Ivan Katavić1, Vjekoslav Tičina2
Abstract
Since 1996 the farming of bluefin tuna (Thunnus thynnus) in Croatia has developed rapidly due to the high prices offered by the Japanese market. Bluefin tuna in the Adriatic Sea are mostly fished by purse seine for farming purposes. After capture they are kept in cages and fed for a 2 to 3-year period. This practice improves the limited fishing quota, by increasing the output tuna product biomass and its market value, without additional capture fishing mortality. This paper traces the tuna farming development in the Adriatic Sea and includes some constraints regarding the environmental impact which industry had to face.
1. Introduction
There are seven different tuna species which live in the various oceans of the world. These are: blackfin tuna ( Thunnus atlanticus), bigeye tuna ( Thunnus obesus), longtail tuna ( Thunnus tonggol), yellowfin tuna ( Thunnus albacares), albacore ( Thunnus alalunga), southern bluefin tuna ( Thunnus maccoyii) and northern bluefin tuna ( Thunnus thynnus). Among them, the most important are albacore and bluefin tuna, which are both captured in the Mediterranean area. There are also some sporadic catches of bigeye tuna, while those of other species have not been reported. Capture quantities of these three species are shown in Table 1. There is a big demand for bluefin tuna due to the quality of its flesh, which is highly valued by the Japanese market. The market price for bluefin tuna can vary from a few dollars per kilogramme to more than 200 USD/kg for fat tuna meat.
Bluefin tuna can reach a length of over 300 cm and weigh more than 600 kg. This fish has a relatively long lifespan, and is believed to live up to 30 years. As it tolerates a wide range of sea temperatures, it has a very wide geographical distribution in the Pacific and Atlantic Oceans. Two main spawning grounds of the northern Atlantic bluefin tuna are located in the Gulf of Mexico and the Mediterranean Sea. During its migration, bluefin tuna can cross the Atlantic Ocean. According to recent tagging results, that suggest the existence of residency and fidelity of tuna to its spawning grounds (De Metrio et al., 2003), it is believed that two sub-populations of bluefin tuna exist in the Atlantic Ocean.
2. Bluefin tuna fisheries in the Adriatic Sea
The northern bluefin tuna (BFT) fisheries in the Atlantic Ocean and in all parts of the Mediterranean area are regulated by the quota system, i.e., by Total Allowable Catch (TAC) regulated by the International Commission for Conservation of Atlantic Tunas (ICCAT). TACs are established for all stock users of bluefin tuna. Management regulations applied to BFT fisheries in the Adriatic are the same as those of the Eastern Atlantic and Mediterranean area. Consequently, there is no partial stock assessment of BFT. The quotas allocated annually by the ICCAT apply to the whole Mediterranean (Adriatic included), and there are no management regulations applying exclusively to the Adriatic. Spawning Stock Biomass (SSB) of the Eastern BFT in the year 2000 was estimated at a level of about 86 percent of SSB during 1970, and the ICCAT set the TAC for the Eastern Atlantic and Mediterranean BFT at 32 000 tonnes for the period from 2003 to 2006. Also, the minimum fish landing size has been increased from 3.2 to 4.8 kg (SCRS/2003/BFT Executive Summary) in order to protect juveniles.
Table 1.Capture quantities (in t) of three tuna species in the Mediterranean fishing area.
| YEAR | Albacore (Thunnus alalunga) | Bluefin tuna (Thunnus thynnus) | Bigeye tun (Thunnus obesus) | TOTAL |
| 1981 | 1.500 | 10.515 | 13996 | |
| 1982 | 1.272 | 15.706 | 18960 | |
| 1983 | 1.235 | 13.650 | 16868 | |
| 1984 | 3.414 | 17.032 | 22430 | |
| 1985 | 4.129 | 17.203 | 23317 | |
| 1986 | 3.712 | 14.560 | 1 | 20259 |
| 1987 | 3.993 | 13.764 | 19744 | |
| 1988 | 4.063 | 17.167 | 23.218 | |
| 1989 | 4.060 | 15.628 | 21.677 | |
| 1990 | 1.896 | 17.207 | 21.093 | |
| 1991 | 2.378 | 19.872 | 2 | 24.243 |
| 1992 | 2.202 | 24.230 | 28.424 | |
| 1993 | 2.130 | 24.901 | 29.024 | |
| 1994 | 1.349 | 39.540 | 42.883 | |
| 1995 | 1.587 | 37.640 | 41.222 | |
| 1996 | 3.125 | 38.100 | 43.221 | |
| 1997 | 2.541 | 33.578 | 1 | 38.117 |
| 1998 | 2.698 | 28.196 | 0 | 32.892 |
| 1999 | 4.851 | 22.825 | 0 | 29.675 |
| 2000 | 5.577 | 23.224 | 30.801 | |
| 2001 | 4.743 | 21.662 | 28.406 |
(Data source: ICCAT Statistical Bulletin, Vol. 32)
Offshore purse-seine fishing activities concerning the bluefin tuna are a very important part of the pelagic fishery within the Adriatic Sea. In Croatia, purse seine is a principal fishing gear used for its capture. Bluefin tuna fishing activities in the Adriatic Sea are described in detail by Tičina (1997; 1999) and Tičina et al. (2002).
The principal fishing grounds for Croatian bluefin tuna purse-seiners are the offshore waters of the central part of the Adriatic Sea. After capture, they are transferred into floating towing cages. This is done in the open sea where the catch has occurred, by simply joining both nets under the sea surface. Once the cages are filled with the right number of tuna they are slowly towed by a tugboat towards the farming locations. The distance between the fishing ground and the farming location can vary from a few to several hundreds of miles (if the fish catch occurs outside the Adriatic Sea, Katavić et al., 2003a).
Purse-seine fishery in the Adriatic has become the principal provider of fish seed for further farming purposes. The Adriatic Sea is an important feeding ground for small-size tuna (mostly up to 3 years of age, Tičina and Kačić, 1998), consequently the fish supplied to the Croatian farms are small. An unusual high amount of small tuna were recently caught in the Adriatic (88,6 percent of fish from 5 to 10 kg in 2003 - preliminary data) which is probably the result of an increase in recruitment in the last years. This could also be related to a reduced number of natural predators of small tuna (sharks, swordfish, giant tuna, etc.) due to a high fishing pressure. However, it should be pointed out that small fish are not preferred by farmers as fish seed, because of higher production risks, additional rearing efforts needed and higher overall production costs as compared with rearing the larger specimen or using them as seed.
3. Bluefin tuna farming in the Adriatic Sea
The first bluefin tuna farming started in Canada in the late 1960s, and the first tuna farming in the Mediterranean took place near Ceuta in the late 1970s. In the Adriatic Sea, the first pilot farming of bluefin tuna started in 1996, applying the technology developed during the farming activities on the southern bluefin tuna in Australia that had been practiced since the 1980s (Miyake et al., 2003).
Since 1996 the farming of bluefin tuna (Thunnus thynnus) in Croatia has developed rapidly. Initially, they were captured by purse seiners and fattened for a period from 4 to 6 months before being harvested and exported to the Japanese market. During this period, they were fed with a variety of small pelagic species. The distribution of fish feed in floating cages is performed manually (i.e., on a wide surface area), and the quantity ranges between 3 and 8 percent according to their bodyweight. However, in the Adriatic Sea an entirely new concept of bluefin tuna farming has developed. Small- to medium-size fish are reared for a period from 2 to 3 years, before being harvested and landed. This practice is aimed at improving the limited fishing quota, by increasing the output tuna product biomass without additional capture fishing mortality (Figure 1) and at raising the value of the product, thus obtaining a better market price. ICCAT quota allocated to Croatia (about 900 tonnes) is not meeting the farmers' needs, consequently more than 50 percent of the fish seed introduced in the fattening cages are imported (Figure 2) from other countries, i.e., EU, Tunisia, etc., (Katavić et al., 2003b). It should also be pointed out that tuna farming does not encourage the catch of small tuna, because there is a higher profit if bigger specimens are used as fish seed.
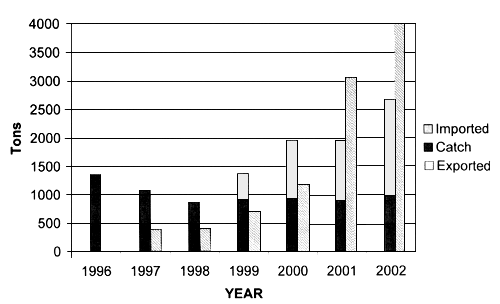
Figure 1. Comparison between Croatian BFT catch and import in relation to exported bluefin tuna from the farms in the Adriatic Sea.
In 2001, on the Eastern Adriatic Sea coast there were six medium- to large-farms with nine rearing sites which included 43 cages (Figure 3). Cages used for tuna farming are constructed as 50 m diameter floating circles with a suspending net of about 20 to 25 m in depth (Katavić et al., 2003a; Miyake et al., 2003).
