T- toxicological evaluation; R-residue and analytical aspects
*New compound
TOXICOLOGY
Methoxyfenozide (N-tert-butyl-N'-(3-methoxy-o-toluoyl)-3,5-xylohydrazide) is a diacylhydrazine insecticide that acts as an ecdysone agonist. Methoxyfenozide has not been evaluated previously by the JMPR. The Meeting noted that the purity of the material tested (> 98%) was higher than that of the material proposed for commercialization (97%), but concluded that the findings were applicable to the proposed technical specification and production material.
Orally administered [14C]-methoxyfenozide is absorbed rapidly, with 58-77% of the dose being excreted within 24 h in rats. Peak plasma concentrations of radioactivity (Cmax) were seen approximately 30 minutes after dosing. Excretion occurs mainly via faeces, after absorption followed by secretion in bile. On the basis of the quantities of radioactivity excreted in the bile and urine, it can be concluded that approximately 60-70% of an orally administered dose of 10 mg/kg bw was absorbed. Absorption and excretion profiles were similar irrespective of dose (10 or 1000 mg/kg bw), single or repeated dosing (over 14 days) or sex, the only differences being evidence of saturation at the high dose and a slightly increased level of urinary excretion in females. Concentrations of radioactivity at Cmax were highest in the liver, with concentration in the adrenals and in the spleen also being higher than that in whole blood.
More than 30 metabolites of methoxyfenozide were identified in rat urine, faeces and bile. The primary reactions were demethylation, glucuronidation and hydroxylation. Less than 5% of the methoxyfenozide administered was cleaved at the amide bridge between the two aromatic rings. Repeated dosing at 10 mg/kg bw for 14 days altered the metabolite profile to a limited extent, with an increase in the concentration of multiple hydroxylated compounds.
Methoxyfenozide has low acute toxicity when administered by the oral, dermal or inhalation routes. The acute LD50 was > 5000 mg/kg bw in rats after oral or dermal administration. Methoxyfenozide was not irritating to rabbit skin, and produced minimal transient irritation of the rabbit eye. Methoxyfenozide did not induce skin in a Magnusson and Kligman maximization test for sensitization in guinea-pigs.
Short-term studies in rats, mice and dogs fed with methoxyfenozide show that these animals tolerated high concentrations of methoxyfenozide in the diet, equivalent to about 1000 mg/kg bw per day, with no marked adverse effects. Effects seen to varying degrees in all species were increased liver weight, hepatocyte hypertrophy and alterations in erythrocyte parameters consistent with a mild haemolytic effect, accompanied by formation of methaemoglobin. Findings were not always consistent between studies in the same species and comparison was also hindered to a certain extent by variations in blood sampling procedure and in the range of parameters investigated. The NOAEL in mice was 2500 ppm, equal to 428 mg/kg bw per day, on the basis of reduced body-weight gain at 7000 ppm, equal to 1149 mg/kg bw per day, in the 90-day study. In rats, the NOAEL was 1000 ppm, equal to 69 mg/kg bw per day, on the basis of increased (by > 10%) relative liver weights and periportal hepatocyte hypertrophy at 5000 ppm, equal to 353 mg/kg bw per day, in the 90-day study in rats. Thyroid follicular cell hypertrophy/hyperplasia seen in the 2-week study in rats receiving 1000 ppm methoxyfenozide, equal to 98 mg/kg bw per day, was not reproduced in the 90-day study. In dogs, increases in the formation of methaemoglobin and abnormal erythrocyte morphology were seen in two 14-day studies, with increases in spleen weights also noted in one of these studies. The overall NOAEL in the 14-day studies in dogs was 500 ppm, equal to 20 mg/kg bw per day, with a LOAEL of 3500 ppm, equal to 154 mg/kg bw per day. No treatment related adverse effects were seen in a 90-day study in dogs receiving doses of up to 5000 ppm, equal to 198 mg/kg bw per day. In a 1-year study in dogs fed with a diet containing methoxyfenozide at concentrations of 0 to 30 000 ppm (0, 60, 300, 3000, 30 000 ppm), there was evidence of haemolysis, methaemoglobinaemia, increased concentrations of bilirubin in blood and urine and increases in numbers of platelets. The presence of increased quantities of iron-positive pigment in the liver and spleen is consistent with phagocytosis of damaged erythrocytes. The NOAEL was 300 ppm, equal to 9.8 mg/kg bw per day, with a LOAEL of 3000 ppm, equal to 106 mg/kg bw per day. Extensive reversibility of haematological effects was demonstrated in dogs examined 4 weeks after the end of a 4-week exposure to 30 000 ppm, equal to 1036 mg/kg bw per day, of methoxyfenozide in the diet. This is consistent with the increases in reticulocytes and bone marrow hyperplasia observed in other studies and indicates that the effects on erythrocytes are not due to a direct effect on stem cells.
The chronic toxicity and carcinogenicity of methoxyfenozide has been investigated in mice and rats at concentrations in the diet equating to >1000 mg/kg bw per day in the groups receiving high doses. There was no treatment related increase in the incidence of any tumour type. There were no significant treatment related non-neoplastic effects. The NOAEL for carcinogenicity and non-neoplastic effects in mice was 7000 ppm, equal to 1020 mg/kg per day, the highest dose tested. The Meeting concluded that methoxyfenozide was not carcinogenic in mice.
In the study in rats, poor survival (< 50% at week 90) in all groups resulted in the study being terminated at 99 weeks. This reduction in the duration of exposure reduces the power of the study, but the study was considered to be adequate for the assessment of carcinogenic potential in rats. Non-neoplastic findings were consistent with those of the short-term studies. Changes in erythrocyte parameters, increases in numbers of platelets, serum gamma-glutamyl transferase activity, liver weight, hepatocyte hypertrophy, glomerular nephropathy, thyroid follicular hyperplasia and erosion of the glandular stomach were seen at doses of 8000 ppm, equal to 411 mg/kg bw per day, and above. The NOAEL for non-neoplastic effects was 200 ppm, equal to 10 mg/kg bw per day. There was no treatment related increase in the incidence of any tumour type. The NOAEL for neoplastic effects was 20 000 ppm, equal to 1045 mg/kg bw per day. The Meeting concluded that methoxyfenozide was not carcinogenic in rats.
Methoxyfenozide (99% pure material) has been investigated in an adequate range of in vitro and in vivo studies of genotoxicity and found to give negative results. The Meeting noted that the purity of the material tested was greater than that of the proposed technical specification, but that the impurity profile (qualitative and quantitative) of the technical material (97%) did not give rise to any significant concerns regarding genotoxicity. The Meeting concluded that methoxyfenozide (technical material) was unlikely to be genotoxic.
In view of the lack of genotoxicity and the absence of carcinogenicity observed in studies in rats and mice, the Meeting concluded that methoxyfenozide is not likely to pose a carcinogenic risk to humans.
A two-generation study of reproductive toxicity in rats treated with methoxyfenozide showed that there were no adverse effects on oestrus cycling, sperm parameters, mating performance, litter size, pup body weight, pup viability or pup gross pathology at doses of up to 20 000 ppm, equal to 1474 mg/kg bw per day. A significant increase in absolute and relative liver weights and altered liver histopathology were seen in parental animals exposed to 20 000 ppm methoxyfenozide. The only compound related effect observed in pups was a slight delay in attainment of vaginal patency, noted in both generations at a dose of 20 000 ppm, the values being outside the range of historical data for the test facility. The developmental delay in attainment of vaginal patency did not have any impact on reproduction at the second mating. There was no evidence that developing pups or secondgeneration parents were especially sensitive to methoxyfenozide. The NOAEL for reproductive effects was 20 000 ppm, equal to 1474 mg/kg bw per day, the highest dose tested. The NOAEL for pup development was 2000 ppm, equal to 143 mg/kg bw per day, based on delayed vaginal patency at 20 000 ppm. The NOAEL for parental toxicity was 2000 ppm, based on increased liver weights and histopathological changes at 20 000 ppm.
The developmental toxicity of methoxyfenozide was investigated in rats and rabbits. Some marginal increases in fetal alterations were noted, but these were within the range of historical control values and not of toxicological concern. There was no evidence of maternal toxicity at the limit dose of 1000 mg/kg bw per day used in both studies. The overall NOAEL was 1000 mg/kg bw per day. The Meeting concluded that methoxyfenozide is not teratogenic.
Methoxyfenozide was tested in studies of neurotoxicity, although there were no signs of neurotoxicity induced by methoxyfenozide in routine studies of toxicity. No evidence of neurotoxicity or neuropathy was seen at 2000 mg/kg bw, the highest dose tested in a study of acute neurotoxicity in rats, or at 20 000 ppm, equal to 1318 mg/kg bw per day, the highest dose in a 90-day study of neurotoxicity in rats receiving repeated doses of methoxyfenozide. No haematological investigations were performed in these studies.
The animal, soil and plant metabolite, N-2,3-hydroxybenzoyl-N'-3,5-dimethylbenzoyl-N'-tert-butylhydrazine (M14) has low acute oral toxicity in mice (LD50 of > 5000 mg/kg bw), and was not mutagenic in an Ames test. It is expected that the metabolites of methoxyfenozide identified in rats will be of no greater toxicity than the parent compound.
Methoxyfenozide is a new compound and there has been only limited exposure of humans to this pesticide. No adverse findings have been identified during routine medical monitoring of workers and operators.
The Meeting concluded that the existing database on methoxyfenozide was adequate to characterize the potential hazards to fetuses, infants and children.
Toxicological evaluation
The Meeting established an ADI for methoxyfenozide of 0-0.1 mg/kg bw based on the NOAELs of 200 ppm, equal to 10 mg/kg bw per day, for effects on erythrocytes plus liver and thyroid hypertrophy in the long-term study in rats, and 300 ppm, equal to 9.8 mg/kg bw per day, for haematological effects in the 1-year study in dogs, and a 100-fold safety factor.
