)methanol. Fifteen metabolites and cyprodinil were identified in the tissues of dosed orally rats. The metabolites were mostly mono-, di- and trihydroxy compounds present as sulfate or glucuronic acid conjugates. The most common sites for hydroxylation were 4-phenyl, 5-pyrimidine and the 6-methyl group. Cleavage at the amino bridge was slight.
Goats. Lactating dairy goats were dosed orally once daily for 4 consecutive days by gelatin capsule with 0.2 mg/kg bw/day per-day of [14C-phenyl]cyprodinil or 0.19 mg/kg bw/day [2-14C-pyrimidine]cyprodinil equivalent to 8.0 and 8.9 ppm cyprodinil in the diet respectively. A parallel high-dose study was conducted with 9.9 and 9.8 mg/kg bw/day per-day equivalent to 267 and 286 ppm cyprodinil in the diet respectively. In the low-dose goats 0.13% and 0.53% of the dose was found in the milk. In the high-dose animals 14C levels were much higher in liver and kidney (0.17-0.28 mg/kg as cyprodinil) than in muscle or fat (0.006-0.01 mg/kg). In the low-dose animals parent cyprodinil at 0.003 (1.7% of the TRR, total radioactive residue) and 0.016 mg/kg (5.8% of the TRR) was identified in liver but not in other tissues. Hydroxylated and conjugated metabolites (4-phenyl and 5-pyrimidine) were identified in the milk, kidney and liver.
Lactating dairy goats were dosed orally directly into the rumen once daily for 4 consecutive days by gelatin capsule with [14C-phenyl]cyprodinil at 4.1 mg/kg bw equivalent to 100 ppm cyprodinil in the diet. Most of the metabolites were products of hydroxylation at the 4-position on the phenyl ring, the 5-position on the pyrimidine ring and on the methyl group, which then formed glucuronic acid or sulfate conjugates. Parent cyprodinil was the major component of the residue in fat (68% of the TRR). No cyprodinil was detected in milk, but 57% of the residue in milk was accounted for by metabolite 4-(4-cyclopropyl-6-methylpyrimidine-2-ylamino)phenol and its glucuronic acid and sulfate conjugates. Metabolites identified in goat tissues and milk were mainly the same as in rat tissues.
Hens. Laying white Leghorn hens were dosed orally once daily for 4 consecutive days by gelatin capsule with 0.4 mg/kg bw of [14C-phenyl]cyprodinil or [2-14C-pyrimidine]cyprodinil, equivalent to 4.7 and 4.5 ppm cyprodinil in the diet respectively. The radiolabel was present at higher levels in the liver and kidney (0.041-0.12 mg/kg) than in other tissues or eggs (0-0.01 mg/kg). A parallel high-dose study was conducted with 19 mg/kg bw/day per-day equivalent to 215 or 226 ppm cyprodinil in the diet. Elimination of the 14C was rapid with 98% and 2% of the daily dose recovered in excreta and cage wash respectively in the first 24 h.
The 14C level in meat was too low for identification. The nature of the residue in skin and fat was also not further examined. Parent cyprodinil was not identified in liver, which had the highest level of 14C. The main identified components of the liver residue were glucuronic acid and sulfate conjugates of 4-(4-cyclopropyl-6-methylpyrimidin-2-ylamino)phenol. Cyprodinil was present at low levels in eggs (0.002 mg/kg in whites to 0.011 mg/kg in yolks, 8-12% of the TRR) from the high-dose experiment.
Plant metabolism
The Meeting received reports of plant metabolism studies on wheat, apples, peaches, tomatoes and potatoes. Cyprodinil is quite persistent and is generally the major identifiable component of the residue. It is slowly absorbed into the plant tissue where it is hydroxylated and conjugated with sugars. Cleavage of the amino bridge is a minor route. In apples much of the residue remains in the peel. Similar metabolic pathways occur in all the studied crops.
Wheat. When wheat plants were treated with [2-14C-pyrimidine]cyprodinil and [14C-phenyl]cyprodinil at the 6-8 leaf stage at 0.75 kg ai/ha and again at the panicle emergence stage at 0.5 kg ai/ha, levels of parent cyprodinil at harvest were grain 0.018 and 0.022 mg/kg, husks 0.37 and 0.44 mg/kg and straw 0.60 and 0.44 mg/kg. Cyprodinil was the major identifiable component of the residue. The pattern of extractable metabolites in wheat straw from cyprodinil with 14C in the two positions was generally similar, demonstrating that the amino bridge was usually intact. Hydrolysis experiments suggested the presence of O- and N-sugar conjugates. Sugar conjugates were identified in straw, husks and grain.
Wheat plants at the 5-leaf stage were treated once with [14C-phenyl]cyprodinil at a rate of 0.75 kg ai/ha in a greenhouse experiment which demonstrated a half-life of approximately 25 days for cyprodinil in the plant, approximately 50% loss of radiolabel in 35 days by volatility, slow but continued uptake of cyprodinil and very little translocation to new growth.
Peach. When peach trees were sprayed with either [14C-phenyl]cyprodinil or [2-14C-pyrimidine]cyprodinil and peaches were harvested 1-day after the last application cyprodinil constituted the major part of the residue. Metabolites were mainly sugar conjugates of hydroxylated cyprodinil. The presence of low levels of 4-cyclopropyl-6-methylpyrimidin-2-ylamine showed the occurrence of limited cleavage at the amino bridge.
Tomato. Greenhouse tomato plants were treated with either [14C-phenyl] or [2-14C-pyrimidine]cyprodinil and tomatoes were harvested 14 days after the second treatment. Cyprodinil was the major part of the residue (55-62%). Approximately 20% of the residue was on the surface with the remainder in the tissue. The metabolic pattern was very similar for the two labels showing that the amino bridge had remained intact. Metabolites resulted from hydroxylation at various positions and subsequent conjugation with sugars.