Recently, the rapid development of tuna farming practices in the Adriatic and other parts of the Mediterranean Sea area caused great concern regarding the sustainability of this new important industry and its impacts. For this reason, an ad hoc GFCM/ICCAT Working Group on sustainable tuna farming/fattening practices has been formed with the aim of developing practical guidelines, in order to address the present problems, and proposing further research. According to the report of the first meeting of the GFCM/ICCAT Working Group, tuna farming currently involves the collection of wild fish, ranging from small to large specimens, and their rearing in floating cages for a period from a few months to a few years. An increase in weight and change in the fat contents of the flesh are obtained through standard fish farming practices. The confinement of the captured fish during a short period of time (2–6 months) helps increase the fat contents of the flesh which strongly influences the price of tuna meat on the Japanese sashimi market, also referred to as «tuna fattening» (SCRS/2003/020).
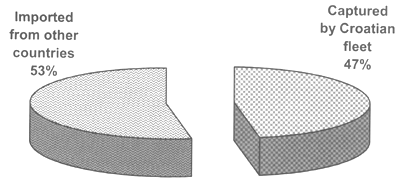
Figure 2. Origins of fish seed entered in the grow-out cages for bluefin tuna farming in the Adriatic Sea during 2000 and 2002 (Katavić et al., 2003b).
Farmed tuna are mainly exported to the Japanese sashimi markets, but this constitutes only 4 percent of the total amount required. From 1998 to 2001 the tuna supply (all species) to the Japanese market ranged from 451 000 to 507 000 tonnes, but the most important is a high valued product called “toro”. “Toro” constitutes only approximately 30 percent of the wild fish catch, but almost the entire quantity of farmed tuna is considered as «toro» (Ottolenghi et al., 2003). The advantages of cultured tuna are its lower prices compared to the wild tuna, and its availability at supermarkets, fresh fish shops and sushi restaurants throughout the year. However, because of the rapid increase in the quantity of farmed bluefin tuna, a serious decline in market prices was observed recently. Due to the fact that the Japanese sashimi market is close to its saturation regarding farmed bluefin tuna (BFT) consumption, it is very likely that the price of farmed products will continue to decrease, unless a new big market is found. One of the major concerns related to BFT farming practices is the negative impact this activity may have on the environment and other activities in the coastal zone (i.e. tourism). This concern stems from the past incidents caused by improper location of the cages. There are also difficulties in estimating (i.e. back calculating) the total biomass and size composition of bluefin catches assigned to fattening and farming, due to lack of information during the rearing period (e.g., accurate data on initial size, rearing period, diet, conversion rates, etc.). There is also much concern regarding the possibility of overexploitation of small pelagic fish stocks used as fish feed for tuna.
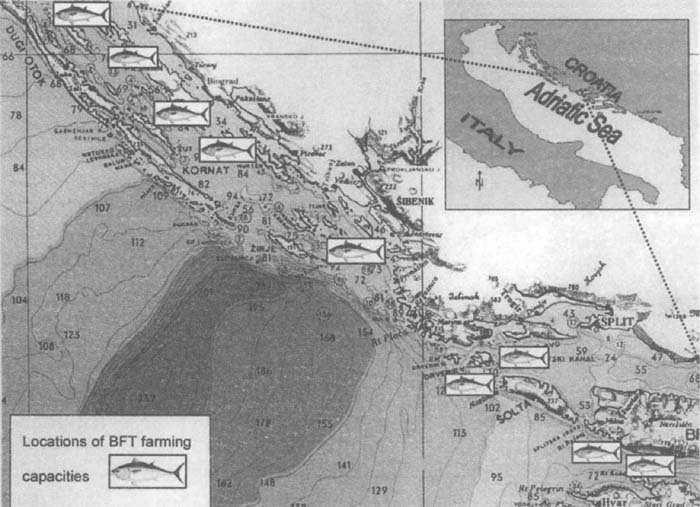
Figure 3. Locations of tuna farming sites along the Eastern Adriatic Sea coast in 2001 (Katavić et al., 2003a).
As all other human activities, tuna farming has a certain local environmental impact. According to the results of some recent studies presented at the International Meeting “Tonno e dintorni” (Castellammare del Golfo, 24–26/10/2003, Italy), no significant environmental changes have been noticed in water columns and sediment at a distance of >100 m from the grow-out floating cages for tuna farming located at 45–50 m depth. In order to avoid negative interactions with other commercial activities, coastal zone management plans should be developed. Another issue of concern was the possible impact this activity could have on small pelagic fish stocks in the Adriatic, since these species are used as fish feed in tuna farming practices. In order to properly assess the availability of the resource and to prevent this problem, small pelagic fish stocks in the Adriatic Sea are monitored by annual acoustic surveys. It should also be pointed out that more than 50 percent of the tuna fish feed is imported from other fishing areas of the Atlantic.
To find out the cause and also to prevent other possible problems, various scientific research projects are currently being carried out on a national and international basis and in close collaboration with tuna farmers. Perhaps, the most important research should be performed on the spawning and breeding of bluefin tuna in captivity, thus enabling an important change from capture based aquaculture to a fully controlled and sustainable aquaculture practice. Also a permanent environmental monitoring contemporarily with studies on improving husbandry and reducing pollution should be carried out.
On the other hand, bluefin tuna farming activities have created many new jobs and currently employ about 500 people. Also, about 30 large bottom trawlers have been fully integrated into tuna farming operations, thus reducing the fishing pressure on an already over-exploited demersal fish stock. Due to its fish aggregating effect, fishing grounds around the areas with tuna cages are among the most favourable for artisanal small-scale fisherman and sport fishing.
4. Conclusions
Tuna farming has important socio-economic and environmental effects and receives considerable public attention. It is a new, rapidly growing activity aimed at increasing the tuna products biomass and also increasing its value on the market, but without increasing fishing mortality, that is already limited by fishing quotas given by ICCAT. However, one of the most important issues is the proper location of the farms so as to avoid environmental problems and negative interactions with other commercial coastal activities.
The main driving force for the development of bluefin tuna farming are high prices of tuna products on the Japanese sushi and sashimi market. Due to the recent expansion of tuna farming in the Adriatic Sea, fuelled by profits related to the Japanese market, the bluefin tuna are mostly fished by purse seine for farming purposes with the aim at obtaining fish seed, and not for canning factories or as fresh (food consumption) to local markets.
Capture-based aquaculture farming activity does not encourage the catch of small tuna, because higher profits are obtained if larger specimens are used as fish seed. However, due to fact that the Adriatic Sea in not a bluefin tuna spawning ground but a feeding ground, tuna catches (made by purse seine) usually does not contain large specimens of tuna (spawners). Consequently, tuna farmers in Croatia are usually unable to get a big size fish seed from local purse seine fishing fleet, but the size of fish seed used for farming usually corresponds to the fish size currently present in the Adriatic Sea.
Tuna farming activities has a positive economic impact on the heavily depopulated Croatian islands, as it helps to create many new jobs where they are mostly needed. Also, it reduces fishing pressure on demersal fish stocks by employing about 30 large bottom trawlers for farming operations. .
A number of different research studies should be carried out with the aim at preventing eventual problems that this activity could cause. However possible achieving of full control of reproduction of bluefin tuna in captivity and breeding of its early life stages, in order to manage its complete lifecycle, would guarantee a sustainable future for this sector of mariculture. To reach this objective, it is of great importance to establish interaction and cooperation between the scientific world and the industry, both at national and international levels. This is the basis for practical future development in accordance with the recent guidelines provided by Code of Conduct for Responsible Fisheries (FAO, 1995).
5. References
Anon. (2003) Report of the First Meeting of the Ad Hoc GFCM/ICCAT Working Group on Sustainable Tuna Farming/Fattening Practices in the Mediterranean (SCRS/2003/020).
Anon. 2003. BFT Executive Summary (SCRS/2003 Doc. No. SCI-006A).
De Metrio, G., Arnold, G.P., de la Serna, J.M., Cort, J.L., Yannopoulos, C., Labini, G.S., Deflorio, M., Buckley, A., Ortiz de Urbina, J.M., Megalofonou, P., Pappalepore, M., & Block, B.A (2003) Where do Atlantic bluefin tuna (Thunnus thynnus) spread after spawning in the Mediterranean Sea? In: ORAY, I.K. and KARAKULAK, F.S. (Eds.), 2003. «Workshop on Farming, Management and Conservation of Bluefin Tuna», Turkish Marine Research Foundation, Istanbul - TURKEY, Publication No. 13: 89–95.
FAO. (1995) Code of Conduct for Responsible Fisheries, Rome.
Katavić, I., Tičina, V., & Franičević V. (2003a) Bluefin tuna (Thunnus thynnus L.) farming on the Croatian coast of the Adriatic Sea - present stage and future plan. In: C.R. Bridges, H. Gordin, A Garcia (eds.) Domestication of the Bluefin Tuna, Thunnus thynnus thynnus. Cahiers Options Mediterranéennes, Vol 60: 101–106.
Katavić, I., Tičina, V., Grubišić L., and Franičević, V. (2003b) Tuna farming as a new achievement in mariculture of Croatia. In: Oray, I.K. and Karakulak, F.S. (Eds.), 2003. «Workshop on Farming, Management and Conservation of Bluefin Tuna», Turkish Marine Research Foundation, Istanbul - TURKEY, Publication No. 13: 10–20.