The Meeting concluded that the toxicological profile of methoxyfenozide required the derivation of an acute RfD. The most appropriate end-point was considered to be haematotoxicity, for which the dog is the most sensitive species. In view of the fact that a 1-day study in dogs was available for the closely related compound, tebufenozide, which has a similar toxicity profile on repeated dosing, the Meeting decided to use this study to establish the acute RfD for methoxyfenozide. An acute RfD of 0.9 mg/kg bw was established, on the basis of the lack of haematological effects at 4300 ppm, equal to 89.4 mg/kg bw, and using a safety factor of 100 (see general item 2.2) the Meeting noted that this value was likely to be conservative since tebufenozide was more potent than methoxyfenozide in producing effects on erythrocytes.
A toxicological monograph was prepared.
Levels relevant to risk assessment
|
Species |
Studya |
Effect |
NOAEL |
LOAEL |
|
Mouse |
78-week study of chronic toxicity and carcinogenicity |
Toxicity and carcinogenicity |
7000 ppmb, equal to 1020 mg/kg bw per day |
- |
|
Rat |
2-year study of chronic toxicity and carcinogenicity |
Toxicity |
200 ppm, equal to 10 mg/kg bw per day |
8000 ppm, equal to 411 mg/kg bw per day |
|
Carcinogenicity |
20 000 ppmb, equal to 1945 mg/kg bw per day |
- |
||
|
Two-generation study of reproductive toxicity |
Parental and offspring toxicity |
2000 ppm, equal to 143 mg/kg bw per day |
20 000 ppm, equal to 1474 mg/kg bw per day |
|
|
Dog |
1-year study of toxicity |
Toxicity |
300 ppm, equal to 9.8 mg/kg bw per day |
3000 ppm, equal to 106 mg/kg bw per day |
|
Single dose study with tebufenozide |
Toxicity |
4300 ppmb, equal to 89.4 mg/kg bw |
- |
a All studies investigated dietary administration of methoxyfenozide
b Highest dose tested
Estimate of acceptable daily intake for humans
0-0.1 mg/kg bw
Estimate of acute reference dose
0.9 mg/kg bw
Studies which would provide information useful for continued evaluation of the compound
Observations in humans
Summary of critical end-points for methoxyfenozide
|
Absorption, distribution, excretion and metabolism in mammals |
|||
|
Rate and extent of absorption: |
About 60-70% within 72 h in the rat (including biliary excretion of 40-65%) at a dose of 10 mg/kg bw |
||
|
Distribution: |
Widely distributed; highest absorbed concentrations after 15 min-2 h in the liver |
||
|
Potential for accumulation |
Low potential: < 0.1% in liver after 5 days |
||
|
Rate and extent of excretion: |
Rapid: 60-80% in 24 h, mainly in the faeces |
||
|
Metabolism in animals |
Extensive (no parent found in urine or bile) Little cleavage of parent |
||
|
Toxicologically significant compounds |
Methoxyfenozide |
||
|
Acute toxicity |
|||
|
Rat, LD50, oral |
> 5000 mg/kg bw |
||
|
Rat, LD50, dermal |
> 5000 mg/kg bw |
||
|
Rat, LC50, inhalation |
> 4.3 mg/l (4-h exposure, nose only, maximum achievable concentration) |
||
|
Skin sensitization |
Not sensitizing (Magnusson and Kligman test) |
||
|
Short term studies of toxicity |
|||
|
Target/critical effect |
Liver (hypertrophy), erythrocytes (methaemoglobin and haemolysis) |
||
|
Lowest relevant oral NOAEL |
10 mg/kg bw per day (1-year study in dogs) |
||
|
Lowest relevant dermal NOAEL |
1000 mg/kg bw per day (28-day study in rats) |
||
|
Lowest relevant inhalation NOAEC |
No data |
||
|
Genotoxicity |
No genotoxic potential |
||
|
Long term studies of toxicity and carcinogenicity |
|||
|
Target/critical effect |
Erythrocytes (reduced parameters), liver (hypertrophy), thyroid (hypertrophy) |
||
|
Lowest relevant NOAEL |
10 mg/kg bw per day (80-90 week study in rats) |
||
|
Carcinogenicity |
No carcinogenic potential |
||
|
Reproductive toxicity |
|||
|
Reproduction target/critical effect |
Delayed attainment of vaginal patency Parental hepatotoxicity |
||
|
Lowest relevant NOAEL for reproductive toxicity |
143 mg/kg bw per day |
||
|
Developmental target/critical effect |
No embryotoxicity or fetotoxicity Not teratogenic |
||
|
Lowest relevant NOAEL for developmental toxicity |
1000 mg/kg bw per day (highest dose tested in rats and rabbits) |
||
|
Neurotoxicity/delayed neurotoxicity |
|||
|
Acute neurotoxicity |
NOAEL: > 2000 mg/kg bw; no neuropathy (rat) |
||
|
90-day study of neurotoxicity |
NOAEL: 1318 mg/kg bw per day (highest dose tested); no neuropathy (rat) |
||
|
Other toxicological studies |
|||
|
Tebufenozide single dose study in dogs |
No effects at 89.4 mg/kg bw (highest dose tested) |
||
|
Metabolite: N-2,3-hydroxybenzoyl-N'-3,5-dimethylbenzoyl-N'-tert-butylhydrazine (M14) |
Acute LD50 > 5000 mg/kg bw in mice treated orally; not mutagenic in an Ames test |
||
|
Medical data |
No adverse effects reported but data limited (new compound) |
||
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
0-0.1 mg/kg bw |
Rat, long-term study; and dog, 1-year study |
100 |
|
Acute RfD |
0.9 mg/kg bw |
Dog, single dose of tebufenozide |
100 |
RESIDUE AND ANALYTICAL ASPECTS
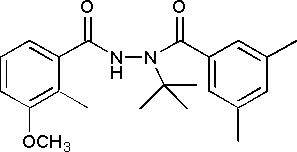
Methoxyfenozide, N-tert-butyl-N'-(3-methoxy-o-toluoyl)-3,5-xylohdrazide or 3-methoxy-2-methylbenzoic acid 2-(3,5-dimethylbenzoyl)-2-(1,1-dimethylethyl)hydrazide, is a substituted dibenzohydrazide and an insecticide that functions by accelerating the moulting process. It was considered for the first time by the present Meeting.
Animal metabolism
The Meeting received information of the metabolism of methoxyfenozide in rats, goats, and hens.
Goats. The metabolism, distribution, and elimination of [14C]methoxyfenozide, labelled in the methoxyphenyl (A) ring, the dimethylphenyl (B) ring, or the tert-butyl group, were studied in lactating dairy goats. The methoxyfenozide was administered orally in gelatin capsules to lactating goats once a day at dietary equivalents of 45, 32, or 61 ppm, for 7 consecutive days. Over the treatment period 81-88% of the administered dose was eliminated in the faeces (74-84%) and urine (5-7%). The major accumulation was in liver, where up to 0.14% of the total dose was found. The total radioactive residue (TRR) was <0.010-0.037 mg/kg in milk, 0.26-1.2 mg/kg in liver, 0.045-0.20 mg/kg in kidney, <0.010-0.017 mg/kg in leg muscle, <0.010-0.023 mg/kg in loin muscle, and 0.018-0.053 mg/kg in fat, expressed as methoxyfenozide. In general, residues were highest in the tert-butyl-labelled samples and lowest in dimethylphenyl-labelled samples.
About 85% of the TRR was extracted from milk, and 28-72% of the TRR was identified. Extraction was 98% from liver, 87-94% from kidney, and 68-98% from muscle. Identification was 22-68% from muscle, 68-84% from fat, 44-76% from kidney, and 40-56% from liver. An additional 50% was characterized in kidney and liver extracts from the tert-butyl labelled residue as lactose and triglycerides (see below).
The milk from the last day of dosing contained methoxyfenozide as the major component (14%-35% of the TRR), the only significant metabolite(s) being a B-ring alcohol-carboxylic acid and/or an A-ring phenol-B-ring alcohol (<10% of the TRR).
Methoxyfenozide was the major component of the radioactive residue in fat (68-81% of the TRR) and muscle (20%) and constituted 2-3% of the TRR in liver and kidney. The major component of the radioactive residue in liver (23-30% of the TRR, 0.075-0.27 mg/kg equivalents) and kidney (25-42% of the TRR, 0.015-0.049 mg/kg) was the glucuronide conjugate of the A-ring phenol formed by the demethylation of the methoxy group of the parent compound. It was present at low levels in the other samples (0.54%-8.1% of the TRR, <0.001-0.004 mg/kg).
The other metabolites identified in milk and most tissues from various labels at low levels were the A-ring phenol or demethylated parent, 0.72-7.4% of the TRR, <0.001-0.069 mg/kg, the B-ring carboxylic acid, 0.25-4.2% of the TRR, <0.001-0.013 mg/kg, the glucuronide conjugate of the A-ring phenol with an additional OH group ortho or para to glucuronide moiety, 0.32-18.2% of the TRR, <0.001-0.024 mg/kg, and the A-ring phenol glucuronide, B-ring monoalcohol, 0.16-14.1% of the TRR, <0.001-0.12 mg/kg.
The metabolite profiles were broadly similar from the three labels, with radioactivity from the tert-butyl group prominent in the fat-soluble fraction. The hexane extracts of tert-butyl-labelled liver and kidney were hydrolysed, and additional analytical procedures demonstrated the incorporation of radioactivity into triglycerides. From the results of HPLC, TLC, LC/MS, and LC/MS/MS analyses, triglyceride structures were proposed for several major molecular ions. Triglycerides accounted for 18% of the TRR (0.21 mg/kg as methoxyfenozide) and 22% of the TRR (0.043 mg/kg) in tert-butyl-labelled liver and kidney respectively. The incorporation of radioactivity into lactose in tert-butyl-labelled milk (23-31% of the TRR, 0.007-0.011 mg/kg) was also demonstrated.