Potato. Greenhouse-grown potato plants were treated 3 times with foliar sprays of either [14C-phenyl] or [2-14C-pyrimidine]cyprodinil at 0.56 kg ai/ha and potato tubers were harvested 14 days after the last treatment. Cyprodinil was not identified as a residue component in the harvested tubers. Phenylguanidine was identified as a metabolite at 0.004 and 0.005 mg/kg, and other metabolites were identified in which the cyclopropyl ring was opened. The total levels of the two compounds 2-anilino-4-(3-hydroxypropyl)-6-methylpyrimidin-5-ol and 2-anilino-4-(2-hydroxypropyl)-6-methylpyrimidinamin-5-ol and their O-sugar conjugates were 0.015 and 0.018 mg/kg in the two experiments. Some of the 14C in potatoes (24% from the phenyl label and 13% from the pyrimidine label) was identified as being incorporated into glucose.
Apple. Golden Delicious apple trees growing in containers were sprayed 3 times with [2-14C-pyrimidine]cyprodinil at 0.050 kg ai/hl and fruit were taken at maturity, 61 days after the last treatment. Of the radiolabel in the whole fruit, 16% was identified, 39% was unextracted and 36% was unidentified and unresolved. Very little residue (<1%) remained on the surface, but most remained in the peel. Parent cyprodinil was the major identified component of the residue at 0.088 mg/kg (11% of the radiolabel). Identified metabolites were 6-cyclopropyl-2-phenylaminopyrimidin-4-ylmethanol and 4-(4-cyclopropyl-6-methylpyrimidin-2-ylamino)phenol present as sugar conjugates, and 4-cyclopropyl-6-methylpyrimidin-2-ylamine.
Environmental fate in soil
The Meeting received information on the fate of cyprodinil during aerobic degradation in a number of soils. At 20°C and moisture levels above 60% field capacity the initial half-life of parent cyprodinil ranged from 11 to 46 days. The rates of loss decreased substantially as the residues aged. Temperature and moisture levels strongly influenced the rate of disappearance with longer half-lives at lower temperatures and moisture levels.
In soil 4-cyclopropyl-6-methyl-pyrimidin-2-ylamine was an important degradation product, demonstrating that amino bridge cleavage occurred readily in soil. This compound and cyprodinil were sufficiently persistent in soil for residues still to be present in the soil at harvest of a root crop.
Hydroxylation at the 3-phenyl position of cyprodinil also produced the important soil product N-(3-hydroxyphenyl)-4-cyclopropyl-6-methylpyrimidin-2-ylamine. This compound has a very short half-life (less than 1 day) when it is incubated independently.
Crop rotation
The Meeting received comprehensive data from confined crop rotation studies with 14C-labelled cyprodinil and from crop rotation trials using unlabelled cyprodinil. In some trials a first crop was treated with cyprodinil while in others bare ground was directly treated with cyprodinil as an extreme case of residues in the soil from the first crop. The normal rotation in the trials was a first crop of wheat followed by a rotation root crop (e.g. sugar beet, radish, turnip), vegetable (e.g. lettuce, mustard) or cereal (e.g. wheat, maize). The rotation crops were sown from approximately 30 days to 1 year after the last treatment of the first crop or bare ground.
Residues of cyprodinil itself at £0.06 mg/kg were detected in rotation crops where the treatment-to-sowing interval (TSI) was 1-12 months, e.g. in wheat husks (0.01 mg/kg, TSI 106 days), wheat grain (0.003 mg/kg, TSI 119 days) and radish root (0.001-0.062 mg/kg, TSI 29-366 days).
An important component of the residue at the longer intervals was identified as (2-amino-6-cyclopropylpyrimidin-4-yl)methanol. It may result from plant uptake of the soil product 4-cyclopropyl-6-methylpyrimidin-2-ylamine followed by metabolic hydroxylation of the methyl group. These two compounds were present at 1.5 and 0.5 mg/kg in wheat fodder from a wheat rotation crop sown 119 days after the cyprodinil treatment. Both compounds were at measurable levels (0.016-0.21 mg/kg) in wheat forage and fodder and radish roots from crops sown 1 year after cyprodinil application.
In the unconfined rotational crop studies with unlabelled cyprodinil, parent cyprodinil was not detected (<0.01 mg/kg) except in wheat plants (0.01 mg/kg). 2-amino-6-cyclopropylpyrimidin-4-ylmethanol and 4-cyclopropyl-6-methylpyrimidin-2-ol were occasionally detected in the range of 0.01-0.13 mg/kg.
The unconfined rotational crop studies suggest that cyprodinil itself will very rarely occur as a residue in rotational crops and then at levels around 0.01 mg/kg.
Analytical methods
The Meeting received descriptions and validation data for analytical methods for cyprodinil and metabolite residues in crops and animal commodities. The methods rely on HPLC and GLC and generally achieve LOQs of 0.01-0.02 mg/kg in crop and animal samples.
Cyprodinil and 6-cyclopropyl-2-phenylaminopyrimidin-4-ylmethanol were taken through the procedures of the US FDA Pesticide Analytical Manual. The compounds were detected by GLC systems with NP detectors and were recovered through procedures for non-fatty foods, but not through those for fatty foods.
Extracts of washed tomato fruits from the metabolism study with [14C-phenyl]cyprodinil were counted for 14C and analysed for cyprodinil by HPLC method REM 141.01. The proportion of cyprodinil in the extract as measured by HPLC was 47% of the total 14C (43-53%, n = 4). The metabolism study had found 55% of the 14C in tomato fruits remaining as unchanged cyprodinil. The good agreement suggests that method REM 141.01 was quantitatively extracting the incurred residue. Aqueous methanol is used for extraction.
Stability of pesticide residues in stored analytical samples
The Meeting received information on the stability of cyprodinil, 4-cyclopropyl-6-methylpyrimidin-2-ol and 2-amino-6-cyclopropylpyrimidin-4-ylmethanol in various substrates (crops, farm animal commodities and processed commodities) at freezer temperatures for 1-2 years. Cyprodinil was generally stable for the duration of the testing, i.e. the decrease in residue level was not evident or was less than 30%. Stability in peach samples was questionable, but low and variable procedural recoveries suggested difficulties with the analyses.