Miyake, P.M., De la Serna, J. M., Di Natale, A., Farrugia, A., Katavić, I., Miyabe N., % V. Tičina (2003) General review of bluefin tuna farming in the Mediterranean Area. ICCAT Coll. Vol. Sci. Pap., 55(1): 114–124.
Ottolenghi, F., Silvestri, C. & A. Lovatelli (2003) An overwiev of world bluefin tuna fishing and farming. In: Oray, I.K. and Karakulak, F.S. (Eds.), 2003. «Workshop on Farming, Management and Conservation of Bluefin Tuna», Turkish Marine Research Foundation, Istanbul - TURKEY, Publication No. 13: 10–20.
Tičina, V. (1997) Bluefin tuna (Thunnus thynnus L.) purse-seine fishing in the eastern Adriatic Sea. ICCAT Coll. Vol. Sci. Pap., 46(2): 126–128.
Tičina, V. (1999) Bluefin tuna (Thunnus thynnus Linnaeus, 1758.) - biology, fishing, management and conservation. (In Croat) Pomorski Zbornik; 37(1); pp. 209–221.
Tičina, V. % Kačić. I. (1998) Preliminary data on age determination of bluefin tuna (Thunnus thynnus L.) caught in the Adriatic Sea. Rapp. Comm. int. Mer Medit. ; 35(2); pp. 486–487.
Tičina, V., Katavić, I., % Franičević, V. (2002) Croatian bluefin tuna catches in the Adriatic during 1999 through 2001 by year/month/size structure. ICCAT Coll. Vol. Sci. Pap., 54(2): 465–471.
2 Institute of Oceanography and Fisheries Setaliste Ivana Mestrovica 63, 21000 Split, Croatia
Eleonora Ciccotti*
Abstract
The European eel, Anguilla anguilla L., is recognised today as an international marine species and a shared resource among European and Mediterranean countries. For this species, major problems exist in relation to a continent-wide decline in recruitment observed in the course of the last decades, and to a contraction in adult eel capture fisheries. In relation to this situation, debate on the possible measures to protect the European eel stock is topical at the present moment, also in relation to a series of steps undertaken by the European Community.
1. Introduction
The European eel ( Anguilla anguilla L., 1758) is a highly migratory amphihaline species. Its life cycle, elucidated in the 1920's by Johannes Schmidt, is considered unique due to the magnitude of the larval migration, but still cannot be considered completely resolved. Spawning takes place, according to Schmidt's findings, in the Atlantic Ocean, and precisely somewhere in the Sargasso Sea where the smallest larvae, the leptocephali, were observed (Schmidt, 1922). After hatching, leptocephali are probably driven towards Europe by the Gulf Stream: this passive migration should take over two years, although recent findings based on glass eel otholith microstructure suggest that the migration is achieved in less than a year (Lecomte-Finiger, 1992; Desaunay and Guérault, 1997). On the continental shelf, leptocephali metamorphose into glass eels, which colonize coastal and inland waters of the Atlantic and Mediterranean coasts, entry in the Mediterranean through the Gibraltar Strait being only supposed. Glass eel ascent, constituting recruitment to most systems - coastal lagoons, estuaries and rivers, streams and channels, lakes and reservoirs - takes place by a mechanism known as selective tidal transport, STT (McCleave and Wippelhauser, 1982). In the course of this phase, glass eels undergo a series of changes, physiological as well as behavioral, darkening as pigmentation develops and becoming able to swim actively, thus reaching the so-called “elver” stage The subsequent stage, the yellow eel, takes place in continental waters, with a duration ranging from 3 to 8 years for males and 5 to 15 years for females. Growth pattern in this phase shows a wide range of variation depending on habitat characteristics. Eels begin gonadal development when still in inland waters and lagoons, becoming silver eels and emigrating towards the sea. Nothing is known about adult migration, that occurs probably deep in the sea: spawning eels have never been observed, thought it is believed that spawning occurs only once, females producing 2–3 millions eggs. No homing behaviour has ever been assumed, and therefore escapement from continental water bodies is considered to contribute to the stock throughout the whole distribution area. The geographic distribution of the European eel comprises most of Europe, ranging from Northern Scandinavia to North Africa, and from the Eastern Mediterranean region to the Azores, the latter being the western limit of its distribution.
On the whole, the species extremely uniform genetics have been considered to confirm panmixia. Recent findings based on microsatellite DNA analyses have suggested the possibility of genetic differences within the stock (Wirth and Bernatchez, 2000) and in particular indicate that there may be population differences on a North-South basis. The genetic variation found is extremely small in comparison to other species. Investigations from different research teams are still under way, but at present the species is still to be considered, from a biological point of view, as a panmictic marine species, and from the point of view of its management as a highly migratory species.
For this species major problems exist, in relation to a continent-wide decline in recruitment observed in the course of the last decades, and to a contraction in adult eel capture fisheries (ICES, 2001; Dekker, 2002a). If compared to other shared species or to other migratory fish, the eel shows some peculiar features. Eel exploitment occurs exclusively within national boundaries, in continental waters, without any interaction between economic zones, typical eel fisheries being mainly small-scale. The spawning process takes place in international waters, and all oceanic life stages are unexploited. Finally, the population is panmictic and the species is a shared resource by practically all European and Mediterranean countries.
Spawning stock management is essential for the sustainable exploitation of the species, but no individual country has any individual responsibility nor the ability to protect it. The necessity to base the establishment of target reference points and the consequent management options on the precautionary approach is evident for this species (Moriarty and Dekker, 1997; ICES, 2001). The majority of reference points require information on several population parameters including age structure, growth, natural mortality, spawning stock size and recruitment size. The limited knowledge and particular population dynamics of the European eel are a major obstacle, in particular with regards to the existence of a relationship between spawning stock and recruitment.
The European eel represents a case for which strict interactions between aquaculture and fisheries exist. Today, a great share of eel supply to the market comes from eel culture, well established in Europe since more than 30 years, and amounting to over 10000 t in 2001. This increase in eel aquaculture production has partially filled the gap created by the wild eel contracting yields. It is well known that the main limiting factor for eel aquaculture lies in seed, i.e. glass eels or elvers to start production cycles, with particular regard to its availability, quality and price. Induced spawning of this species is to be considered out of reach for the next future, despite the basic and applied research going on at present on the reproductive biology of anguillid eels.
In relation to this situation, debate on the possible measures to protect the European eel stock is topical at present, also in relation to a series of steps undertaken by the European Community.
In the present paper, a general review of eel exploitation is given at European and Mediterranean level, with emphasis on the links between capture fisheries and aquaculture, and paying special attention to the situation in the Adriatic region. This might prove to be important in consideration of the wide geographical distribution of the eel, and in relation to the fact that management options shall have to be translated into appropriate local-system targets (ICES, 2001).
2. Eel fisheries in Europe and in the Mediterranean region
Target stages of eel fisheries throughout its entire distribution area varies from recruiting glass eel to escaping silver eel, and this applies also to eel fisheries in the Mediterranean. Fisheries for eel in single countries reflect traditions of availability and market or consumption customs. Where glass eel ascent is intense, such as in the large tidal estuaries of the Atlantic coast of France, Spain, Portugal and in the Severn estuary in England, specific glass eel fisheries have developed for direct human consumption and for restocking inland waters or to be used as seed for aquaculture. Yellow eel are fished in inland and coastal waters throughout Europe and northern Africa, fishing gears being a variety of lines, nets and traps. A third set of fisheries focuses on the emigrating silver eels which are easily caught in intercepting barriers, nets and traps on their downriver and coastal emigration routes. Silver eel fisheries are found all over Europe, but most predominantly in Scandinavia and in Mediterranean lagoons.
Large scale fisheries for eel are rare and account for less than 5 percent of the total European catch (Dekker, 2002a). The remaining fisheries can be considered small-scale, throughout Europe and the Mediterranean, and can be commercial, semi-commercial or recreational. The processing and trade industries are organised in larger size companies and operate on an international scale (Dekker, 2002a).
With reference to yellow and silver eel fisheries in the Mediterranean region, inland fisheries are found in main rivers and lakes in most countries, but no formal information is available about these rural, small scale, scattered fisheries. Egypt is the most southern place where a commercial eel fishery is known to exist, the Nile and related waters in the valley having a very large eel stock (Dekker, 2002b). Eel fisheries are concentrated in coastal lagoons and lakes, but considerable fisheries are also found in the many branches of the Nile, where fisheries target yellow eel only. The Tunisian eel fishery focuses on yellow and silver eel, the same applies to Marocco, with particular reference to the lagoon of Nador.
In the Adriatic region, eel is exploited in inland waters in Albania (Shkodra lake and Shkopte lake, an artificial basin connected with the Mat river) and in Italy. In this country, largest inland eel fisheries are from the great Alpine lakes in the northern regions, but the eel is also an important target species for professional fisheries in some volcanic lakes of Central Italy. In all those environments, eel fisheries have been sustained by restocking.