Hens. After oral doses of [methoxyphenyl-14C]methoxyfenozide (A-ring), [dimethylphenyl-14C]methoxyfenozide (B-ring), or [tert-butyl-14C]methoxyfenozide to laying hens for 7 consecutive days at the equivalent of 58-68 ppm in the diet, the TRRs were 0.005-0.10 mg/kg in eggs, 0.28-1.57 mg/kg in liver, 0.009-0.027 mg/kg in dark muscle, 0.007-0.014 mg/kg in light muscle, 0.042-0.072 mg/kg in fat, and 0.042-0.052 mg/kg in skin with fat. Total recovery of the administered dose ranged from 84 to 93%. In general, 14C residues were highest in tert-butyl-labelled samples and lowest in dimethylphenyl-labelled samples, as in the ruminant study.
Approximately 81-98% of the TRR was characterized or identified in eggs and tissues from all labels except tert-butyl-labelled light muscle (TRR = 0.014 mg/kg, 48% characterized or identified). Methoxyfenozide was identified in eggs and tissues with all labels except the dimethylphenyl label in liver. It was the major residue in methoxyphenyl- and dimethylphenyl-labelled dark muscle, fat, and skin with fat, and in tert-butyl-labelled fat and skin with fat (11-55% of the TRR, 0.001-0.032 mg/kg), and was a minor residue in eggs, methoxyphenyl- and tert-butyl-labelled liver, and tert-butyl-labelled dark and light muscle (0.26-8.1% of the TRR, <0.001-0.006 mg/kg). The glucuronide conjugate of the A-ring phenol was identified in eggs and tissues with all labels except methoxyphenyl- and dimethylphenyl-labelled dark muscle. It was a major metabolite in eggs, methoxyphenyl- and dimethylphenyl-labelled liver, and tert-butyl-labelled light muscle and skin with fat (10-30% of the TRR, 0.001-0.054 mg/kg), and was present at low levels in the other samples (1.8-9.7% of the TRR, 0.001-0.007 mg/kg). The A-ring phenol or demethylated parent was identified in eggs and tissues with all labels at levels of 1.5-11% of the TRR (<0.001-0.044 mg/kg). Two other metabolites were identified at significant levels: the glucuronide conjugate of the A-ring phenol with an additional -OH group ortho or para to glucuronide moiety (1.1-9.5% of the TRR, <0.001-0.009 mg/kg), and the A-ring phenol glucuronide-B-ring monoalcohol 2.2-28% of the TRR, <0.001-0.047 mg/kg). The B-ring carboxylic acid was also identified at <6% of the TRR in eggs and various tissues.
As in goats the metabolite profiles were broadly similar from the three labels, and the incorporation of radioactivity into triglycerides in tert-butyl-labelled liver (£56% of the TRR, £0.89 mg/kg) and kidney (£32% of the TRR, £0.18 mg/kg) was demonstrated.
The Meeting concluded that the metabolism of methoxyfenozide is similar in poultry and ruminants. The major component in muscle, fat, and milk was the parent, which was present at low levels (<5% of the TRR) in eggs, liver, and kidney. The main metabolite in eggs, liver, and kidney is the glucuronide conjugate of the A-ring phenol. The residues were concentrated in the fat relative to the muscle by factors of about 2-5. This is consistent with the partition coefficient whose log value of 3.7 suggests slightly greater solubility in fat than muscle.
The metabolites found in rats were qualitatively the same as those in the goat and hens.
Plant metabolism
Studies on cotton, apples, grapes and rice were reported.
Cotton. The total radioactive residues were 0.072, 0.054, and 0.057 mg/kg in hulled kernels and 0.089, 0.107, and 0.162 mg/kg in hulls and lint 21 days after two applications each of A-ring-, tert-butyl- and B-ring-labelled [14C]methoxyfenozide at 1 kg ai/ha. The TRRs in the whole cotton seed calculated from the total weight of kernels, hulls and lint were 0.081 mg/kg (methoxyphenyl label), 0.080 mg/kg (tert-butyl label), and 0.11 mg/kg (dimethylphenyl label). In whole cotton plants, the TRR decreased from 72 mg/kg (methoxyphenyl label), 60 mg/kg (tert-butyl label), and 86 mg/kg (dimethylphenyl label) in immature plants harvested after 7 days to 17 mg/kg (methoxyphenyl label), 13 mg/kg (tert-butyl label), and 17 mg/kg (dimethylphenyl label) in mature plants harvested after 21 days.
Solvent extractions released ³75% of the TRR, and about 65-86% of the TRR was characterized or identified in whole cotton seed. Methoxyfenozide was the only residue identified, accounting for 46% of the TRR (0.038 mg/kg) in methoxyphenyl-labelled, 67% (0.054 mg/kg) in tert-butyl labelled, and 57% (0.063 mg/kg) in dimethylphenyl-labelled whole cotton seed.
Apples. The total radioactive residues were 0.23 and 0.28 mg/kg in or on apples collected 14 and 36 days (normal harvest) after two applications of [methoxyphenyl-14C]methoxyfenozide at 1 kg ai/ha.
Over 93% of the TRR was characterized or identified in apples. Methoxyfenozide was the major residue identified, accounting for 91% of the TRR (0.26-0.27 mg/kg) in apples collected after 14 and 36 days. Two other metabolites identified in 14- and 36-day apples were the B-ring monoalcohol at 1.4% of the TRR (0.004 mg/kg) and the B-ring dialcohol at 0.08% of the TRR and 0.11% of the TRR (both 0.003 mg/kg). The half-life of methoxyfenozide on apple foliage and fruit was estimated at 23 + 8 days and 12 + 9 days respectively.
Grapes. The total radioactive residues were 0.75 mg/kg in grapes collected 27 days (normal harvest) after two applications of [tert-butyl-14C]methoxyfenozide at 1 kg ai/ha. The TRR was 110 mg/kg in grape foliage at harvest.
Approximately 93% of the TRR were characterized or identified. Methoxyfenozide was the major residue identified, accounting for 81% of the TRR (0.60 mg/kg). Two metabolites identified were the B-ring monoalcohol at <2.3% of the TRR (<0.017 mg/kg), and the glucose conjugate of the A-ring phenol at 3.6% of the TRR (0.027 mg/kg).
The TRRs in grapes and grape foliage sampled between the first and second applications and after the second application to harvest and beyond (for foliage) were monitored. The half-lives of methoxyfenozide were determined to be 13-21 days on grapes and 11-26 days on foliage.
Rice. Radiolabelled methoxyfenozide (A-ring, B-ring, and tert-butyl) was applied to rice 70 and 107 days after planting. The B-ring- and tert-butyl-labelled compounds were applied at 0.6 kg ai/ha in both applications. The A-ring material was applied first at 0.62 kg ai/ha, then at 0.31 kg ai/ha. Samples of grain (panicles) and foliage were collected 62 days after the second treatment. The panicles were separated into chaff and brown rice. The radioactive residue in plants ranged from 6.6 to 10 mg/kg, in grain 0.52 to 0.71 mg/kg, and in straw 21-44 mg/kg.
Solvent extraction released 88-91% of the TRR from rice straw. The major component was methoxyfenozide, 65-69% of the TRR. Identified metabolites were the B-ring-monoalcohol, 0.9-1.4% of the TRR, the A-ring phenol or demethylated methoxyfenozide, 2.7-2.9% of the TRR, the A-ring phenol B-ring monoalcohol 2.1-2.3%, the B-ring carboxylic acid 1.2-1.6%, and the glucose conjugate of the A-ring phenol 1.5-2.4%. A total of 75-78% of the TRR was identified.
The main component of the residue in the grain was again methoxyfenozide, 52-59% of the TRR. Identified metabolites were the B-ring monoalcohol (1.1-4.1% of the TRR), the A-ring phenol (3.2-7.5%), the B-ring carboxylic acid (1.6-2.9%), the B-ring dialcohol (0.4-0.7%), and the glucose conjugate of the A-ring alcohol (1.8-2.3%).
Summary. The Meeting concluded that the metabolism studies on apples, rice, grapes, and cotton adequately elucidate the nature of the residue from the foliar application of methoxyfenozide to various types of crop. The major component of the residue is methoxyfenozide, typically 50-90%. The metabolism of methoxyfenozide in plants is slow but occurs via the same pathways as in animals. The primary routes of metabolism involve demethylation of the A-ring methoxy group to produce a phenol which is then conjugated with sugars, and the oxidation of the methyl groups on the B-ring to produce alcohols, acids, and combinations of alcohol and acids. B-ring alcohols and acids also form glucose conjugates.
Environmental fate in soil
The aerobic degradation of radiolabelled methoxyfenozide was studied in four soils at a concentration of 0.75 kg ai/ha over a one-year period. The results demonstrate that methoxyfenozide is very persistent in soil, with 59-75% of the applied dose remaining after one year. Calculated first-order half-lives ranged from 340 to 1100 days, depending on the soil. The major degradation pathway of methoxyfenozide in soil leads to incorporation into soil natural products, mainly humic and fulvic acids and to a lesser extent humins. Degradation also proceeds by oxidation of a methyl substituent of the B-ring to the acid (up to 3.2% of the applied dose), followed by mineralization to carbon dioxide (5.5% of the applied dose). No other degradation product exceeding 2% of the applied radioactivity was identified. Total recoveries during the test period ranged from 90 to 123%.
Confined rotational crop studies were conducted with methoxyfenozide labelled in the A-ring, B-ring, and tert-butyl group. Three applications of each, formulated as an emulsifiable concentrate, were made to bare soil at a total application rate of 2.2 kg ai/ha, equivalent to the maximum registered rate in the USA. Mustard, white radish and wheat were planted in the treated soil at three different plant-back intervals, 30, 90 and 365 days (nominal) after the last application. Crops were harvested at an intermediate stage and at maturity.