2-Amino-6-cyclopropylpyrimidin-4-ylmethanol in radish roots was unstable in freezer storage. Levels dropped below 10% of their initial value within 3 months.
Definition of the residue
Parent cyprodinil is the major identifiable component of the residue when it is used on crops, and is reasonably persistent. It is a very minor residue in animal commodities where it is readily hydroxylated to derivatives that form glucuronic acid and sulfate conjugates. Parent cyprodinil was identified in the liver, fat and muscle of dosed goats and in the eggs from dosed hens.
The log POW of cyprodinil is 4.0, which suggests that it is probably fat-soluble. Cyprodinil is metabolized quickly so that it does not tend to accumulate in fat. In the dairy cow feeding study at 50 ppm feed dry weight, residues were not detected (<0.01 mg/kg) in the fat or muscle, but were just detected (0.013 mg/kg) in the liver. In the goat metabolism study, cyprodinil levels were higher in the liver than in the fat. Levels of parent cyprodinil were higher in the fat than in the muscle, so residues in the fat are appropriate for monitoring residues in meat.
The Meeting agreed to classify cyprodinil as fat-soluble.
The relevant residue for analysis and enforcement is parent cyprodinil. The same residue would be used for the estimation of dietary intake.
Definition of the residue (for compliance with MRLs and for estimation of dietary intake):
cyprodinil.
The definition applies to plant and animal commodities.
The residue is fat-soluble.
Supervised trials
The Meeting received supervised trials data for apples, pears, stone fruits, grapes, strawberries, raspberries, onions, cucumbers, egg plant, tomatoes, sweet peppers, lettuce, beans, peas, kidney beans, barley, rye, wheat, almonds and the straw and fodder of barley, rye and wheat.
In some trials residues were measured on samples taken just before the last application as well as just after it (the "zero day" residue). The former residue expressed as a percentage of the latter provides a measure of the contribution of previous applications to the final residue in use patterns involving multiple applications.
In fruits (pear, peach, plum, grapes, strawberries) the average carryover of residue was approximately 35%, which suggests that 2 applications are likely to produce a higher residue level than one application, but 3 or more applications should not produce residue levels significantly different from 2. In vegetables the carryover was lower and less consistent: peas (pods) 0%, beans, lettuce, cucumbers and peppers approximately 10% and tomatoes 36%, suggesting that the number of applications may influence the residue in tomatoes but probably not in other crops.
Residue data were evaluated only where labels (or translations of labels) describing the relevant GAP were available to the Meeting.
Apples. No labels were available for the use of cyprodinil on apples in France or Switzerland, so the residue data from those countries could not be evaluated.
GAP for apples in the USA allows 4 foliar applications of 0.26 kg ai/ha to apples until the end of flowering with 72 days PHI. Cyprodinil residues in apples from 10 US trials meeting these conditions were <0.02 (5), 0.02 (3), 0.022 and 0.024 mg/kg.
The Meeting estimated a maximum residue level and an STMR for cyprodinil in apples of 0.05 and 0.02 mg/kg respectively.
Pears. Cyprodinil may be applied to pears in Italy at 0.38 kg ai/ha and harvested 14 days after the last application. In 5 trials in Italy and one in France that matched Italian GAP cyprodinil residues in pears were 0.03, 0.05, 0.13, 0.33, 0.51 and 0.61 mg/kg.
In Spain cyprodinil may be used on pears at 0.38 kg ai/ha with harvest permitted 14 days after the last application. In 2 trials matching Spanish GAP the cyprodinil residues were 0.19 and 0.34 mg/kg.
Cyprodinil may be used at 0.26 kg ai/ha on pears in the USA with a PHI of 72 days. In 6 US trials matching GAP cyprodinil residue levels were <0.02 (4), 0.025 and 0.027 mg/kg.
The data sets from Europe and the USA appeared to be from different populations and so were not combined. The 8 residues from Europe in rank order (median underlined) were 0.03, 0.05, 0.13, 0.19, 0.33, 0.34, 0.51 and 0.61 mg/kg.
The Meeting estimated a maximum residue level and an STMR for cyprodinil in pears of 1 and 0.26 mg/kg respectively.
Stone fruits
In Italy cyprodinil may be applied to apricots at 0.38 kg ai/ha with harvest 7 days after the last application. In trials in Greece and Italy matching these conditions residue levels in apricot pulp (Greece) and whole fruit (Italy) were 0.22 and 0.03 mg/kg respectively.
The US maximum registered use for sour cherries is 0.53 kg ai/ha with a PHI of 2 days and a maximum seasonal treatment of 1.1 kg ai/ha. US trials with an application rate of 0.56 kg ai/ha (5 applications) and a PHI of 1-day did not exactly match GAP, but cyprodinil is a reasonably persistent residue so 1-day data were considered adequate. The 5 applications were excessive compared with the allowed seasonal maximum but in view of the previous information on carryover of cyprodinil residues the Meeting agreed that the conditions of the residue trials were sufficiently close to GAP to allow evaluation for cherries. The same argument applies to the US trials on peaches and plums. Residue levels in the cherries from the 11 trials were 0.40, 0.46, 0.58, 0.68, 0.78, 0.98, 1.4, 1.5, 1.5, 1.7 and 1.7 mg/kg.
In Italy cyprodinil may be applied to peaches at 0.38 kg ai/ha with a 7 days PHI. Cyprodinil residues from 2 trials in Greece and 5 trials in Italy meeting these conditions (0.30 kg ai/ha accepted as GAP, residues at 14 days higher than at 7 days in some cases) were 0.12, 0.13, 0.14, 0.20, 0.37 (pulp), 0.45, and 0.58 mg/kg. In a single trial on nectarines in Italy according to GAP the residue was 0.36 mg/kg.
French GAP allows an application rate of 0.19 kg ai/ha and a PHI of 14 days for cyprodinil use on peaches. In 2 trials where the application rate was 0.23 kg ai/ha (sufficiently close to 0.19 kg ai/ha) the residues 14 days after treatment were 0.09 and 0.1 mg/kg.