Glass eel fisheries in the Mediterranean region are small scale, if compared to the big, commercial, ship based fisheries of the Atlantic, and fishing is always carried out by handheld nets (dipnets), or by fixed fykenets of varying dimensions (with or without wings), in estuaries and low river stretches, channel mouths and lagoon openings. In Spain glass eel fisheries are present on the Mediterranean side (Ebro Delta, Albufera de Valencia, S'Albufera de Mallorca), but in this country the Atlantic regions (the Basque country and on a minor basis the Asturias) play the central role in glass eel fishing, as well as in trading and consumption. The same applies to France, where glass eel fisheries are not present on the Mediterranean coast at all, while glass eel fisheries are known to occur in Marocco, on both the Atlantic and the Mediterranean coast (Dekker, 2002b). No consistent glass eel fisheries are known to be present in Greece, apart some small scale experiences in the western areas, despite a growing interest towards its exploitation, and glass eel stage is not exploited in Turkey nor in Tunisia and Egypt, but in both countries, glass eel entry is observed (Dekker, 2002b).
In the Adriatic area, glass eel recruitment occurs in many systems in Albania but no fishery is known to exist. In Italy, recruitment to most Adriatic lagoons is extremely reduced today, and eel production in these environments is sustained by restocking. In Italy most of the glass eel yield comes from the Central and Southern Thyrrenhian area. Fishing sites are channel mouths, estuaries and lagoon openings, frequented not only by locally-based fishermen but occasionally also by fry fishermen from other regions, who reach those sites with trucks equipped with oxygenated tanks to collect mullet, sea bass, and sea bream fry, and glass eels. Local fishermen are usually single or co-operative fishermen that are equipped with boats and facilities to store the product alive. Destination of glass eels ought to be seeding for aquaculture or restocking. Despite the fact that trading for direct human consumption is forbidden in Italy, a certain amount of glass eels for consumption reaches some traditional markets in Tuscany.
The most distinctive exploitation pattern for eel in the Mediterranean is coastal lagoon fishery. Coastal lagoon management has always been based on the intercept of seasonal migrations of these species between sea and brackish water areas: ascent of juveniles to lagoons, more suitable for growth, and return of adult fish to sea for changing environmental conditions, primarily temperature, or reproduction. To exploit these periodic movements, large areas were enclosed, and permanent capture systems were consequently developed and improved. In coastal lagoons, such as the Sardinian ponds or the French or North African lagoons, artisanal fisheries are well developed, whereas management is simple and mostly based on natural fry ascent.
Referring to the Adriatic region, in Albania, the Kanavasta lagoon and the Narta lagoon, respectively 3900 ha and 2670 ha, are known to yield eel, and on a minor basis two smaller lagoons, Orikumi and Vilun. In Italy the whole North Adriatic area, and in particular the lagoon of Venice and related valli, in particular Comacchio, were strongly linked to eel production up to the 1970s, and in the Southern Adriatic the lagoons of Lesina and Varano.
Fishing equipment for eel catching in lagoons includes a variety of other instruments ranging from single fyke nets to groups of fyke nets, traps, baskets and fish hooks, depending on sites, local traditions, fishermen skill etc. Systems consisting of arrangements of nets and fykenets, constituting barriers that close the lagoon from one shore to the other, are used in some lagoons, such as the “paranze” from the lagoon of Lesina in the Southern Adriatic, Italy. Those systems, large up to 100 m in length, have been exported by Italian fishermen to other Mediterranean lagoons, in Northern Africa and in Albania.
Most of silver eel captures take place at fish barriers (Italian lavoriero, French bordigue), devices based on the principle of V-shaped traps. The structure (shape, number of chambers), size and design, building materials (from reeds to concrete and metal) have greatly evolved through the centuries and differ among countries, in relation to local traditions and degree of technology attained. The basic principle of its functioning is the same, i.e. intercept the fish when moving to reach the sea: in the case of silver eel, most captures take place in winter in coincidence with seaward migration. Fishing efficiency by these devices can be considered to attain 100 percent.
Eel yields in coastal lagoons environments depend primarily on environmental quality, even more than on recruitment. Those two features both influence management operations with reference to fishing efforts and to restocking. Thus observed yields can be extremely variable, from the 6 kg/ha observed in Comacchio in the mid '1980s, to the 300 kg/ha obtained in Monaci coastal lake in 1984 by means of restocking with small yellow eels.
Italian vallicoltura differs from coastal lagoon management practiced in other similar environments in the Mediterranean by a more active running. This includes stocking and active hydraulic management (Ciccotti et al., 2000).
3. Eel culture
Eel exploitation on a “culture” basis has a long standing tradition in the whole Mediterranean area, right in relation to coastal lagoon management. The eel became an important commercial species in Italy by the 1300s, when it was first extensively reared in the lagoon of Venice and in the whole upper Adriatic region, with the vallicoltura. The famous Comacchio valli reached a peak in prosperity in 1800 thanks to the eel and its processing industry. Extensive culture played a major role in European eel production, namely in Italy, up to the 1970s, when the whole sector was struck down by a parasitic disease, “Argulosis”, caused by the ectoparasite Argulus giordanii. This event, together with an increasing market demand, led to the first trialss towards intensive eel farming in open systems based on the on-growing in earthen or concrete ponds, on the basis of the Japanese technology already well established, and using either ground-waters or warm effluent waters. Limiting factors were seed weaning technologies and food conversion rates during the out-growing phase, together with the need of frequent grading operations. During the 1980s, advances in feed preparation technology and improvements of farming techniques (engineering and water treatment technology, disease management) enhanced the potential for successful farming, mostly in Italy but also in other southern European countries. Eel culture production shows a steady growing trend through the second half of last century (Figure 1). Up to the mid 1990s, Italy was the leading country, with 3000 t/y, about 47 percent of total European production (Ciccotti et al., 2000). The Netherlands are now the leader country. In this country as in Denmark the biggest investments have been made in the last decade, following the setting up of efficient heathed recyrculated systems, rising production from 500 tonnes (1988) to over 5000 tonnes (Ciccotti and Fontenelle, 2001). Hence, European eel farming has shifted towards higher productions, with improved intensive farming performances and reduced impacts on the environment.
4. Status of the stock in Europe and in the Mediterranean region
The general picture on the status of eel stocks and fisheries throughout Europe displays declining recruitment (Figures 2 and 3) and reduced yields (Figure 4), apparent both for capture fisheries and for scientific indices.
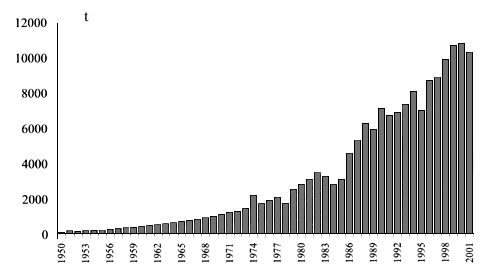
Figure 1: Cumulated eel aquaculture production in the European countries, 1950–2000 (from FAO, 2003).
The conclusion that recruitment has declined in the past decades is based on compilations of time-series data, covering varying time intervals, from 19 river catchments in 12 countries, and derived from both fishery-dependent sources (i.e. catch records) and fishery-independent surveys across much of the geographic range of the European eel. No upward trends are present in any of these European data sets: over the last two decades of all time-series, downward trends were evident, reflecting the rapid decrease after the high levels of the 1970s. In the 1980s, the trend was clearly downwards; after the '90s fairly stable low levels, recent years show a continued decrease.
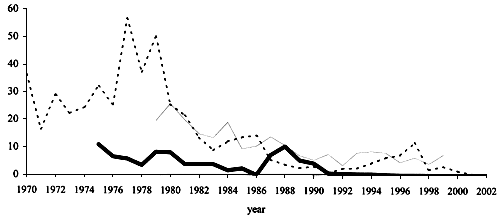
Figure 2. Recruitment trends at three sites in Europe (from ICES, 2003). - Den Oever, The Netherlands (index, dotted line); Gironde, France, NW Atlantic (CPUE, thin line); Tiber, Italy, Mediterranean (catch in tons, thick line).
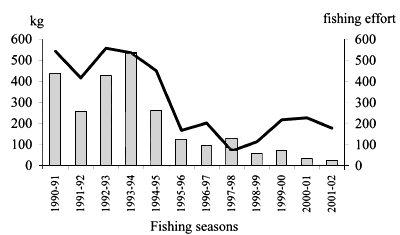
Figure 3. Recruitment monitoring at the Tiber river estuary: total catch (left axis, bars) and fishing effort (as fishing days × number of nets) (from Ciccotti, 2002).
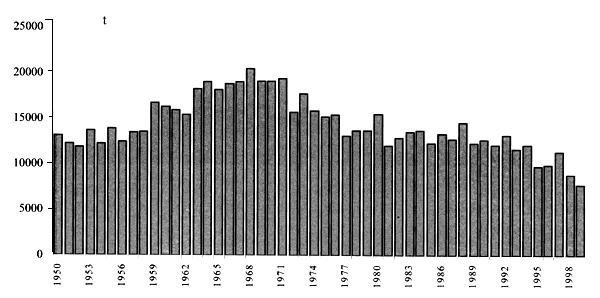
Figure 4: Total eel capture in Europe, 1950–2000 (from FAO, 2003).