Total radioactive residue levels were <0.05-0.3 mg/kg in all crops except wheat forage and straw, 1-3 mg/kg. Residue levels were similar in immature and mature crops harvested from the same plant-back intervals, except in wheat. In general, the total residues found in mature and immature crop samples decreased significantly with increasing plant-back time. The highest TRRs, averaging about 3 mg/kg, were found in wheat straw samples at 30 days plant-back. Wheat forage residues averaged only about one-third those found in wheat straw. Wheat grain residues, at <0.05 mg/kg, were the lowest in any of the crops investigated. In general, most of the residue in all crops was readily extractable, but wheat straw and grain contained a large amount of bound residue.
In mustard leaves at 30 days plant-back, the parent methoxyfenozide was present up to 0.027 mg/kg (about 21% of the TRR) and individual metabolites were all below 0.01 mg/kg.
In radish leaves at 30 days plant-back, individual residues (up to 21 components were characterized) were all <0.05 mg/kg. The parent compound was found at up to 0.013 mg/kg (about 18% of the TRR). The N-glycosyl conjugate of the B-ring monoalcohol was detected at up to 0.035 mg/kg (about 13%) while the glucose conjugate of the A-ring phenol was detected at up to 0.028 mg/kg, (12~13% of the TRR). Radish roots contained the highest levels of unmetabolized parent of any of the crops investigated. Methoxyfenozide was the main residue in mature roots of radishes planted 30 days after treatment, from 0.022 to 0.033 mg/kg (up to 41% of the TRR).
Residues in wheat forage ranged from 0.72 to 1.5 mg/kg at a 30-day plant-back. The parent compound was a very minor component, less than 1% of the TRR (maximum 0.009 mg/kg). The two major components of the extracted residue were the malonylglycosyl conjugate of the A-ring phenol (up to 0.70 mg/kg, 48% of the TRR) and the glucose conjugate of the A-ring phenol (up to 0.36 mg/kg, 24% of the TRR).
Wheat straw contained the highest residues of any crop, 2-4 mg/kg at 30 days plant-back. Only about 45-50% of the straw residue was extractable. The main residue in the extract was the A-ring phenol (demethylated methoxyfenozide), which was present at up to 1.4 mg/kg and up to about 37% of the TRR.
Wheat grain contained the lowest residue levels, about 0.05 mg/kg. Only 11-23% of the grain residue was extractable. The main component of the extractable residue was the A-ring phenol, at a concentration of 0.006 mg/kg (about 15% of the TRR).
Potentially quantifiable residues of methoxyfenozide transformation products were found in wheat forage and straw at a 365-day plant-back interval. These included the A-ring phenol and its glucose and malonylglycosyl conjugates.
Residues of methoxyfenozide were determined in rotational crops at two trial locations (Texas and California) in the USA. Leaf lettuce, used as the cover crop, was planted in plots at each location for subsequent planting of rotational crops. Five applications of methoxyfenozide 80W were made at 7-10 days intervals to the lettuce crop at 0.45 kg ai/ha per application with a season total of 2.2 kg ai/ha. The leaf lettuce cover crop was harvested and removed from the plot 1-3 days after the last application. Rotational crops representative of leafy crops (mustard greens), fruiting vegetables (tomatoes), cucurbits (cucumbers), root vegetables (turnips), cereal grains (wheat), legumes (soya beans), and bulb crops (onions) were planted 6-7 days after the last application.
The methoxyfenozide contents of the rotational crops harvested at maturity were mustard greens 0.12 mg/kg, turnip tops 0.004 mg/kg, turnip roots 0.021 mg/kg, onions 0.055 mg/kg, wheat hay 0.031 mg/kg, wheat straw 0.057 mg/kg, soya beans 0.02 mg/kg, soya forage 1.2 mg/kg, soya hay 1.1 mg/kg, tomatoes <0.02 mg/kg, wheat grain <0.02 mg/kg. The wheat and soya samples were also analysed for the A-ring phenol and its glucose conjugate. Neither was detected in wheat grain or soya beans (<0.05 mg/kg), but both were found in the animal feed commodities at levels as high as 4.1 mg/kg of the conjugate and 2.2 mg/kg of the phenol.
The Meeting concluded that methoxyfenozide persists in the soil. The major pathways of transformation are incorporation into soil natural products and slow oxidation of the methyl substituent of the B-ring to form the acid. The Meeting also concluded that methoxyfenozide and/or its degradation products may accumulate in rotational crops, particularly in forages and fodders.
Environmental fate in water
The hydrolytic stability of methoxyfenozide was evaluated in sterile buffer solutions at pH 5, 7, and 9. The concentration of combined unlabelled and tert-butyl-labelled material was 1 µg/ml. Samples were incubated in the dark at 25ºC for 30 days. Methoxyfenozide was found to be stable for the 30-day test period. The calculated half-life values were 600 days at pH 5, 1600 days at pH 7 and 700 days at pH 9.
Methods of analysis
Numerous methods were presented for the determination of methoxyfenozide in plant commodities, both for data collection in field trials and processing studies and for monitoring and enforcement of national MRLs. Enforcement methods included independent laboratory validations. Methods that determine both methoxyfenozide and the glucuronide conjugate of the A-ring phenol were reported for meat, milk, fat, poultry, and eggs.
In all methods, an extraction suitable for the sample is followed by a standard clean-up, such as solid-phase extraction and Florisil chromatography, and analysis by HPLC. Detection is by UV (240 nm), MS (367.3 ion) or MS/MS (369 ® 149). HPLC-MS and HPLC-MS/MS are used for metabolites. The methods typically have limits of quantification of 0.01- 0.05 mg/kg for methoxyfenozide in plant commodities, 0.01 mg/kg for methoxyfenozide in milk, eggs, and meat, and 0.01-0.02 mg/kg for the glucuronide conjugate of the A-ring phenol.
Enforcement methods based on the above procedures were developed with HPLC and UV detection as the primary analytical method for many plant commodities (apples, pears, grapes, peppers, tomatoes, leafy vegetables, brassica vegetables, pecans, almonds, almond hulls, sweet corn, corn forage and fodder, cotton seed, cotton gin trash) and some animal commodities (milk, fat, bovine muscle, chicken liver, eggs) and HPLC-MS and/or HPLC-MS/MS as the confirmatory method and the primary method for the glucuronide conjugate of the A-ring phenol in animal commodities and for methoxyfenozide in bovine liver and kidney and chicken muscle. The methods underwent successful independent laboratory validations. Methods were also radio-validated using samples from the goat metabolism and confined rotational crop studies.
Neither the parent nor metabolites are determined by multiresidue methods.
The Meeting concluded that adequate methods exist for the determination of methoxyfenozide in a variety of plant commodities with a limit of quantification of 0.01 or 0.02 mg/kg in most instances, and for the determination of methoxyfenozide in poultry and bovine commodities at LOQs of 0.01 mg/kg. It was noted that the method for methoxyfenozide in bovine liver and kidney and chicken muscle for methoxyfenozide is HPLC-MS and/or HPLC-MS/MS and may not be practicable for some laboratories.
Stability of residues in stored analytical samples
The stability of methoxyfenozide in numerous plant commodities and of methoxyfenozide and the glucuronide conjugate of the A-ring phenol in bovine and poultry commodities stored frozen at about -20°C was reported. Methoxyfenozide was stable for at least the indicated periods in the following commodities: apples 365 days, apple juice 283 days, wet apple pomace 302 days, tomatoes 372 days, head lettuce 365 days, cotton seed 9 months, refined cotton seed oil 12 months, cotton gin trash 6 months, maize grain 397 days, maize meal 127 days, maize oil 184 days, milk 106 days, bovine muscle 165 days, bovine liver 261 days, bovine kidney 265 days, eggs 93 days.
The Meeting concluded that methoxyfenozide is stable for 6-12 months in various plant commodities and for about 100 days in animal commodities stored frozen.
Definition of the residue
Studies of metabolism in a variety of crops and of its environmental fate demonstrated that methoxyfenozide did not undergo extensive transformation. The major residue found in soil and plants is the parent compound, methoxyfenozide. The main residue in goat milk and tissues (except liver and kidney) is again methoxyfenozide, as it is in poultry tissues except liver, kidney, and eggs. In goat and poultry liver and kidney as well as in eggs, the A-ring phenol glucuronide is the main residue and the parent residue is about 10% of the metabolite and below the typical level of quantification. The glucuronide conjugate constituted a significant proportion of the total radioactive residues in eggs (30%), goat kidney (42%), goat liver (29%), hen liver (20%), and hen kidney (36%). However, the feeding studies described below revealed approximately equal concentrations of methoxyfenozide and the metabolite in poultry eggs and bovine kidney and liver.
The distribution of methoxyfenozide between fat and muscle in the ruminant and poultry metabolism studies indicate that it is fat-soluble. The methoxyfenozide concentration in fat was approximately 10 times that in muscle in goats and about 3-8 times that in muscle in hens. The log of the partition coefficient, 3.7, also indicates solubility in fat.
The Meeting concluded that the residue should be defined as methoxyfenozide for compliance with MRLs and for dietary intake estimation in both plant and animal commodities.
The compound is fat-soluble in its distribution between meat muscle and fat, but not in its distribution in milk.
Results of supervised trials on crops
Oranges and mandarins. Supervised field trials were conducted on oranges and mandarins in various parts of Europe, but there is no finalized GAP so the trials could not be evaluated.