The US maximum registered use for peaches is 0.53 kg ai/ha with a PHI of 2 days and a maximum seasonal treatment of 1.1 kg ai/ha. US trials with an application rate of 0.56 kg ai/ha (5 applications) and a PHI of 1-day were accepted as valid (see discussion of cherries). Cyprodinil residues in the 13 acceptable trials were 0.26, 0.59, 0.60, 0.67, 0.68, 0.80, 0.83, 0.88, 0.92, 1.0, 1.0, 1.2 and 1.3 mg/kg.
In France cyprodinil is registered for use on plums at 0.19 kg ai/ha with a 14 days PHI. Trials at 0.23 kg ai/ha were accepted as within maximum GAP. Residue levels in plums in 4 French trials matching GAP were 0.08 and 0.14 mg/kg in the pulp and 0.06 and 0.13 mg/kg in the whole fruit. The Meeting accepted that residue levels in the pulp were a reasonable approximation to residue levels in the whole fruit. The residue in plums from a Swiss trial matching French GAP was 0.14 mg/kg.
In Italy cyprodinil may be applied to plums at 0.38 kg ai/ha with a 7 days PHI. Cyprodinil residues from 2 trials in Italy meeting these conditions were 0.12 and 0.13 mg/kg.
The US maximum registered use for cyprodinil on plums is 0.53 kg ai/ha with a PHI of 2 days and a maximum seasonal treatment of 1.2 kg ai/ha. US trials with an application rate of 0.56 kg ai/ha (5 applications) and a PHI of 1 day were accepted as valid, as with cherries. Cyprodinil residues in the 9 acceptable trials were 0.067, 0.080, 0.10, 0.19, 0.22, 0.43, 0.50, 0.54 and 0.65 mg/kg.
No relevant GAP was available for evaluation of the plum trials in Germany and the remaining trials in Switzerland.
The Meeting, while recognizing that the residues on plums generally appeared lower than on cherries and peaches, agreed to pool the stone fruit data and estimate a group maximum residue level for stone fruits.
The combined European stone fruit residues in rank order (median underlined) were 0.03, 0.06, 0.08, 0.09, 0.10, 0.12, 0.12, 0.13, 0.13, 0.14, 0.14, 0.14, 0.14, 0.20, 0.22, 0.36, 0.37, 0.45 and 0.58 mg/kg. The combined US residues were 0.067, 0.08, 0.10, 0.19, 0.22, 0.26, 0.40, 0.43, 0.46, 0.5, 0.54, 0.58, 0.59, 0.6, 0.65, 0.67, 0.68, 0.68, 0.78, 0.8, 0.83, 0.88, 0.92, 0.98, 1.0, 1.0, 1.2, 1.3, 1.4, 1.5, 1.5, 1.7 and 1.7 mg/kg.
The two sets of data were apparently from different populations. The Meeting estimated a maximum residue level and an STMR of 2 and 0.68 mg/kg respectively for stone fruits, on the basis of the US data.
Grapes. Cyprodinil may be used on grapes in Chile at 0.38 kg ai/ha with harvest 2 days after the second application. The PHIs in the trials were 7 and 21 days, which were not sufficiently close to the recommended 2 days.
In France, cyprodinil may be used at 0.45 kg ai/ha with harvest 50 days after a single application. The trials with application rates of 0.38-0.50 kg ai/ha and PHIs of 42-89 days were accepted as complying with maximum GAP. A decline study suggested that residues were quite persistent. Residues in grapes from 16 trials were 0.02, 0.05, 0.06, 0.12, 0.16, 0.17, 0.18, 0.18, 0.24, 0.29, 0.31, 0.33, 0.36, 0.37, 0.44 and 0.78 mg/kg.
In Italy, cyprodinil may be used twice on grapes at 0.30 kg ai/ha with a 21 days PHI after the second application. In 3 trials in Italy at 0.38 kg ai/ha and 21 or 28 days PHI the residues were 0.51, 0.64 and 0.75 mg/kg.
In Spain, cyprodinil may be used twice on grapes at 0.38 kg ai/ha with a 21 days PHI after the second application. In 5 trials in Spain matching GAP the residues were 0.39, 0.54, 0.70, 1.1 and 2.1 mg/kg.
In Switzerland, a single application may be used on grapes at 0.45 kg ai/ha. The label did not specify a PHI, so it was difficult to decide which trials accorded with maximum GAP. No labels were available for GAP in South Africa or Germany.
In the USA, cyprodinil may be used on grapes at 0.53 kg ai/ha with a 7 days PHI. No more than 1.1 kg ai/ha is permitted per crop. Residue data from the trials at 0.56 kg ai/ha with a 7 days PHI but with 4 applications instead of the permitted 2 were accepted as relevant because the residue level would be mainly influenced by the last 2 applications. Cyprodinil residues in grapes from the 12 US trials were <0.02, 0.48, 0.52, 0.66, 0.82, 0.85, 0.94, 0.95, 0.96, 1.3, 1.4 and 1.8 mg/kg.
The residue data from the USA, Italy and Spain appear to be from similar populations and can be combined. Residues from the French trials (longer PHI) appear to be substantially lower and constitute a different population. The data from the USA, Italy and Spain were combined for evaluation giving residues in 20 trials in rank order (median underlined) of <0.02, 0.39, 0.48, 0.51, 0.52, 0.54, 0.64, 0.66, 0.7, 0.75, 0.82, 0.85, 0.94, 0.95, 0.96, 1.1, 1.3, 1.4, 1.8 and 2.1 mg/kg.
The Meeting estimated a maximum residue level and an STMR for cyprodinil in grapes of 3 and 0.79 mg/kg respectively.
Strawberries. In France, cyprodinil may be used on strawberries at 0.38 kg ai/ha with harvest 3 days after a single application. Trials in France (8), Germany (3) and Italy (1) were accepted as matching GAP when the application rate and PHI were correct but the number of applications was 3 and in one trial was 4. Residues on strawberries from the 12 trials were 0.10, 0.11, 0.18, 0.25, 0.27, 0.29, 0.30, 0.32, 0.33, 0.41, 0.43 and 1.2 mg/kg.