As a general picture, total European glass eel catch was estimated (Moriarty, 1996) to amount to about 920 tonnes, and a total commercial yield from European eel capture fisheries of 22–-30 000 tonnes. Glass eel catches then only represents 2.7 percent of the total yield in weight, but accounts for more than 2.4 billions of recruits in number (Feunteun, 2002). An estimate of the whole glass eel catch for the Mediterranean does not seem feasible. Official landing statistics do not discriminate among stages, while national institutions seem lacking because of high rates of illegal fishing, not reporting or underreporting in most countries. Mediterranean glass eel yield is anyway for sure only a minor quota of the whole European catch. However, the apparent decline in recruitment reported for all Europe is confirmed for the Mediterranean area, where one of the monitoring sites is located on the Tiber river, Latium, central Italy (Ciccotti et al., 2000; Ciccotti, 2002).
The effects of this decline on the eel stock are not easy to document, long-term surveys in single systems being scarce and sometimes not informative, because management practices such as restocking are carried out. Contractions inyellow and silver catches in many systems from all countries, and collapse of some fisheries, have been reported (Moriarty and Dekker, 1997). Inconsistencies of national catches as quoted by official statistics have been underlined (Moriarty and Dekker, 1997; ICES, 2001), because of illegal and unreported catches, as well as lack of coverage of many areas in several countries, or variations in fishing effort. Anyway, even if catch return data do not necessarily reflect the status of the eel stock, it is felt that to some extend trends in the reported data will reflect true changes in fishing yields (ICES, 2001). Examination of reported landings (Figure 4) in Europe points to a decrease of yield in most countries during the last 20 years.The same source has been used to outline a picture of the trend of eel yields in the Mediterranean, with minor corrections. On the whole, a decreasing trend can be evidenced for global yields, in particular for Mediterranean marine production (Figure 5) that can be considered to coincide with coastal lagoons yields because in the Mediterranean no real marine fisheries exist. In inland waters (Figure 6) the decrease is less evident, but it must be considered that in some countries inland stocks can be sustained by restockings, or data be mixed with aquaculture, or even that fishing effort statistical records have begun only recently.
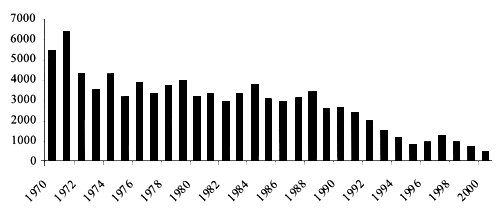
Figure 5. Eel capture from coastal areas in the Mediterranean area, 1970–2000 (from FAO, 2003).
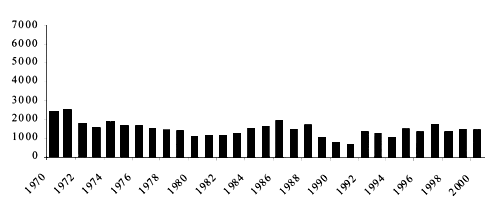
Figure 6. Eel capture from inland waters in the Mediterranean area, 1970–2000 (from FAO, 2003).
If individual countries are considered, some show market decreasing trends. For example, in Italy in coastal lagoon production a decreasing trend is evident, which took place during the '80s, with yields decreasing from an average of 1.500 tonnes in the '70s to about 500 tonnes in the '90s to < 300 in 2000 (Ciccotti et al., 2000). The main limiting factor in eel production in lagoons today, apart from the habitat changes related to coastal waters eutrophication and pollution, is seed availability for stocking. National glass eel catches are used for lagoon restocking, and the fall in recruitment and the consequent decline of glass eel fisheries cannot be compensated for by imported seed, also because of rises in prices. This, together with the fact that the eel life cycle in lagoons is long (average seven years) and hence non-competitive with the aquaculture product, means that other species are given preference when local management strategies are formulated.
In inland waters, eel populations are believed to be reduced owing to the presence of numerous dams, most of which have inadequate fishways or none at all and are therefore impassable. Recruitment to most lakes has been considerably reduced by the construction of dams along the effluent rivers. Considering that nowhere, for eel fisheries, standardised survey campaigns as compared to other marine shared species can be carried out, the most effective information can be derived from single systems followed for a consistent period of time, but this has rarely been achieved on a sound basis within the Mediterranean.
A case for which data are available refers to the North Adriatic area, i.e. the already mentioned Valli di Comacchio, renown in relation to its eel production, for which official data of fish production have been available since 1781. The trend in the course of those two centuries has always been characterised by fluctuations ranging from 6 to > 30kg/ha, attributable to such environmental problems as hypersalinity and freezing of the valli. The average annual yield of eel per hectare was 14.3kg, about 78 percent of the total fish production. Higher yields were obtained after 1964, coinciding with restocking and seeding practices whereas, from the late 1970s, production has been considerably lower (5–7kg/ha), attributed to falling recruitment in the Comacchio lagoons. From 1990, owing to the above-mentioned environmental problems and to internal problems of the Consorzio Valli di Comacchio, eel production has reached its historical minimum, falling to less than 5kg/ha, stockings having been completely abandoned. Catches consist now only of large eel, the older individuals still inside the lagoon (Ciccotti, 1997).
The hypotheses brought forward as possible explanations of the causes of the widespread decline in recruitment are diverse, from antrophogenic to natural. Among the latter, the main hypothesis is a dependence of recruitment decline by a change in the oceanic circulation. The parallel decline of the recruitment of the American eel ( Anguilla rostrata) in some of its distribution area are thought to support this model. Spreading in Europe of the swimbladder parasite Anguillicola crassus, that causes swimbladder dysfunction and hence may influence the migration of mature eels, have also been called in, as well as predation by the greatly increased European populations of cormorants or other predators. With regards to anthropogenic factors, the impact of fisheries on the population cannot be adequately described. Habitat loss, wetland reclamation in coastal and estuarine environments has been considered to be considerable throughout Europe, even if the process has occurred gradually, mainly during the second half of the last century (ICES, 2001; Feunteun, 2002), and a large part of the European inland water habitat has been made inaccessible to eels by hydroelectric dams or other obstructions to upstream migration. Finally, the spread of environmental contaminants has been considered to possibly contribute to the recruitment failure. Eels accumulate organochlorines and other fat soluble substances readily, and this may impair the migration and affect the survival of the larvae. A concern expressed more recently is the spread of endocrine disruptors (ICES, 2001).
5. Interactions with aquaculture, globalisation of the market
From the late '90s, European eel aquaculture has become involved at a global scale, due to growing interactions with Asian eel aquaculture, whose production amounts to more than 180000 tonnes (Table 1) and to a globalization of processing industries and of markets. A large and rapid increase in Chinese eel culture led, as a primary consequence, to the fact that the growing Asian productive capacity increased the demand for glass eel from anywhere, and from Europe in particular, because of the current shortage of Japanese glass eels. Up to the mid 1990s, notwithstanding the decline in recruitment, the dependence of European aquaculture on glass eel or elvers from the wild was not really considered a major problem. Indeed, the demand of glass eel for European aquaculture purposes was estimated to amount to about 40 t/year. When the Far East aquaculture asked for more Anguilla anguilla, this upset the EU glass eel fishery scenario and markets. At a first stage (1997–1998), there was a sharp increase in seedlings prices within EU (more than 300 €/kg paid to fishermen in France). This new trend seriously alarmed producers, while a consequent increase in fishing effort on glass eels was observed by commercial fishers, the demand for glass eels being also an incentive for poaching. Then, a double impact on the wild stock should be expected: (i) by an increased fishing effort on glass eels and (ii) a reduction of available stocks for enhancement. Restocking in open waters to sustain wild eel fisheries throughout Europe is often carried out by national fishery authorities, in particular in northern countries (Sweden, Ireland, Denmark as well as France). Higher price for glass eel reduced stocking practices everywhere, thus affecting both local fisheries and local wild eel stocks. As a secondary consequence, Asian aquaculture has known an outburst of production that brought about repercussions on the European market, because exceeding supply led Europe to turn prices down.
Table 1. The world-wide production (tonnes per year) of anguillid eels in fisheries and aquaculture, averaged over the 1990s (from Dekker, 2002a, based on FAO statistics).
| Area | species | Fishing yield | Aquaculture production |
| Europe and N. Africa | A. anguilla | 15 262 | 18 101 |
| America | A. rostrata | 1 480 | 100 |
| Asia, east | A. japonica | 1 300 | 187 875 |
| Asia, southeast | Anguilla spp. | 8 385 | 1 579 |
| Australia and New Zealand | A. dieffenbachii and A. australis | 2 241 | 100 |
| Total | > 28 668 | > 207755 |
6. International framework and ongoing actions on eel management
The unique status of the eel was defined in 1976, when a joint ICES/EIFAC Working Party on Eels was established, that has met in alternate years since then. Within the WP, the above mentioned monitoring system for eel recruitment was set up, that allowed to document and follow the decline of supply following the abundance of the 1970's (Moriarty, 1990; 1996). The general concern by fishermen, fish culturists and scientists alike on the decline in recruitment and fishery yields of the eel led to the establishment of a EC Concerted Action AIR A94–1939, to pursue a project entitled Enhancement of the European eel fishery and conservation of the species, whose final Report (Moriarty and Dekker, 1997) contained an account of the eel fishery in Europe, a review of scientific data of significance relative to local stocks and maked recommendations for future management.