Apples. Supervised field trials were conducted in the USA and in Europe. GAP in the USA requires WP 800 g/kg or SC 240 g/l, 0.34 or 0.28 kg ai/ha, 1.1 kg ai/ha per season, 14-day PHI. This corresponds to no more than 4 applications per season. All US trials were with 6 applications at 14-day intervals with a total application of 2.0 kg ai/ha. Two of the trials were conducted as residue decline studies. The half-life of the methoxyfenozide residue was about 20 days, and since it was found in the apple metabolism study that the half-life of the total radioactive residue was 12 + 9 days it can be estimated that the residue contribution from the first two applications would have decreased to £20% of the initial value by the time of the last application (56-70-day interval). The trials may therefore be considered as being according to maximum GAP. The residues of methoxyfenozide from trials according to maximum GAP in the USA in ranked order were 0.20, 0.23, 0.25, 0.30, 0.36, 0.37, 0.40, 0.43, 0.52, 0.56, 0.60, 0.62, 0.62, 1.0, 1.0 mg/kg. The HR is 1.0 mg/kg and the STMR is 0.43 mg/kg.
The proposed GAP in northern and southern Europe, Austria, Belgium, France, Germany, Greece, Hungary, Ireland, Italy, Luxembourg, The Netherlands, Portugal, Spain, and Switzerland have not been adopted by the respective national authorities. The recently finalized GAP for the UK specifies a 240 g/l SC formulation applied at 0.6 l product/ha (0.14 kg ai/ha in 1500 l water/ha) for large trees and 0.4 l product/ha (0.096 kg ai/ha in 1000 l water/ha) for smaller trees, 3 applications and a 14-day PHI. The spray concentration is 0.0096 kg ai/hl. The residues found on apples from trials complying with maximum UK GAP in France, Italy, Spain, Belgium, and Germany were <0.05 (3), 0.06, 0.06, 0.10, 0.11, 0.11, 0.13, 0.13, 0.15, 0.15 and 0.23 mg/kg.
The residues in the European trials appear to be from a different population from those in the US trials, which are higher.
Pears. Supervised field trials were conducted in the USA and Europe. GAP in the USA is the same as for apples. In the US trials, as with apples, there were six applications, each at 0.34 mg/kg. Two residue decline studies on pears in Europe indicated a residue half-life of 14-20 days. Assuming a 20-day half-life, the residues from the first two applications would have decreased to £20% of the initial value by the time of the last application, so the trials may be considered as complying with maximum GAP. The residues of methoxyfenozide on pears from 10 trials at maximum GAP in the USA in ranked order were 0.27, 0.31, 0.35, 0.36, 0.39, 0.50, 0.68, 0.74, 0.74 and 0.92 mg/kg. The HR is 0.92 mg/kg and the STMR is 0.44 mg/kg.
Proposed GAP in northern and southern Europe, Austria, Belgium, France, Germany, Greece, Hungary, Ireland, Italy, Luxembourg, The Netherlands, Portugal, Spain, and Switzerland is SC 240 g/l, 0.0096 kg ai/hl, 3 applications, 14-day PHI. However, this GAP has not been finalized. Maximum GAP for the UK specifies a 240 g/l SC formulation applied at 0.6 l product/ha (0.14 kg ai/ha) in 15 l water/ha, 3 applications, and a 14-day PHI. For small trees (foliar canopy height 2 m or less), the application rate is reduced to 0.096 kg ai/ha in 10 hl water/ha. The spray concentration is 0.0096 kg ai/hl in both cases. The ranked order of residues in pear trials at the maximum UK GAP in Italy (2), France (1), and Germany (1) is 0.07, 0.08, 0.09, 0.14 and 0.15 mg/kg.
The European residues again appear to be from a different population from those in the US trials, which are higher.
The residues from the 25 apple and pear trials in the USA are comparable, and can be combined to give 0.20, 0.23, 0.25, 0.27, 0.30, 0.31, 0.35, 0.36 (3), 0.37, 0.40, 0.43, 0.50, 0.52, 0.56, 0.60, 0.62, 0.62, 0.68, 0.74 (2), 0.92 and 1.0 (2) mg/kg. The Meeting estimated an STMR of 0.43 mg/kg and a maximum residue level of 2 mg/kg for pome fruit. The HR is 1.0 mg/kg.
Stone fruit. Trials were reported from the USA and various countries in Europe for the period 1998-1999. GAP for peaches, cherries, nectarines, and plums in the USA is SC 240 g/l or WP 800 g/kg, 0.28 kg ai/ha, 1.1 kg ai/ha per season, 7-day PHI. GAP for cherries specifies SC 240 g/l, 0.28 kg ai/ha, 0.95 kg ai/ha per season, 7-day PHI. Compliance with the 1.1 kg ai/ha/season rate requires no more than 4 applications per season. All US trials were conducted with 6 applications at 14-day intervals. Several decline studies conducted on peaches, cherries, and plums in the USA and Europe showed residue half-lives of 7 to 21 days, and the half-life of methoxyfenozide in the grape metabolism study was 13-21 days. The residues from the first two applications would therefore have decreased to £20% of the initial value by the last application. As the fruits would also have been rather small at the time of the first two applications, the first two would have a minimal effect on the residue level compared with the final four applications, and the trials may be considered to accord with maximum GAP.
The ranked order of residues on peaches from 9 trials at maximum GAP is 0.32, 0.50, 0.54, 0.64, 0.78, 0.88 (2), 0.98 and 1.4 mg/kg. The STMR is 0.78 mg/kg and the HR 1.4 mg/kg.
The ranked order of residues on cherries from 7 trials at maximum GAP is 0.19, 0.26, 0.28, 0.34, 0.43, 0.52 and 0.56 mg/kg, with an STMR of 0.34 mg/kg and an HR of 0.56 mg/kg.
The ranked order of residues on plums from 7 trials at maximum GAP is 0.13, 0.14, 0.16, 0.19, 0.29, 0.30 and 0.34 mg/kg, with an STMR of 0.19 mg/kg and an HR of 0.34 mg/kg.
The trials on peaches and nectarines in France, Italy, Spain, and Greece could not be evaluated because there is no finalized relevant GAP.
The Meeting noted the identical use patterns for peaches, cherries, and plums and decided to combine the residues to give 0.13, 0.14, 0.16, 0.19 (2), 0.26, 0.28, 0.29, 0.30, 0.32, 0.34 (2), 0.43, 0.50, 0.52, 0.54, 0.56, 0.64, 0.78, 0.88 (2), 0.98, 1.4 mg/kg.
The Meeting estimated an STMR of 0.34 mg/kg and a maximum residue level of 2 mg/kg for stone fruit. The HR is 1.4 mg/kg.
Grapes. Supervised field trials on grapes were reported from Europe and the USA. Trials on table grapes in Greece, Italy, France, Spain, and Portugal, and on wine grapes in Portugal, France, Spain, Italy and Germany could not be evaluated as there is no finalized GAP. GAP in the USA is WP 800 g/kg or SC 240 g/l, 0.28 kg ai/ha, 0.84 kg ai/ha per season, 30-day PHI. The ranked order of residues in 15 trials at maximum GAP is <0.02, 0.20, 0.20, 0.21, 0.26, 0.26, 0.32, 0.33, 0.34, 0.39, 0.45, 0.46, 0.52, 0.52, 0.84 mg/kg.
Using only the US data with finalized GAP, the Meeting estimated an STMR of 0.33 mg/kg and a maximum residue level of 1 mg/kg. The HR is 0.84 mg/kg.
Broccoli. Eight supervised field trials were reported from the USA. GAP is WP 800 g/kg or SC 240 g/l, 0.28 kg ai/ha, 1.2 kg ai/ha per season, 1-day PHI. The ranked order of residues is 0.52, 0.70, 0.76, 0.89, 0.98, 1.4, 1.6, 1.6 mg/kg. The Meeting estimated an STMR of 0.94 mg/kg and a maximum residue level of 3 mg/kg. The HR is 1.6 mg/kg.
Cabbage. Nine supervised field trials on head cabbage were reported from the USA. The GAP is the same as for broccoli. The ranked order of residues is 0.56, 0.57, 0.67, 0.88, 0.93, 2.2, 3.3, 3.4, 6.2 mg/kg. The Meeting estimated an STMR of 0.93 mg/kg and a maximum residue level of 7 mg/kg. The HR is 6.2 mg/kg.
Tomato. Supervised field trials were reported from Australia, Germany, Belgium, The Netherlands, Spain, Portugal, Italy, France, and the USA. GAP in Australia is SC 240 g/l, 0.03 or 0.04 kg ai/hl, (0.3 or 0.4 kg ai/ha, calculated), 3 applications, 0-day PHI. Nine trials were conducted at maximum GAP (0.04 kg ai/hl and/or 0.4 kg ai/ha), and the ranked order of residues is 0.13, 0.14, 0.21, 0.26, 0.56, 0.57, 0.73, 1.0, 1.6 mg/kg.
Glasshouse trials in Germany, Belgium, The Netherlands, Spain, Portugal, Italy, and France. These trials could not be evaluated for lack of finalized GAP.
GAP in the USA is SC 420 g/l or WP 800 g/kg, 0.28 kg ai/ha, 1.2 kg ai/ha per season, 1-day PHI. Thirteen trials were conducted at maximum GAP, with residues in ranked order of 0.052, 0.088, 0.12, 0.12, 0.13, 0.14, 0.16, 0.19, 0.20, 0.28, 0.33, 0.94, 1.8 mg/kg.
As the residues from Australia and the USA represent similar use patterns and are from the same population the values were combined, giving 0.052, 0.088, 0.12, 0.12, 0.13, 0.13, 0.14, 0.14, 0.16, 0.19, 0.20, 0.21, 0.26, 0.28, 0.33, 0.56, 0.57, 0.73, 0.94, 1.0, 1.6, 1.8 mg/kg.
The Meeting estimated an STMR of 0.20 mg/kg and a maximum residue level of 2 mg/kg for tomatoes. The HR is 1.8 mg/kg.