The Spanish maximum registered use for cyprodinil on strawberries is 0.38 kg ai/ha with a PHI of 7 days. In 4 trials matching GAP, residues in strawberries were 0.42, 0.75, 0.86 and 1.9 mg/kg.
Swiss registered uses allow application at 0.45 kg ai/ha with a 14 days PHI. Cyprodinil residues in 2 Swiss trials at 0.38 kg ai/ha (considered as matching GAP) were 0.12 and 0.24 mg/kg.
The US trials, with an application rate of 0.56 kg ai/ha, could not be evaluated because US GAP allows only 0.38 kg ai/ha.
In summary, cyprodinil residues from the available 18 trials in rank order (median underlined) were 0.10, 0.11, 0.12, 0.18, 0.24, 0.25, 0.27, 0.29, 0.30, 0.32, 0.33, 0.41, 0.42, 0.43, 0.75, 0.86, 1.2 and 1.9 mg/kg.
The Meeting estimated a maximum residue level and an STMR for cyprodinil in strawberries of 2 and 0.31 mg/kg respectively.
Raspberries. Swiss registered uses for cyprodinil on raspberries allow application at 0.45 kg ai/ha with a 14 days PHI. Cyprodinil residues in 4 German trials at 0.38 kg ai/ha and 13-14 days PHI, approximating Swiss GAP, produced residues of 0.23, 0.26, 0.26 and 0.38 mg/kg.
The Meeting estimated a maximum residue level and an STMR for cyprodinil in raspberries of 0.5 and 0.26 mg/kg respectively.
Onions. Supervised residue trials on onions reported from France, Germany and Italy. Swiss GAP allows application at 0.38 kg ai/ha but no PHI is specified. The Meeting agreed that data on the bulbs harvested 0-7 days after the last treatment would be accepted as equivalent to GAP data. Cyprodinil residues in bulbs from 8 trials in rank order were <0.02 (3), 0.05, 0.08, 0.09, 0.12 and 0.28 mg/kg.
The Meeting estimated a maximum residue level and an STMR for cyprodinil in bulb onions of 0.3 and 0.065 mg/kg respectively.
Cucumber and summer squash. In Spain, cyprodinil may be applied at a spray concentration of 0.038 kg ai/hl with harvest 7 days after the last of 3 applications. In 4 Spanish greenhouse trials matching GAP, residues in cucumbers were 0.05, 0.07, 0.10 and 0.12 mg/kg.
The registered use in Italy allows cyprodinil application to cucumbers at 0.30 kg ai/ha and a PHI of 7 days. In a field trial in Greece and two field trials in Spain with cyprodinil application at 0.38 kg ai/ha and 7 days PHI (conforming to Italian GAP) the residues were <0.02, 0.04 and 0.10 mg/kg. In a greenhouse trial in Greece and two greenhouse trials in Switzerland (0.38 kg ai/ha, complying with Italian GAP) the residues were 0.05, 0.09 and 0.12 mg/kg.
In summary, residues from field uses were <0.02, 0.04 and 0.10 mg/kg, and from greenhouse uses 0.05, 0.05, 0.07, 0.09, 0.10, 0.12 and 0.12 mg/kg. The Meeting agreed to combine the 10 trials for evaluation, giving <0.02, 0.04, 0.05, 0.05, 0.07, 0.09, 0.10, 0.10, 0.12 and 0.12 mg/kg.
The registered use in Italy on summer squash is the same as on cucumber. The Meeting agreed to extrapolate the cucumber values to summer squash.
The Meeting estimated a maximum residue level and an STMR for cyprodinil in cucumbers and summer squash of 0.2 and 0.08 mg/kg respectively.
Egg plant. The registered use in Italy allows cyprodinil application to egg plant at 0.30 kg ai/ha and a PHI of 7 days. In 2 Italian greenhouse trials on egg plant at 0.38 kg ai/ha and 7 days PHI the residues were 0.02 and 0.08 mg/kg.
The registered use of cyprodinil in Spain allows a spray concentration of 0.038 kg ai/hl on egg plant with a PHI of 7 days. The residues in 2 greenhouse crops with this use pattern in Spain were 0.06 and 0.10 mg/kg.
In summary the residues in egg plants were 0.02, 0.06, 0.08 and 0.10 mg/kg.
The Meeting noted that egg plant is not a major crop and agreed to estimate a maximum residue level and an STMR for cyprodinil in egg plant of 0.2 and 0.07 mg/kg respectively on the limited database.
Tomato. The registered use in Italy allows cyprodinil application to tomatoes at 0.30 kg ai/ha and a PHI of 7 days. Applications at 0.38 kg ai/ha were considered GAP. In greenhouse and tunnel trials in Greece (2), Italy (3), Spain (2), Switzerland (1) and the UK (2) complying with Italian GAP, cyprodinil residues were 0.31, 0.13, 0.12, 0.14, 0.08, 0.10, 0.12, 0.16, 0.11 and 0.08 mg/kg respectively.
The registered use of cyprodinil in Spain allows a spray concentration of 0.038 kg ai/hl with a PHI of 7 days. The residues in 2 covered crops with this use pattern in Spain were 0.13 and 0.17 mg/kg.
In Switzerland, cyprodinil may be applied to tomatoes at 0.30 kg ai/ha with harvest 3 days later. Residues in 2 glasshouse trials and one field trial in Switzerland with an application rate of 0.38 kg ai/ha were 0.16, 0.25 and 0.15 mg/kg.
The Meeting agreed to combine the data from the 15 tomato trials. The residues in rank order (median underlined) were 0.08, 0.08, 0.10, 0.11, 0.12, 0.12, 0.13, 0.13, 0.14, 0.15, 0.16, 0.16, 0.17, 0.25 and 0.31 mg/kg.
The Meeting estimated a maximum residue level and an STMR for cyprodinil in tomatoes of 0.5 and 0.13 mg/kg respectively.