The EC in 1997 requested ICES to provide information about the status of the eel, in order to ensure a sustainable development of the eel fisheries within the European Union, and in 1998, having acknowledged that the eel stock is outside safe biological limits, has requested to provide escapement targets and other biological reference points. Since then the ICES/EIFAC Working Group on Eels (1999; 2001; 2002, 2003) has been working on the defining of reference points for European eel management use.
With specific reference to the Mediterranean, in 2002 the STECF Subgroup on Mediterranean included the eel within the species for which a scientific evaluation and critical review of the background information were performed, in consideration of the fact that this resource is shared among the majority of Mediterranean countries. Eel in fact is included among the shared stocks in the Community Action Plan for the conservation and sustainable exploitation of fisheries resources in the Mediterranean Sea under the Common Fisheries Policy(COM (2002) 535).
In conclusion, concern about the conservation of this species has been growing in the course of the last few years and the need for conservation and management measures has been clearly identified by scientists, managers, and even by the public opinion. The International Council for the Exploration of the Sea (ICES) in October 2002 pointed out the urgent need for a recovery plan for European eel, that should include measures to reduce exploitation of all life stages and restore habitats.
At present few conservation measures are being taken at national level in some Community and non-Community countries. However, given the fundamental trans-boundary migration pattern of European eel, national measures are not sufficient to ensure adequate conservation of this species in Europe. In May 2003 a Regional Workshop on Action Plans for Eels was organised by the European Commission, to provide the scientific and technical background information for the Development of a Community Action Plan for the management of European Eel, that was published October 1st, 2003 (COM (2003) 573).
This document, on the basis of the current evaluation of the eel situation, considers the legal background for management, ranging from the Community framework for action to the UNCLOS specific article (67) for catadromous species, with reference to Coastal States responsibilities and obligations to ensure ingress and egress of fish. The need to ensure that rivers do not become barriers (through pollution or public works) for the movement of the species through its natural habitat brings in also the possible role of Water Framework Directive (EC) No 2000/60. One of the key elements of the Directive is the introduction of River Basin Management on a Europe-wide scale including international co-ordination in transboundary river basins. In this respect, the WFD could joint in the objectives of the eel action programme, by using the management system and the river basin authorities when setting targets and implementing eel action programmes.
In considering the possible management measures and the rebuilding plan targets, the overall approach of the document is centred on the ICES advice. The strong need for urgent management action is acknowledged, also supported by the precautionary principle, which suggests that high-risk situations need urgent protective measures. In many areas, the quickest and most effective measure to increase the survival of eel will prove to be a reduction in fishing, whereas environmental improvements may take some years to show results. A number of actions are identified that are intended to develop a comprehensive basis for rebuilding eel stocks, based on locally-appropriate actions and targets. This rebuilding and management approach requires substantial acquisition of new scientific data before it can be fully implemented. Therefore, the Commission will urgently seek to identify a wide range of precautionary measures for rapid implementation, while the rebuilding plan is being developed.
In conservation terms, the main objective of eel management actions is identified in allowing an adequate escapement of silver eel. Possible local targets for eel conservation and management are reviewed, with regards to recruitment/settlement targets, stocking targets and particularly escapement targets. Local management actions that could contribute to the latter could include: i) managed escapement of silver eels from inland waters to the sea; ii) prohibition of certain fishing gears particularly likely to catch silver eel; iii) construction of eel passes in dams and hydroelectric plants. The need for a data collection system is pointed out, in connection to the necessity to measure outcomes of the various management instruments. The international dimension of actions and the necessity to extend to the eel the existing regional agreements is also considered, GFCM among others being indicated as an appropriate forum for such discussion.
7. Conclusions
The conservation and management of eels is a very wide-ranging issue, as it depends upon both commercial exploitation and preservation of its natural habitats. Both environmental considerations and fisheries management issues need to be taken into account. The possible effect of trade on the conservation of this species adds an additional dimension to the problem. Without doubt, a number of uncertainties, when dealing with eel, arise not only on its biology, but also on the feasibility, as well as on the chances of success of possible management strategies. A process has begun, that shall bring about a management plan, as well as emergency actions in the immediate future, to ensure eel conservation within a framework of sustainability of the related socio-economic activities.
From the information given in the present paper, some points can be drawn for the European eel in the Mediterranean. The continent-wide decline in recruitment is confirmed, and there are strong evidences of contracting stocks, emerging from both official landing statistics and from long-term observations in selected systems. The establishment of a long-term monitoring program for glass eel recruitment at the regional level seems then opportune, as well as a regional survey data monitoring program for eel fishery, that could provide elements for the identification of key index systems for eel stock assessment.
Some distinctive features of exploitation, with regards to Mediterranean coastal lagoons, and in particular to the Adriatic region, provide a key to the setting up of a relevant geographical management unit. In these environments traditional management practices were finalised to sustain local eel stocks and environmental characteristics are such that very high productions can be attained if recruitment is consistent. On the other hand, silver eel fishing at the fish barrier, typical of the Italian tradition in the North Adriatic and spread also in other Mediterranean areas, can be considered to control completely the escapement. An opportunity to resume the coastal lagoon management model seems then opportune also as a potential instrument for eel conservation: the sustaining and implementing of these traditional “enhanced fisheries”, based on the rational use of glass eel fisheries and contemplating local escapement quotas, could give a contribution to overall escapement at the Regional scale.
8. References
Ciccotti, E. (1997) Italy. In: Moriarty C. & W. Dekker (eds.), Management of European eel fisheries. Fisheries Bulletin (Dublin), 15: 91–100.
Ciccotti, E, Busilacchi, S. and Cataudella, S. (2000) Eel, Anguilla anguilla (L.), in Italy: recruitment, fisheries and aquaculture. Dana, 12: 7–15
Ciccotti, E. & Fontenelle, G. (2001) A review of eel, Anguilla anguilla, aquaculture in Europe: Perspectives for its sustainability. J. Taiwan Fish. Res., 9(1&2): 27–43.
Ciccotti, E. (2002) Monitoring of glass eel recruitment in Italy. In: Monitoring of glass eel recruitment, W. Dekker ed., Netherlands Institute of Fisheries research, Ijmuiden, The Netherlands, report C007/02 WD: 227–236.
Commission of the European Communities (2002) Communication from the Commission to the Council and the European Parliament laying down a Community Action Plan for the conservation and sustainable exploitation of fisheries resources in the Mediterranean sea under the Common Fisheries Policy. COM (2002) 535 Final, 37 pp.
Commission of the European Communities (2003) Communication from the Commission to the Council and the European Parliament. Development of a Community Action Plan for the Management of European Eel. COM (2003) 573 Final, 15 pp.
Dekker, W. (2002a) Status of the European eel stock and fisheries. Proceedings of the International Symposium 'Advances in Eel Biology', Tokyo (Japan) 28–30 September 2001.
Dekker, W. (ed.) (2002b) Monitoring of glass eel recruitment. Report C007/02-WD, Netherlands Institute of Fisheries Research, IJmuiden, 256 pp.
Desaunay, Y. & D. Guerault (1997) Seasonal and long-term changes in biometrics of eel larvae: a possible relationship between recruitment variation and North Atlantic ecosystems productivity. J. Fish Biol. 51, 317–339.
Feunteun E. (2002) Management and restoration of European eel population ( Anguilla anguilla): An impossible bargain. Ecological Engineering, 18: 575–591.
Fontenelle, G., Feunteun, E,. & C. Briand (1997) France. In: Moriarty C. & W. Dekker eds.), Management of European eel fisheries. Fisheries Bulletin (Dublin), 15: 77–90.
ICES. (2001) Report of the ICES/EIFAC Working Group on Eels. ICES C.M. 2002/ACFM:03.
ICES. (2002) Report of the ICES/EIFAC Working Group on Eels. ICES C.M. 2003/ACFM:06.
ICES. (2003) Report of the ICES/EIFAC Working Group on Eels. ICES C.M. 2004/ACFM:09.
Lecomte-Finiger, R. (1992) Growth history and age at recruitment of European glass eels (Anguilla anguill a) as revealed by otolith microstructure. Mar. Biol. 114: 2054–2210.
McCleave, J.D. & G.S. Wippelhauser (1987) Behavioral aspects of selective tidal stream transport in juvenile American eels. American Fisheries Society Symposium Series 1:138–150.
Moriarty, C. (1990) European catches of elver of 1928–1988. Internationale Revue der gesamten Hydrobiologie 75(6):701–706.
Moriarty, C. (1996) The European eel fishery in 1993 and 1994. First report of a working group funded by the Europea Union Concerted Action AIR A94–1939. Fisheries Bulletin (Dublin), 14, 52 pp.
Moriarty, C. & W. Dekker (eds.) (1997) Management of European eel fisheries. Fisheries Bulletin (Dublin), 15: 77–90.
Schmidt, J. (1922) The breeding places of the eel. Philosophical Transactions Royal Society 211: 179–208
Wirth, T. & L. Bernatchez (2000) Genetic evidence against panmixia in the European eel. Nature, 409: 1037–1040.