Peppers. Supervised field trials were reported from the USA on peppers (bell and non-bell) and from Portugal, Spain, Italy, France, and The Netherlands on bell peppers. The 14 glasshouse trials in Europe could not be evaluated as there is no GAP. GAP in the USA is SC 240 g/l or WP 800 g/kg, 0.30 kg ai/ha, 1.1 kg ai/ha per season, 1-day PHI. The ranked order of residues on peppers from 13 trials at maximum GAP is 0.041, 0.049, 0.050, 0.12, 0.14, 0.16, 0.16, 0.20, 0.26, 0.36, 0.40, 0.48, 0.94 mg/kg. The residues in non-bell peppers are in italics.
The Meeting estimated an STMR of 0.16 mg/kg and a maximum residue level of 2 mg/kg for peppers. The HR is 0.94 mg/kg.
Egg plants. Two trials were reported from Malaysia, one within 75% of maximum GAP with a residue value of 0.13 mg/kg.
The Meeting considered one trial insufficient to estimate a maximum residue level.
Sweet corn. Supervised field trials were reported from the USA, where GAP is 0.13 kg ai/ha in a minimum of 94 l/ha (ground and aerial) before tasselling and in a minimum of 190 l/ha after tasselling (ground equipment) for SC 240 g/l, and 0.28 kg ai/ha in 94 l/ha through silking and 0.13 kg ai/ha thereafter for WP 800 g/kg, 1.1 kg ai/ha per season, 3-day PHI. The 14 trials were conducted at 0.28 kg ai/ha. But the residues (kernels + cob with husk removed) were all <0.02 mg/kg.
The Meeting estimated an STMR of 0 mg/kg and a maximum residue level of 0.02 mg/kg (*) for sweet corn (corn-on-the-cob). The HR was estimated as 0.02 mg/kg.
Lettuce (head and leaf). Supervised field trials were reported from the USA on leaf and head lettuce. GAP for leaf and head lettuce is 0.28 kg ai/ha, 1.1 kg ai/ha per season, 1-day PHI. Eight trials on leaf lettuce at maximum GAP gave the ranked order of residues of 3.4, 8.2, 10, 12, 13, 17, 18, 18 mg/kg.
The Meeting estimated an STMR of 12.5 mg/kg and a maximum residue level of 30 mg/kg for leaf lettuce. The HR is 18 mg/kg.
Eight trials on head lettuce at maximum GAP gave the ranked order of residues for head lettuce with wrapper leaves of 1.6, 4.8, 5.4, 6.0, 6.2, 6.5, 7.9, 9.6 mg/kg.
The Meeting estimated an STMR of 6.1 mg/kg and a maximum residue level of 15 mg/kg for head lettuce. The HR is 9.6 mg/kg.
Spinach. Supervised field trials were reported from the USA where GAP is the same as for lettuce. Eight trials at maximum GAP showed the ranked order of residues 9.8, 10, 12, 14, 16, 18, 23, 43 mg/kg.
The Meeting estimated an STMR of 15 mg/kg and a maximum residue level of 50 mg/kg for spinach. The HR is 43 mg/kg.
Mustard greens. Supervised field trials were reported from the USA. GAP is the same as for lettuce. Seven trials at maximum GAP gave the ranked order of residues 10, 12, 14, 16, 17, 17, 18 mg/kg.
The Meeting estimated an STMR of 16 mg/kg and a maximum residue level of 30 mg/kg for mustard greens. The HR is 18 mg/kg.
Soya beans. Two supervised trials on soya beans were reported from Brazil. The residue was 0.03 mg/kg in the one trial according to GAP.
The Meeting considered one trial inadequate to estimate a maximum residue level.
Long beans. Two supervised trials were reported from Malaysia, one within 75% of the maximum GAP with a residue of <0.05 mg/kg.
The Meeting considered one trial inadequate to estimate a maximum residue level.
Celery. Supervised trials for the foliar application of methoxyfenozide to celery were reported from the USA. GAP is SC 240 g/l or WP 800 g/kg, 0.28 kg ai/ha, 1.1 kg ai/ha per season, 1-day PHI. Seven trials were according to GAP. An eighth trial was rejected because of a disease which made the celery unmarketable. The ranked order of residues is 0.48, 0.72, 3.0, 3.4, 5.5, 7.2, 7.8 mg/kg.
The Meeting estimated an STMR of 3.4 mg/kg and a maximum residue level of 15 mg/kg. The HR is 7.8 mg/kg.
Rice. Residues in two supervised trials in Japan at maximum GAP were <0.02 (2) mg/kg.
The Meeting concluded that an STMR and/or maximum residue level could not be estimated from the limited data base.
Maize (field corn). Numerous supervised field trials were reported from the USA, where GAP is SC 240 g/l or WP 800 g/kg, 0.13 kg ai/ha in a 47 l/ha minimum spray volume (ground and aerial), 1.1 kg ai/ha per season, 21-day PHI. Methoxyfenozide residues in maize grain from 24 trials conducted at between 2 and 3 times maximum GAP (0.24-0.36 kg ai/ha) were all <0.02 mg/kg. One additional trial at a similar rate yielded 0.033 mg/kg on one of duplicate samples.
Two supervised trials were reported from Mexico, where GAP is SC 240 g/l, 0.04 kg ai/ha, 4 applications, 30-day PHI. The PHIs were 77 and 156 days.
Two supervised trials were reported from Brazil, where GAP is 0.043 kg ai/ha, 1 application, 7-day PHI. The residues were 0.11 and 0.40 mg/kg.
Although the trials in Brazil produced the highest residues, the Meeting considered two trials inadequate for the estimation of an STMR and/or maximum residue level. Moreover, the description of the plants at the time of the last application (0.7 m high) suggests harvest of an immature commodity.
The Meeting estimated an STMR and HR of 0.02 mg/kg and a maximum residue level of 0.02 mg/kg (*), based on the US trials.
Cotton seed. Supervised trials on cotton were reported from Australia, Mexico, and the USA.
GAP in Australia is SC 240 g/l, 0.6 kg ai/ha, 3 applications at 10-day intervals, 28-day PHI. Eight trials at maximum GAP yielded residues in the seed of <0.05, <0.05, <0.05, <0.05, 1.6, 3.2, 4.8, 4.9 mg/kg. Those values below the LOQ are from delinted cotton seed with little or no boll opening. Those with finite values are for undelinted seed with substantial boll opening at the last application.
GAP in Mexico is SC 240 g/l, 0.12 kg ai/ha, 2 applications, 14-day PHI. Residues in two trials at maximum GAP were 0.02 and 0.11 mg/kg.
GAP in the USA is WP 800 g/kg, 0.45 kg ai/ha in a minimum of 19 l water/ha (aerial) or 47 l water/ha (ground), 1.1 kg ai/ha per season, 14-day PHI. The 1.1 kg ai/ha seasonal rate limits the number of applications at maximum rate to 2, but all US trials were conducted with 5 applications at 14-day intervals for a total seasonal rate of 2.2 kg ai/ha. As the trials in Australia indicate, however, the state of the boll (closed or open) is much more likely to affect the residue level than additional applications 28-70 days before harvest. Eighteen trials were at maximum GAP, and the ranked order of residues in undelinted seed is 0.013, 0.08, 0.10, 0.14, 0.22, 0.26, 0.26, 0.37, 0.41, 0.50, 0.51, 0.51, 0.52, 0.52, 0.98, 1.1, 1.2, 1.3 mg/kg.
The trials in Australia, Mexico, and the USA were combined to give the ranked order <0.05 (4), 0.013, 0.020, 0.08, 0.10, 0.11, 0.14, 0.22, 0.26, 0.26, 0.37, 0.41, 0.50, 0.51, 0.51, 0.52, 0.52, 0.98, 1.1, 1.2, 1.3, 1.6, 3.2, 4.8, 4.9 mg/kg.
The Meeting estimated an STMR of 0.46 mg/kg and a maximum residue level of 7 mg/kg for cotton seed, with an HR of 4.9 mg/kg.
Tree nuts. Supervised trials on almonds and pecans were reported from the USA. GAP is SC 240 g/l or WP 800 g/kg, 0.43 kg ai/ha, 1.1 kg ai/ha per season, 14-day PHI. The ranked order of residues from trials at maximum GAP is <0.02 (3), 0.024, 0.027, 0.034 mg/kg in pecans and <0.020, 0.020, 0.021 (2), 0.036 0.074 mg/kg in almonds.
The combined ranked order of residues in pecans and almonds is <0.02 (4), 0.020, 0.021 (2), 0.024, 0.027, 0.034, 0.036, 0.074 mg/kg
The Meeting estimated an STMR of 0.021 mg/kg and a maximum residue level of 0.1 mg/kg for tree nuts. The HR is 0.074 mg/kg.
Animal feed items
Almond hulls. In the US trials on almonds described above the ranked order of residues in hulls is 6.4, 10 (2), 16, 26, 35 mg/kg.
The Meeting estimated a maximum residue level of 50 mg/kg on a dry weight basis (90% dry matter), and an STMR of 13 mg/kg (not dry weight) for almond hulls.
Maize forage and fodder. Supervised field trials in the USA on maize are described above. The GAP application rate is 0.13 kg ai/ha with a 21-day PHI. As all the trials were at twice this rate (0.24-0.36 kg ai/ha) and showed finite residues at the 21-day PHI they were not evaluated. The evaluation of residues in sweet corn forage and fodder is described below.
Sweet corn forage and fodder. Supervised field trials were reported from the USA for the foliar application of methoxyfenozide to sweet corn according to the following GAP: SC 240 g/l or WP 800 g/kg, 0.13 kg ai/ha in a minimum of 94 l/ha (ground and aerial) before tasselling and in a minimum of 190 l/ha after tasselling for SC, 0.28 kg ai/ha through silking and 0.13 kg ai/ha thereafter for WP in 94 l water/ha (aerial and ground) before tasselling and in 190 l water/ha after tasselling, 1.1 kg ai/ha per season, 3-day PHI for forage and 21-day PHI for fodder. The 14 trials were conducted at 0.28 kg ai/ha/application. The ranked order of residues in forage for the 12 trials with the WP formulation at maximum GAP is 0.20, 0.52, 1.4, 1.5, 3.4, 4.4, 4.6, 6.1, 6.2, 7.2, 15, 22 mg/kg.