Sweet peppers. The registered use in Italy allows cyprodinil application to sweet peppers at 0.30 kg ai/ha and a PHI of 7 days. Applications at 0.38 kg ai/ha were considered GAP. In a field trial (F) in Italy and a field trial and greenhouse and tunnel trials in Spain matching Italian GAP the residues were 0.02 (F), 0.05, 0.09 (F), 0.11 and 0.19 mg/kg.
The registered use of cyprodinil in Spain allows a spray concentration of 0.038 kg ai/hl with a PHI of 7 days. The residues in 3 covered crops with this use pattern in Spain were 0.12, 0.28 and 0.29 mg/kg.
The Meeting agreed to use the data from the covered crops for the evaluation, giving residues in rank order (median underlined) of 0.05, 0.11, 0.12, 0.19, 0.28 and 0.29 mg/kg.
The Meeting, noting that residues in sweet peppers were very similar to those in tomatoes from the same use pattern, estimated a maximum residue level and an STMR for cyprodinil in sweet peppers of 0.5 and 0.16 mg/kg respectively.
Lettuce. Cyprodinil is registered in France for use on lettuce in the field or glasshouse at 2 x 0.19 kg ai/ha with harvest 14 days after the second application. In 7 supervised trials in France with 3 applications of 0.23 kg ai/ha on lettuce in greenhouses and 14 days PHI, residues were 1.1, 2.7, 2.8, 2.8, 2.9, 4.1 and 6.4 mg/kg.
In Italy cyprodinil is registered for use on lettuce in the field or glasshouse at 3 x 0.26 kg ai/ha with harvest 14 days after the third application. In 3 supervised trials in greenhouses in Italy matching GAP cyprodinil residues in lettuce were 1.3, 2.0 and 2.2 mg/kg. Two field trials in Italy matching GAP produced residues of 0.06 and 0.18 mg/kg.
Cyprodinil may be used in Spain at 3 x 0.23 kg ai/ha with harvest 14 days after the third application. In 3 field trials on cos lettuce matching GAP the residues were <0.02, 1.0 and 1.1 mg/kg.
Lettuce trials in Germany and Switzerland could not be evaluated because there was no relevant label-supported GAP for German uses and the Swiss GAP did not specify a PHI.
The trials in Italy suggested that residues from glasshouse uses would be higher than from field uses and should be evaluated separately. The Meeting decided to use the greenhouse lettuce data to support the evaluation.
In summary, cyprodinil residues in lettuce from 7 greenhouse trials in France and 3 trials in Italy in rank order (median underlined) were 1.1, 1.3, 2.0, 2.2, 2.7, 2.8, 2.8, 2.9, 4.1 and 6.4 mg/kg.
The Meeting noted that the 10 trials covered 9 varieties of lettuce and decided to make recommendations for both head and leaf lettuce. The Meeting estimated maximum residue levels and STMRs of 10 and 2.75 mg/kg respectively for cyprodinil in head and leaf lettuce.
Beans. Supervised residue trials with cyprodinil on beans were evaluated against Austrian GAP for dwarf beans (0.38 kg ai/ha and 14 days PHI). Residues in pods from trials approximating this use pattern in 14 trials in France were 0.07, 0.10, 0.10, 0.11, 0.11, 0.13, 0.14, 0.14, 0.15, 0.18, 0.19, 0.20, 0.26 and 0.29 mg/kg.
In Spain cyprodinil may be sprayed on beans at a concentration of 0.038 kg ai/hl with harvest 14 days after a third application. Residues in pods from 5 trials in Spain matching this use pattern were 0.09, 0.09, 0.11, 0.12 and 0.12 mg/kg.
In summary, the residues in beans from the 19 supervised trials in rank order (median underlined) were 0.07, 0.09, 0.09, 0.10, 0.10, 0.11, 0.11, 0.11, 0.12, 0.12, 0.13, 0.14, 0.14, 0.15, 0.18, 0.19, 0.20, 0.26 and 0.29 mg/kg.
The Meeting estimated a maximum residue level and an STMR for cyprodinil in beans in pods, except broad bean and soya bean, of 0.5 and 0.12 mg/kg respectively.
Peas. No relevant information was available on labels for the evaluation of the pea data.
Barley. Cyprodinil is registered in France for use on barley as a foliar spray at 0.48 kg ai/ha, with timing specified by a growth stage instruction (use until end of earing). The instruction was interpreted as a PHI of approximately 35-50 days for the purpose of evaluating the trials. Trials in France and Germany were considered to comply with French GAP with application rates in the range of 0.36-0.61 kg ai/ha and with PHIs of 40-50 days. Cyprodinil residues in barley grain from 41 trials meeting these conditions in rank order (median underlined) were <0.02, 0.07, 0.09, 0.11, 0.13, 0.14, 0.18, 0.22, 0.24, 0.25, 0.28, 0.31, 0.32, 0.36, 0.36, 0.40, 0.44, 0.48, 0.54, 0.55, 0.58, 0.58, 0.65, 0.67, 0.73, 0.74, 0.74, 0.75, 0.76, 0.77, 0.93, 1.1, 1.2, 1.2, 1.3, 1.3, 1.4, 1.5, 1.8, 1.9 and 2.0 mg/kg.
The Meeting estimated a maximum residue level and an STMR for cyprodinil in barley of 3 and 0.58 mg/kg respectively.
Rye. No labels were available for uses of cyprodinil on rye so the data could not be evaluated.
Wheat. Cyprodinil is registered in France for use on wheat as a foliar spray at 0.60 kg ai/ha, with timing specified by the instruction to use until the end of earing, interpreted as a PHI of approximately 45-60 days for the purpose of evaluating the trials. Trials in France, Germany, Switzerland and the UK were considered to conform to French GAP with application rates in the range of 0.45-0.75 kg ai/ha and with PHIs of 42-61 days. Cyprodinil residues in wheat grain from 29 trials meeting these conditions in rank order (median underlined) were <0.02, <0.02, 0.02, 0.02, 0.03, 0.03, 0.03, 0.04, 0.05, 0.052, 0.06, 0.06, 0.06, 0.06, 0.07, 0.07, 0.07, 0.08, 0.08, 0.10, 0.10, 0.11, 0.11, 0.13, 0.13, 0.13, 0.14, 0.16 and 0.32 mg/kg.