Giuseppe Prioli*
* M.A.R.E. Soc.Coop. A.r.l. Via E. Toti, 2-Cattolica (RN), Italy. Email: [email protected]
Abstract
General information (catch statistics, species cultured, culture methods) on shellfish fisheries and culture in the Mediterranean is given with particular reference to the Adriatic Sea. Details on shellfish market and legislative framework are also reported. Interactions between shellfish culture and both bivalve molluscs harvesting and capture fisheries are identified in different sectors: political-management, environmental, economical and social.
1. Introduction
Bivalve molluscs represent an important resource within Mediterranean production setting. In 2000, bivalve production amounted to 284.000 tonnes, corresponding to 15 percent of total fishery and aquaculture yield which in the same year reached 1.850.000 tonnes. Out of the total production, 1.490.000 tonnes came from capture fishery and 360.000 tonnes from aquaculture (190.000 tonnes represented by molluscs, the rest by fish and crustaceans). Within capture fisheries production, bivalve mollusc catches contributed with 93.000 tonnes, covering 6 percent of the total (Figure 1).
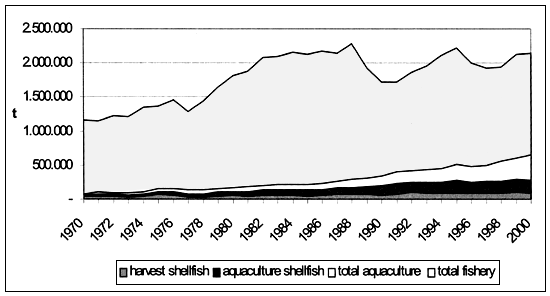
Figure 1. Total fishery and aquaculture production in Mediterranean area (FAO-Fishstat).
2. Shellfish fisheries: general information, reported catch statistics in the Mediterranean, with particular reference to the Adriatic Sea
Bivalve molluscs fisheries production amounted to 93.000 tonnes in 2000, out of which 43.000 tonnes of mussels (Mytilus galloprovincialis), 47.000 tonnes of clams, mainly stripped venus (Chamelea gallina), and 350 tonnes of oysters (Pacific cupped oyster and European flat oyster). Compared to 1970 figures, the production has increased by 120 percent (FAO-Fishstat) with variable fluctuations (Figure 2).
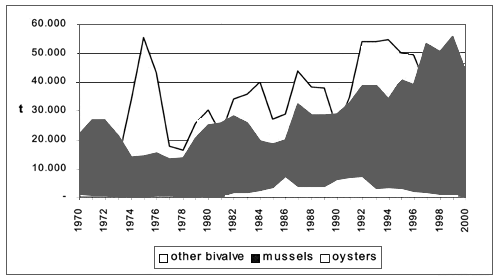
Figure 2. Bivalve molluscs fishery production in Mediterranean area by species FAO-Fishstat).
Adriatic contribution to total Mediterranean production is 59.000 tonnes, or 64 percent, out of which 27.000 tonnes are mussels, 32.000 tonnes clams (stripped venus) and 13 tonnes are oysters (European flat oyster) (Figure 3). It is difficult to quantify Japanese littleneck clams caught in the wild.
Catches of other bivalve molluscs species such as scallops (Pecten jacobaeus) and queen scallops (Aequipecten opercularis and Proteopecten glaber), in the past quite abundant in the northern Adriatic, are not reported in FAO sources consulted (Fishstat). While today scallops and queen scallops production appears to be quite marginal, in 1991 they were estimated to 2.500 tonnes and 100 tonnes respectively.
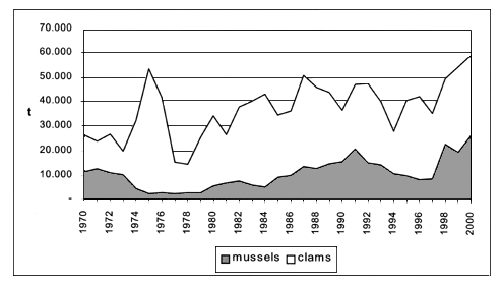
Figure 3. Molluscs fishery production in Adriatic area (FAO-Fishstat).
According to the figures reported so far, bivalve molluscs fishery seems to be quite significant in the Adriatic area, especially in the north west basin where best edaphic and trophic conditions for propagation of these species are met. Major rivers flowing into this part of the Adriatic Sea, together with extended lagoons along the coast and muddy and sandy bottoms characterized by minor slopes, are the main factors that make this area rich in biocenosis with important bivalve molluscs species. The potential of these areas is still not fully explored. It is worthwhile to cite the presence of extended Anadara inaequivalvis beds, an allochthonous species involuntarily introduced in Adriatic towards the end of the '60s, that now proliferate between 1 and 10 miles from the coast, and has yet not found a valuable market utilization.
Mussels are usually harvested (collected) by hand and less frequently, where rich mussels beds on lagoon bottoms are present, through bottom trawl fishery. The most exploited areas are the ones close to the rocky coastal parts, among which Conero promontory in the Marche region stands out. Equally important are the quantities collected on methane-producing platforms during cleaning and maintenance activities.
Clams are usually caught by vessels equipped with a hydraulic dredge. In 2000, out of 728 dredge boats registered in Italy, 685 were operative along the Adriatic coast. This fishery system operates on sandy bottoms within 1 mile from the coast. Normative applied to this capture system contains the following indications: gears dimensions, catches limit, vessels dimensions, engine power, clam size. Fishing areas are managed by compartmental management consortiums to which all fishermen are affiliated. Some of these vessels are used or other bivalve molluscs fisheries as well, such as smooth callista (Callista chione) and razor-clams (Solen spp. e Ensis spp.).
Western coast clams production (stripped venus) is only reported for Albania referring to the period 1987–1996. The trend shows a progressive decrease from the initial amount of 700 t (FAO Fishstat). Although reduced clam beds are present along the northern coast of this country, collection of any kind is not allowed.
Capture fisheries of Pectinidae (scallops and queen scallops) has nowadays become marginal. In the past Pectinidae species were collected in the northern Adriatic with bottom trawl gears called “rapidi”, vessels equipped with fixed dredges originally constructed for flat fish fisheries (Mattei and Pellizzato, 1997).
Although farming activities account for the largest part of clam production, natural harvesting of Japanese littleneck clams (Tapes semidecussatus) can be practiced according to gear regulations in specific areas, identified by hygienic and sanitary parameters
3. Shellfish culture: general information, species cultured, culture methods used, seed used in grow out, pathologies, environmental impact.
Total cultured shellfish production in the Mediterranean area and in the Black sea amounted to 196.000 tonnes in 2001 (FAO Fishstat), showing a remarkable increase compared to the 16.000 tonnes estimated in 1970 (Figure 4). Three species are dominant in this production: mussels (Mytilus galloprovincialis) with 131.000 tonnes, Japanese littleneck clams (Tapes semidecussatus) with 55.000 tonnes, and Pacific oysters (Crassostrea gigas) with 9.500 tonnes.
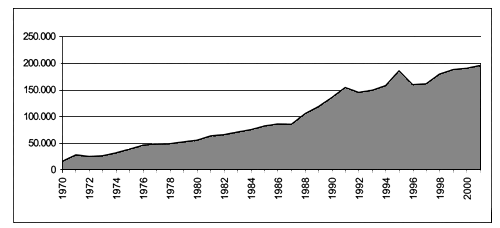
Figure 4. Bivalve molluscs culture production in Mediterranean and Black sea (FAO-Fishstat).
As far as the Adriatic area is concerned, shellfish culture has developed along both the eastern and western coasts. However, major productive sites are concentrated in the western areas, from Trieste to the Gargano promontory, where in 2000 the production has been estimated to 65.000 tonnes of mussels and 53.000 tonnes of clams, roughly equivalent to 50 percent and 96 percent of total Mediterranean production respectively (Prioli, 2001). Production contribution from other countries such as Slovenia, Croatia, Montenegro and Albania must be added to these quantities: catch of mussels and oysters (Ostrea edulis) reached 3.400 tonnes in 2001.
In the open sea, both mussels and oysters are farmed using the long-line systems, which sometimes differ among regions, as the bi-tri ventia in Friuli V. G. In lagoon basins of both coasts fixed systems prevail instead (Prioli 2001).
A total number of shellfish farms in the Adriatic is estimated to be 220, out of which 177 are located along the western, and 43 along the eastern coast. Out of the total number, 171 farms produce mussels and a minor quantity of oysters, while the other 49 produce Japanese littleneck clams (Table 1).
On the eastern coast Croatia is the country with the highest number of farms, mainly distributed between Istria, Mali Ston bay and the Krka river estuary. Three farms are situated close to Piran in Slovenia, while in Montenegro mussels culture farms can be found in Boka Kotorska bay. In Albania there is a slowdown in mussels productivity: in Butrinti lagoon, where the major mussel culture was practiced, there are 80 farms that were in use until the '90s, and much concern is now expressed by the government to make them operative again.