The Meeting estimated a maximum residue level of 50 mg/kg on a dry weight basis (48% dry matter), and an STMR of 4.5 mg/kg (not dry weight) for maize forage.
The ranked order of residues in fodder for the 11 trials with the WP formulation at maximum GAP is 1.0, 2.0, 4.9, 5.9, 8.2 (2), 8.4, 9.4, 20 (2), 46 mg/kg.
The Meeting estimated a maximum residue level of 60 mg/kg on a dry weight basis (83% dry matter) and an STMR of 8.2 mg/kg (not dry weight) for maize fodder.
Cotton gin by-products. Eight of the 18 cotton trials in the USA at maximum GAP described above included the determination of methoxyfenozide residues in cotton gin trash. The ranked order of residues in the gin trash is 3.8, 7.1, 9.4, 9.9, 12, 15, 17, 18 mg/kg.
The Meeting estimated an STMR of 11 mg/kg and an HR of 18 mg/kg for cotton gin by-products (trash containing 90% dry matter).
Fates of residue during processing
Processing studies on oranges, apples, grapes, tomatoes, maize (field corn), fresh prunes, and cotton seed were reported.
Two orange processing trials were conducted in Spain and one in Italy. Oranges were converted to marmalade and in two trials juice. The processing factors for juice were 0.3 and 0.2, average 0.25, and for marmalade 0.8, 0.5 and 1.1, average 0.8.
Single processing studies were conducted on apples in Germany, Belgium, France, and the USA. The processing factors were as follows: apple sauce, 0.4, 0.4, 0.4, average 0.4; apple juice, 0.4, 0.4, 0.4, 0.2, average 0.3; apple pomace (wet), 2, 2, 2, 6, average 3; apple pomace (dry), 7, 8, 7, average 7.
From the STMR of 0.43 mg/kg for pome fruit and the average processing factors for apple juice and wet pomace the Meeting estimated STMR-Ps of 0.13 mg/kg for apple juice and 1.3 mg/kg for apple pomace (wet). From the HR of 1 mg/kg for pome fruit and the average processing factor of 7 for dry apple pomace, the Meeting estimated a maximum residue level of 7 mg/kg for apple pomace (dry).
A processing study on the preparation of peach preserves was reported from Italy, but neither the RAC nor the preserves contained a quantifiable residue.
A study on processing plums to dried prunes was reported from the USA. The processing factor was 1.3, which when applied to the HR and STMR for stone fruits (1.4 and 0.34 mg/kg), provides a maximum residue level, an STMR-P and an HR-P of 3, 0.44 and 1.8 mg/kg respectively for prunes (dried plums).
Processing studies were conducted on grapes in France, Italy, Germany, Portugal, Greece, and the USA. The processing factors for grape juice were 0.3 (USA), 0.4 (Portugal), 0.3 (France), 0.2 (Greece), and 0.1 (Italy), average 0.3; for dried grapes (raisins) 1.3 (USA), 2.4 (Greece), 3.1 (Italy), and 2.1 (Italy), average 2.2; for wine 0.4 (USA), 0.3 (USA), 1.3 (Italy), 0.2 (Germany), 0.3 (Germany), 0.4 (Germany), 0.3 (Germany), 0.4 (Portugal), 0.4 (France) and 0.3 (France), average 0.4.
From the processing factors and the STMR for grapes (0.33 mg/kg) the Meeting estimated STMR-Ps for grape juice, raisins, and wine of 0.10 mg/kg, 0.73 mg/kg, and 0.13 mg/kg respectively.
From the processing factor for raisins (2.2) and the STMR and HR for grapes (0.33 and 0.84 mg/kg), the Meeting estimated a maximum residue level of 2 mg/kg, and STMR-P of 0.73 mg/kg and an HR-P of 1.8 mg/kg for dried grapes (raisins).
Tomato processing studies were carried out in the USA, Belgium, Germany, and Italy. The processing factors for juice were 0.2 (USA), 0.3 (Belgium), 0.4 (Germany), 0.4 (Germany) and 0.4 (Italy), average 0.3; for tomato paste 0.7 (USA), 2.2 (Belgium), 1.7 (Germany), 3.0 (Germany) and 3.4 (Italy), average 2.2; and for tomato pomace 3.6 (Belgium). The processing factor for peeling was 0.3 (average of 0.2 (Belgium), 0.4 (Germany), 0.4 (Germany), and 0.2 (Italy)).
From the processing factors and the STMR for tomato (0.20 mg/kg), the Meeting estimated STMR-Ps for tomato juice (0.060 mg/kg), tomato paste (0.44 mg/kg), and peeled tomatoes (0.06 mg/kg).
Wet milling and dry milling studies on maize grain were reported from the USA, but the residue on the RAC was not quantifiable (0.0067 or <0.02 mg/kg). The only processed commodities with quantifiable residues were aspirated grain fractions (0.59 mg/kg) and refined oil from wet milling (0.036 mg/kg). Processing factors could not be calculated.
Cotton seed processing studies were reported from Mexico and the USA. In the four trials in Mexico, only crude oil was analysed. The processing factors were hulls 0.14, crude oil 0.47 (USA), 0.5 (Mexico), 0.37 (Mexico), 0.13 (Mexico) and 0.14 (Mexico), average 0.32; cotton seed meal 0.45.
From the STMR for cotton seed (0.46 mg/kg) and the processing factors, the Meeting estimated STMR-Ps for cotton seed oil (crude) of 0.15 mg/kg, for cotton seed hulls of 0.064 mg/kg, and for cotton seed meal of 0.21 mg/kg.
Residues in the edible portions of food commodities
The residue levels in peel and pulp were determined in the citrus trials in Europe. The pulp residue was <0.05 mg/kg in all mandarin and orange trials. No GAP is available for citrus fruits in Europe.
Pome fruit (apple and pear) trials included studies on peeling and washing. Washing reduced methoxyfenozide residues by an average factor of 0.76 (range 0.4-1). Peeling had a more dramatic effect, with an average factor of 0.15 (range 0.1-0.3).
In the head cabbage trials in the USA, residues were determined both with and without the wrapper leaves. The wrapper leaves are commonly removed before sale at the retail level and/or by the consumer. The average reduction factor was 0.09 (range 0.006-0.46).
In the head lettuce field trials in the USA, residues were determined both with and without the wrapper leaves, which are normally removed. The average reduction factor was 0.014 (range 0.0072-0.021).
Residues in animal commodities
Dietary burden in animals
The Meeting estimated the dietary burden of methoxyfenozide residues in farm animals on the basis of diets listed in Appendix IX of the FAO Manual. Calculation from MRLs, HRs, and STMR-Ps provides the levels in feed appropriate for recommending MRLs for animal commodities, while calculation from STMR and STMR-P values for feed is suitable for estimating STMRs for animal commodities.
Estimated maximum dietary burdens of livestock
|
Commodity |
Codex group |
Residue |
Basis |
% Dry matter |
Residue, dry wt (mg/kg) |
Diets (%) |
Residue contribution (mg/kg) |
||||
|
Beef cattle |
Dairy cattle |
Poultry |
Beef cattle |
Dairy cattle |
Poultry |
||||||
|
Almond hulls |
AM |
50 dry wt |
MRL |
|
50 |
|
|
|
|
|
|
|
Apple pomace, wet |
AB |
1.3 |
STMR-P |
40 |
3.25 |
15 |
5 |
0 |
0.49 |
0.16 |
|
|
Maize grain |
GC |
0.02 |
MRL |
88 |
0.023 |
|
|
80 |
|
|
0.018 |
|
Maize forage |
AF |
50 dry wt |
MRL |
|
50 |
40 |
50 |
0 |
20 |
25 |
|
|
Maize fodder |
AS |
60 dry wt |
MRL |
|
60 |
|
|
|
|
|
|
|
Cotton seed |
SO |
7 |
MRL |
88 |
8.0 |
25 |
25 |
0 |
2.0 |
2.0 |
|
|
Cotton seed hulls |
AM |
0.064 |
STMR-P |
90 |
0.07 |
|
|
|
|
|
|
|
Cotton seed meal |
- |
0.21 |
STMR-P |
89 |
0.24 |
|
|
20 |
|
|
0.05 |
|
Cotton gin by-products |
AM |
18 |
HR |
90 |
20 |
20 |
20 |
0 |
4.0 |
4.0 |
|
|
TOTAL |
|
100 |
100 |
100 |
26 |
31 |
0.07 |
||||
Estimated median dietary burdens of livestock
|
Commodity |
Codex group |
Residue (mg/kg) |
Basis |
% Dry matter |
Residue dry wt (mg/kg) |
Diets (%) |
Residue contribution (mg/kg) |
||||
|
Beef cattle |
Dairy cattle |
Poultry |
Beef cattle |
Dairy cattle |
Poultry |
||||||
|
Almond hulls |
AM |
13 |
STMR |
90 |
14 |
|
|
0 |
|
|
|
|
Apple pomace, wet |
AB |
1.3 |
STMR-P |
40 |
3.25 |
40 |
20 |
0 |
1.3 |
0.64 |
|
|
Maize grain |
GC |
0.02 |
STMR |
88 |
0.023 |
|
|
80 |
|
|
0.018 |
|
Maize forage |
AF |
4.5 |
STMR |
40 |
11 |
40 |
50 |
0 |
4.4 |
5.5 |
|
|
Maize fodder |
AS |
8.2 |
STMR |
83 |
11 |
|
|
0 |
|
|
|
|
Cotton seed |
SO |
0.46 |
STMR |
88 |
0.52 |
|
10 |
0 |
|
0.052 |
|
|
Cotton seed hulls |
AM |
0.064 |
STMR-P |
90 |
0.071 |
|
|
0 |
|
|
|
|
Cotton seed meal |
- |
0.21 |
STMR-P |
89 |
0.24 |
|
|
20 |
|
|
0.05 |
|
Cotton gin by-products |
AM |
11 |
STMR |
90 |
12 |
20 |
20 |
0 |
2.4 |
2.4 |
|
|
TOTAL |
|
100 |
100 |
100 |
8.1 |
8.6 |
0.07 |
||||
The estimated maximum dietary burdens of methoxyfenozide for beef cattle, dairy cattle, and poultry are 26 ppm, 31 ppm, and 0.07 ppm respectively, and the estimated median dietary burdens 7.5 ppm, 7.8 ppm, and 0.07 ppm respectively.