The Meeting estimated a maximum residue level and an STMR for cyprodinil in wheat of 0.5 and 0.07 mg/kg respectively.
Almonds and almond hulls. In the USA cyprodinil may be used on almonds in blossom at 0.53 kg ai/ha with harvest 150 days later. Cyprodinil residues were below the LOQ in almonds (<0.02 mg/kg) and almond hulls (<0.05 mg/kg) in 5 trials in the USA matching GAP conditions.
The Meeting estimated maximum residue levels and STMRs for cyprodinil in almonds of 0.02* and 0.02 mg/kg respectively, and in almond hulls of 0.05* and 0.05 mg/kg respectively.
Cereal straw and fodder. The barley trials that were evaluated for grain residues were evaluated for residues in barley straw. Residues in barley straw determined in 29 of the trials, in rank order, were 0.15, 0.15, 0.17, 0.18, 0.20, 0.22, 0.24, 0.32, 0.33, 0.33, 0.34, 0.39, 0.39, 0.40, 0.41, 0.42, 0.42, 0.45, 0.46, 0.51, 0.55, 0.61, 0.67, 0.82, 0.84, 0.87, 1.1, 1.7 and 2.5 mg/kg.
Residues in wheat straw in 29 of the wheat trials in rank order were <0.05, 0.06, 0.06, 0.088, <0.10, 0.10, 0.13, 0.19, 0.19, 0.22, 0.26, 0.28, 0.31, 0.32, 0.32, 0.39, 0.50, 0.54, 0.58, 0.65, 0.71, 0.80, 0.95, 1.0, 1.1, 1.7, 2.3, 2.5 and 5.8 mg/kg.
The Meeting decided to combine the data from barley and wheat straw to recommend an MRL for straw and fodder of cereal grains. The residues in rank order (median underlined) were <0.05, 0.06, 0.06, 0.088, <0.10, 0.10, 0.13, 0.15, 0.15, 0.17, 0.18, 0.19, 0.19, 0.20, 0.22, 0.22, 0.24, 0.26, 0.28, 0.31, 0.32, 0.32, 0.32, 0.33, 0.33, 0.34, 0.39, 0.39, 0.39, 0.40, 0.41, 0.42, 0.42, 0.45, 0.46, 0.50, 0.51, 0.54, 0.55, 0.58, 0.61, 0.65, 0.67, 0.71, 0.80,0.82, 0.84, 0.87, 0.95, 1.0, 1.1, 1.1, 1.7, 1.7, 2.3, 2.5, 2.5 and 5.8 mg/kg.
The Meeting estimated a maximum residue level and an STMR for cyprodinil in straw and fodder (dry) of cereal grains of 10 and 0.395 mg/kg respectively.
Processing
The Meeting received information on the fate of cyprodinil residues during the brewing of beer, production of fruit juices, vinification, wheat milling and baking, drying of plums and grapes and the production of strawberry jam and tomato paste. Cyprodinil was shown to be hydrolytically stable under food processing conditions.
The processing factors (PF) shown below were calculated from the trials data. The number of trials is shown in parentheses. The factors are the mean values excluding those where residues were undetectable except for beer. Cyprodinil residues were not detected in beer in 17 trials with LOQs of 0.01, 0.005 and 0.002 mg/kg. Estimated processing factors ranged from <0.002 to <0.17 and depended on the LOQ and the residue level in the barley. The value reported (<0.01) is a best estimate.
|
RAC |
Processed product |
PF |
No. |
|
Apples |
wet pomace |
3.5 |
(7) |
| |
juice |
0.03 |
(2) |
|
Barley |
beer |
<0.01 |
(17) |
|
Grapes |
juice |
0.15 |
(22) |
| |
wine |
0.078 |
(46) |
| |
raisins |
2.1 |
(15) |
|
Plums |
dried prunes |
1.7 |
(10) |
|
Tomatoes |
juice |
0.17 |
(1) |
| |
paste |
0.86 |
(1) |
|
Wheat |
bran |
3.0 |
(1) |
| |
flour |
0.27 |
(1) |
| |
whole meal flour |
0.92 |
(1) |
| |
whole-grain bread |
0.52 |
(1) |
The Meeting used the processing factors to estimate maximum residue levels and STMR-Ps for processed commodities.
The processing factor for raisins (2.1) was applied to the highest residue level in grapes (2.1 mg/kg) to calculate a residue of 4.4 mg/kg. The Meeting estimated a maximum residue level of 5 mg/kg for cyprodinil in dried grapes (currants, raisins and sultanas).
The processing factor for dried prunes (1.7) was applied to the highest residue level in stone fruits (1.7 mg/kg) to calculate a residue of 2.9 mg/kg. The Meeting estimated a maximum residue level of 5 mg/kg for cyprodinil in dried prunes.
The processing factor for wheat bran (3.0) was applied to the highest residue level in wheat (0.32 mg/kg) to calculate a residue of 0.96 mg/kg. The Meeting estimated a maximum residue level of 2 mg/kg for cyprodinil in wheat bran.
The processing factors were applied to the STMRs for the raw commodities to produce the following STMR-P values: wet apple pomace 0.07 mg/kg; apple juice 0.0006 mg/kg; beer 0.0058 mg/kg; grape juice 0.12 mg/kg; wine 0.062 mg/kg; dried grapes 1.7 mg/kg; apricot juice 0.3 mg/kg; dried prunes 1.2 mg/kg; tomato juice 0.022 mg/kg; tomato paste 0.12 mg/kg; wheat bran 0.21 mg/kg; wheat flour 0.019 mg/kg; wheat wholemeal 0.064; wholemeal bread 0.036 mg/kg.
Farm animal dietary burdens
The Meeting estimated the dietary burdens of cyprodinil for livestock from the residues in animal feeds resulting from its use.