Feeding studies
Three cows at each level were dosed orally with the equivalent of 16, 54, or 180 ppm in the diet for 28 consecutive days. Milk was collected daily and analysed on days 1, 2, 4, 7, 10, 14, 17, 21, 24, and 28. The cows were slaughtered within 24 h of the last dose, and tissues were collected and analysed for methoxyfenozide and the glucuronide conjugate of the A-ring phenol.
All milk samples from the 16 and 54 ppm feeding levels were below the LOQ (0.01 mg/kg) for methoxyfenozide. Methoxyfenozide was detected (>0.003 mg/kg) in some samples, the highest values being 0.0063 mg/kg in day 28 milk at the 16 ppm feeding level and 0.0076 mg/kg in day 7 milk at the 54 ppm level. Quantifiable residues were found in milk from the 180 ppm group. The residue reached a plateau of 0.03-0.05 mg/kg on days 7-10 and an average of 0.027-0.030 mg/kg for days 10-28. The highest residue was 0.10 mg/kg from a single cow on day 7.
Day 28 milk from the 180 ppm level was separated into cream and skimmed milk. The residue in the cream was 0.12 mg/kg, in the skimmed milk 0.0054 mg/kg. The residue in the whole milk was 0.028 mg/kg. The concentration factor for cream relative to whole milk is 4.3. This does not represent a significantly higher solubility in milk fat than in whole milk. No information was provided on the residues in cream from the other feeding levels.
Fat, muscle, liver, and kidney from each of the cows at each of the feeding levels were analysed for methoxyfenozide. The glucuronide conjugate of the A-ring phenol was also determined in liver and kidney.
In fat, methoxyfenozide was quantifiable at all feeding levels: 0.011 mg/kg maximum(<0.01 mg/kg average) at 16 ppm; 0.082 mg/kg maximum (0.041 mg/kg average) at 54 mg/kg; 0.44 mg/kg maximum (0.28 mg/kg average) at 180 ppm.
Methoxyfenozide was not detected in muscle at the 16 and 54 ppm feeding levels (limit of detection, 0.003 mg/kg). At the 180 ppm level, the maximum residue was 0.10 mg/kg and the average was estimated at 0.0073 mg/kg (LOQ 0.01 mg/kg).
Methoxyfenozide residues were not quantifiable in liver (<0.01 mg/kg) at the 16 ppm feeding level. The highest residue was 0.0094 mg/kg. At the 54 ppm feeding level the highest methoxyfenozide residue was 0.030 mg/kg, average 0.028 mg/kg, while the highest and average residues of the glucuronide conjugate of the A-ring phenol were 0.035 mg/kg and 0.026 mg/kg respectively. At the 180 ppm feeding level, the highest and average residues of methoxyfenozide were 0.15 mg/kg and 0.13 mg/kg, and of the glucuronide conjugate of the A-ring phenol 0.12 mg/kg and 0.10 mg/kg.
In kidneys, methoxyfenozide was not detected at the 16 ppm feeding level, and was below the limit of quantification at the 54 ppm feeding level. At the 180 ppm level, the highest and average methoxyfenozide residues were 0.034 mg/kg and 0.026 mg/kg, and those of the glucuronide conjugate of the A-ring phenol 0.046 mg/kg and 0.029 mg/kg.
The Meeting noted that the methoxyfenozide and glucuronide conjugate residues were approximately equal in kidney and liver in the feeding studies, whereas in the metabolism study the metabolite concentration was about 10 times that of the parent.
In a poultry feeding study 10 or 12 hens in each of three feeding groups were dosed orally at the equivalent of 2.4, 7.6, and 24 ppm methoxyfenozide for 28 consecutive days. Eggs were collected each day by group, and the hens were killed within 24 h of the last dose. Tissues were analysed for methoxyfenozide.
At the 2.4 ppm feeding level, residues in eggs were below the limit of detection on days 1, 3, and 7, as they were at the 7.6 ppm level except in one of three samples (0.0032 mg/kg) on day 1. The estimated limit of detection for methoxyfenozide was 0.003 mg/kg.
At the 24 ppm feeding level, residues of methoxyfenozide in eggs were below the limit of detection over the entire 28 days, except in one sample on day 10 (0.0054 mg/kg) and one sample on day 17 (0.0030 mg/kg). Residues of the glucuronide conjugate of the A-ring phenol became detectable on day 7 but never reached the limit of quantification (0.01 mg/kg).
Residues of methoxyfenozide never reached the limit of detection (0.003 mg/kg) in fat, muscle, or liver at any feeding level. At the 7.6 and 24 ppm feeding levels the glucuronide conjugate of the A-ring phenol in liver averaged 0.013 and 0.021 mg/kg respectively.
Maximum residue levels in animal commodities
The Meeting agreed that the residues in cows from the 54 ppm feeding level could be extrapolated to the 31 ppm maximum dietary burden for dairy cattle to estimate maximum residue levels for ruminant commodities. The 16 ppm feeding level could not be used as the residues were undetectable or unquantifiable.
The Meeting further agreed that the residues from the 16 mg/kg feeding level, all below the LOQ, could be used without extrapolation as the residues at the 8.1 and 8.6 median feeding levels for beef and dairy cattle respectively.
|
Dietary burden (ppm) Feeding level [ppm] |
Methoxyfenozide residue1 (mg/kg) |
||||||||
|
Milk2 |
Muscle |
Liver |
Kidney |
Fat |
|||||
|
Mean |
Highest |
Mean |
Highest |
Mean |
Highest |
Mean |
Highest |
Mean |
|
|
MRL dairy/beef cattle (31) |
0.0027 |
0.0017 |
|
0.017 |
|
0.0022 |
|
0.047 |
|
|
[54]3 |
0.0047 |
<0.003 |
|
0.0305 |
|
0.0038 |
|
0.0820 |
|
|
STMR dairy cattle (7.8) |
0.0041 |
|
0.003 |
|
0.0075 |
|
0.003 |
|
0.0082 |
|
[16]4 |
0.0041 |
|
<0.003 |
|
0.0075 |
|
<0.003 |
|
0.0082 |
1 Methoxyfenozide only
2 Day 28
3 Extrapolation
4 Direct application of the 16 ppm level, owing to high uncertainty in the values (undetectable or below the LOQ)
The Meeting estimated a maximum residue level for milk of 0.01 mg/kg and an STMR of 0.0041 mg/kg. Although methoxyfenozide distributed preferentially in the fat of muscle, the concentration factor from whole milk to cream at the 180 ppm feeding level was only 4.3 to 1. No measurements were made on cream at the feeding level of relevance, 54 ppm. The Meeting estimated maximum residue levels for mammalian meat (fat) of 0.05 mg/kg and for mammalian offal of 0.02 mg/kg, and STMRs for mammalian muscle at 0.003 mg/kg, for offal at 0.0075 mg/kg, and for mammalian fat (trimmable) at 0.0082 mg/kg. The HRs are 0.017 mg/kg for mammalian offal, 0.0017 mg/kg for mammalian meat muscle, and 0.047 mg/kg for mammalian meat fat.
The Meeting concluded that residues of methoxyfenozide are unlikely in poultry commodities at the maximum and median dietary burden levels of 0.07 ppm. At the lowest feeding level, 2.4 ppm, methoxyfenozide and the glucuronide conjugate of the A-ring phenol were generally undetectable in eggs (<0.003 mg/kg). Methoxyfenozide was detected in one sample at the 2.4 ppm feeding level and in several samples at higher feeding levels, so the estimated maximum residue level is 0.01 mg/kg and the STMR 0.000 mg/kg in eggs. At all feeding levels, residues were undetectable (<0.003 mg/kg) in muscle and fat, so the estimated maximum residue level is 0.01 (*) mg/kg for poultry meat, and the STMR 0.000 mg/kg for poultry muscle and fat. In liver, residues were undetectable at all feeding levels (<0.003 mg/kg). The maximum residue level for poultry offal is therefore 0.01(*) mg/kg and the STMR 0.000 mg/kg. The HRs are 0.003 mg/kg for eggs and 0.000 mg/kg for poultry offal and meat.
DIETARY RISK ASSESSMENT
Long-term intake
The International Estimated Daily Intakes of methoxyfenozide based on the STMRs estimated for 42 commodities for the five GEMS/Food regional diets were in the range of 0-9% of the maximum ADI (Annex 3). The ADI is 0-0.1 mg/kg bw. The Meeting concluded that the long-term dietary intake of residues of methoxyfenozide is unlikely to present a public health concern.
Short-term intake
The acute RfD for methoxyfenozide is 0.9 mg/kg body weight. The international estimate of short term intake (IESTI) for methoxyfenozide was calculated for food commodities for which maximum residue levels, STMRs and/or HR values were established at this Meeting. The results are shown in Annex 4.
The IESTI for spinach is 310% of the acute RfD for the children. The information provided to the Meeting precludes an estimate that the short-term dietary intake of spinach by children would be below the acute reference dose. The Meeting noted that a conservative acute RfD was established and that a refinement is possible.
For all the other commodities considered, the percentage of the acute RfD varied from 0% to 100%. The Meeting concluded that short-term intake of residues of methoxyfenozide in these commodities, when used in ways that have been considered by the JMPR, is unlikely to present a public health concern.