Maximum farm animal dietary burden
|
Commodity |
group |
residue |
basis |
% dry matter |
residue, on dry wt |
Chosen diets, % |
Residue contribution, |
||||
|
Beef cattle |
Dairy cattle |
Poultry |
Beef cattle |
Dairy cattle |
Poultry |
||||||
|
Almond hulls |
AM |
0.05 |
MRL |
90 |
0.055 |
|
|
|
|
|
|
|
Apple pomace, wet |
AB |
0.07 |
STMR-P |
40 |
0.18 |
25 |
|
|
0.045 |
|
|
|
Barley |
GC |
3 |
MRL |
88 |
3.4 |
50 |
40 |
75 |
1.7 |
1.36 |
2.55 |
|
Straw and fodder of cereal grains |
AS |
10 |
MRL |
88 |
11.4 |
251 |
601 |
|
2.85 |
6.84 |
|
|
Wheat |
GC |
0.5 |
MRL |
89 |
0.57 |
|
|
|
|
|
|
|
Wheat bran |
CM |
0.21 |
STMR-P |
88 |
0.24 |
|
|
|
|
|
|
| |
|
|
|
|
TOTAL |
100 |
100 |
75 |
|
|
|
| |
|
|
|
|
|
Maximum dietary burden |
4.6 |
8.2 |
2.6 |
||
1 barley hay
STMR farm animal dietary burden
|
Commodity |
group |
residue |
basis |
% dry matter |
residue, on dry wt |
Chosen diets, % |
Residue contribution, |
||||
|
Beef cattle |
Dairy cattle |
Poultry |
Beef cattle |
Dairy cattle |
Poultry |
||||||
|
Almond hulls |
AM |
0.05 |
STMR |
90 |
0.055 |
|
|
|
|
|
|
|
Apple pomace, wet |
AB |
0.07 |
STMR-P |
40 |
0.18 |
25 |
|
|
0.045 |
|
|
|
Barley |
GC |
0.58 |
STMR |
88 |
0.66 |
50 |
40 |
75 |
0.33 |
0.26 |
0.50 |
|
Straw and fodder of cereal grains |
AS |
0.395 |
STMR |
88 |
0.45 |
251 |
601 |
|
0.11 |
0.27 |
|
|
Wheat |
GC |
0.07 |
STMR |
89 |
0.079 |
|
|
|
|
|
|
|
Wheat bran |
CM |
0.21 |
STMR-P |
88 |
0.24 |
|
|
|
|
|
|
| |
|
|
|
|
TOTAL |
|
|
|
|
|
|
| |
|
|
|
|
|
STMR dietary burden |
0.48 |
0.53 |
0.50 |
||
1 barley hay
The cyprodinil dietary burdens for estimations of MRLs and STMRs in animal commodities (residue levels in animal feeds expressed on dry weight) are beef cattle 4.6 and 0.48 mg/kg, dairy cattle 8.2 and 0.53 mg/kg and poultry 2.6 and 0.50 mg/kg.
Farm animal feeding studies
A feeding study on lactating dairy cows was reported, which provided information on likely residues in animal tissues and milk resulting from residues in the animal diet.
Lactating Holstein cows were dosed daily by gelatin capsule with cyprodinil at the equivalent of 5, 15 and 50 ppm in the dry-weight diet for 28 consecutive days. Milk was collected throughout and a cow from each dosing group was slaughtered for tissue collection on days 28, 29 and 30. Cyprodinil residues were not detected (LOQ 0.01 mg/kg) in the milk (days 0, 1, 3, 7, 14 and 21), kidney or fat of cows from the highest dose group (50 ppm), nor in milk (day 26) or muscle from any group. Cyprodinil was present in liver (highest residue 0.013 mg/kg) from the highest dose group but not from the other groups.
Maximum residue levels in animal commodities
The Meeting noted that no cyprodinil residues (<0.01 mg/kg) were detected in milk, kidney, fat or muscle from animals dosed for 28 days at 50 ppm, which was substantially above the maximum dietary burdens for beef and dairy cattle (4.6 and 8.2 mg/kg). Cyprodinil residues were present in liver at 0.013 mg/kg in the 50 ppm dosing group, but not the 15 ppm group.
Maximum residue levels at the LOQs of suitable analytical methods would be appropriate for the animal commodities. Residue levels in tissues (except liver) and milk were essentially zero. The level of cyprodinil residues in liver was also very low but was detected at a high dose. The data for liver and kidney were used to support a maximum residue level for edible offal.
The Meeting estimated maximum residue levels of 0.01* mg/kg for cyprodinil in meat (fat) from mammals other than marine mammals and for mammalian edible offal, and a maximum residue level of 0.0004* F mg/kg for milks (equivalent to 0.01* mg/kg in the milk fat).
The Meeting estimated STMRs of 0 mg/kg for cyprodinil in muscle and fat from mammals other than marine mammals and for milks and 0.01 mg/kg for mammalian edible offal.
The Meeting noted that in the metabolism studies on laying hens cyprodinil itself was not detected in the tissues (except in kidney at 0.001 mg/kg) even at the high feeding levels of 215 and 226 ppm. Cyprodinil was detected in eggs at 0.002-0.011 mg/kg from birds dosed at the high level. The feeding levels in the metabolism study were almost 100 times the maximum dietary burden (2.6 mg/kg), so the Meeting agreed that the expected level of cyprodinil residues in poultry tissues and eggs was essentially zero.
The Meeting estimated maximum residue levels of 0.01* mg/kg and STMRs of 0 mg/kg for cyprodinil in poultry meat (fat), edible offal of poultry, and eggs, and an STMR of 0 mg/kg for cyprodinil in poultry muscle.
DIETARY RISK ASSESSMENT
Long-term intake
The International Estimated Daily Intakes of cyprodinil, based on the STMRs estimated for all commodities, for the five GEMS/Food regional diets were in the range of 0-10% of the ADI of 0.03 mg/kg bw (Annex 3). The Meeting concluded that the long-term intake of residues of cyprodinil resulting from its uses that have been considered by the JMPR is unlikely to present a public health concern.
Short-term intake
The Meeting decided that an acute RfD was unnecessary and concluded that the short-term intake of cyprodinil residues is unlikely to present a public health concern.