In Italy most farms are situated in the northern Adriatic, but a strong development is being registered in the southern regions, especially Marche and Abruzzo. Veneto and Emilia Romagna represent the most significant productive pole of the Adriatic area, exclusive as far as clams production is concerned and nationally important for mussels production. This is well demonstrated by the number of personnel employed in this sector (Table 1).
Environmental conditions are a critical factor in shellfish culture. Aquaculture farms are situated in areas where the requirements for a high trophic level, good water quality and maintenance of farm equipment have to be met.
Nutrients supply, as well as the abundance of phytoplankton and organic particles gradually decrease from the Po delta southward. On the eastern coast nutrients are more available around estuarine and coastal lagoons. Trophic conditions are a limiting factor in shellfish culture, and this gives rise to the differentiation between Adriatic and Tyrrenic coast, as in the latter only few sites close to the shore are suitable for shellfish farming. Water quality is another important factor. Although it is not a strictly restrictive factor because contaminated molluscs can receive purification treatment after the harvest, it does weigh upon production cost. This is the reason why during the past 20 years offshore shellfish culture has much increased in many Adriatic regions. The development of new technologies, protected from mechanical stress induced by strong waves in the open sea, has extended the frontiers of shellfish culture allowing the exploitation of new areas, as well as the overcoming of restrictions connected to the oscillating environmental conditions in lagoons, both trophic and physio-chemical.
The main limit to shellfish culture development in the eastern Adriatic countries at present might be imputable to insufficient sanitary control measures and to inadequate implementation of European Community normative. This practice is precluding export to the main consumer countries and limiting productive potential. It is reasonable to foresee an increase of production in these countries once these deficiencies are overcome.
Table 1. Bivalve molluscs culture farms in Adriatic.
| Regions | Culture farms | Staff | ||
| Total | Mussels and oysters | Clams | ||
| Friuli-Venezia Giulia | 60 | 56 | 4 | 55 |
| Veneto | 56 | 29 | 27 | 1800 |
| Emilia-Romagna | 42 | 24 | 18 | 1200 |
| Marches | 7 | 7 | 0 | 32 |
| Abruzzo | 4 | 4 | 0 | 10 |
| Molise | 2 | 2 | 0 | 8 |
| Apulia | 6 | 6 | 0 | 220 |
| Italy (total) | 177 | 128 | 49 | 3.325 |
| Slovenia | 3 | 3 | na | |
| Croatia | 28 | 28 | na | |
| Montenegro | 12 | 12 | na | |
| Albania | na (80) | na (80) | na | |
| Total in Adriatic | 220 | 171 | 49 | 3.325 |
Rearing of some mollusc species such as mussel, oyster and Japanese littleneck clam, can start either from juveniles collected in the wild or from larvae captured through specific collectors. For Japanese littleneck clam and oyster, it is also possible to use spat obtained through breeding from specialised hatcheries mostly situated abroad: France, Britain, United States, and partly in Italy.
The pathologies that affect bivalve molluscs in Italy are the ones commonly known in Europe. However, serious epidemic cases in either reared or wild populations have never been recorded in Italy so far. Two sole infective cases of Bonamia ostreae on Ostrea edulis have been registered in the waters offshore Chioggia and close to Apulia coast. Other diseases so far reported are: microcytosis affecting Crassostrea gigas and Ostrea edulis; Perkinsosi, affecting Crassostrea gigas, Tapes semidecussatus, Tapes decussatus; MSX disease affecting Crassostrea gigas (Cerchia, 2003, personal communication).
The environmental impact of shellfish culture is related to the type of equipment used, to the culture method and, most importantly, to the farming site. As far as mussel farming with long line method is concerned, recent studies on culture sites offshore carried out in 2001 by ISMAR-CNR in Ancona, didn't show the presence of significant quantity of organic waste on the bottom, probably thanks to strong sea currents. Nevertheless, the same currents might damage equipment items such as buoys or ropes or cause the loss of the mesh tubing that remain floating on the bottoms after the loss of mussels.
More evident effects on the environment can be observed when farms are situated in closed areas, such as the Gulf of Trieste, or even more in lagoons. Another undesirable effect is the involuntary release of larvae in the environment related to oyster farming with suspended methods. The case of the larvaeparous species (Ostrea edulis), and its following settlement in the wild appears to be especially critical.
Further impact can be generated by the transport of live shellfish, both juveniles and adults. Most oysters or Philippines clams spat comes from non-Mediterranean countries, and this can determine the introduction of pathogens and undesirable phytoplankton, algal and bivalve species. In case of adult product, especially mussels in mesh tubes, the associated flora and fauna can also be transferred.
A significant impact was generated by voluntary introduction of allochthonous species such as Crassostrea gigas (or C. angulata) and Tapes semidecussatus. Both species have well adapted in most of the northern Adriatic lagoons and also along the coast. The spread of Japanese littleneck clam has been so considerable and fast as to influence the economy and the environment of large areas with remarkable social effects. Crassostrea gigas on the other hand has been less invasive although today it is present along the entire coast.
The presence of Japanese littleneck clam has led to a different lagoon environmental management that favours allocation of areas to aquaculture farmers, while harvesting in the wild decreases. In this new setting, activities aimed at improving sustainable exploitation of resources such as hydraulic interventions, bottom cleaning and seeding, are carried out.
4. Shellfish market: fresh and processed products, Mediterranean market capacity
In 2001, reported global amount of imported bivalve molluscs products was 444.343 t, 135.723 tonnes of which, or 31 percent, was of Mediterranean origin. Mediterranean countries contributed with 25 percent of fresh and frozen products and with 53 percent of processed products to the total imports (Table 2) (FAO-Fishstat).
Table 2. Global and Mediterranean imports of processed and fresh/frozen bivalve molluscs, quantity expressed in tonnes (FAO-Fishstat; 2001).
| Product | Commodity | All countries | Mediterranean countries | % |
| Fresh/frozen | Import | 354.162 | 87.937 | 25% |
| Processed | Import | 90.181 | 47.786 | 53% |
| Totale | Import | 444.343 | 135.723 | 31% |
As shown in Figure 5 imports of fresh or frozen shellfish have gradually raised on a global scale, increasing from 135.567 tonnes in 1976 to 354.162 tonnes in 2001 (equivalent to 160 percent growth), whereas, during to the same period, the increase of 52 percent in the Mediterranean countries is much less significant.
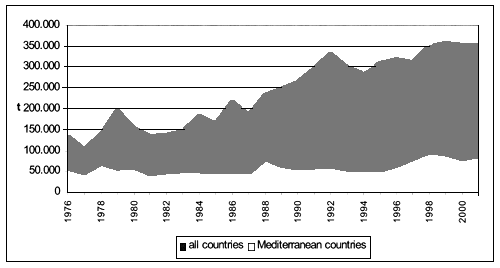
Figure 5 - Global and Mediterranean imports of fresh/frozen bivalve molluscs (FAO-Fishstat; 2001).
Different considerations can be made analysing the import trend of processed products. As it can be observed in Figure 6, the import increase registered for Mediterranean countries, from 8.037 tonnes to 47.786 tonnes, is equivalent to 495 percent, while the global trend reaches 208 percent increase, going up from 29.292 tonnes in 1976 to 90.181 tonnes in 2001.
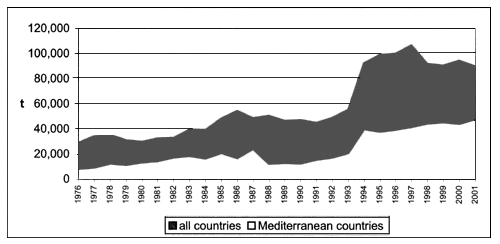
Figure 6 - Global and Mediterranean imports of processed bivalve molluscs (FAO-Fishstat; 2001).
Global quantity of fresh and processed products exported reached 483.141 tonnes in 2001, of which 15 percent, or 74.046 tonnes, were of Mediterranean origin (Table 3).
Table 3. Global and Mediterranean exports of processed and fresh/frozen bivalve molluscs, quantity expressed in tonnes (FAO-Fishstat; 2001).
| Product | Commodity | All countries | Mediterranean countries | % |
| Fresh/frozen | Export | 386.184 | 59.449 | 15% |
| Processed | Export | 96.957 | 14.597 | 15% |
| Total | Export | 483.141 | 74.046 | 15% |
The increase of global exported fresh/frozen bivalve molluscs for the period 1976–2001 is quite significant as well, increasing from 90.447 tonnes to 386.184 tonnes, which is equivalent to 327 percent growth (Figure 7). Export of the Mediterranean countries increased by 335 percent, from 13.673 tonnes in 1976 to 59.449 tonnes in 2001
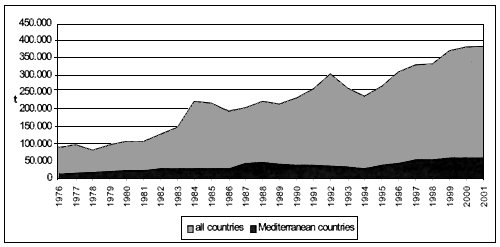
Figure 7. Global and Mediterranean exports of fresh/frozen bivalve molluscs (FAO-Fishstat; 2001).
Processed bivalve molluscs exports in Mediterranean countries rise from 5.706 t to 14.597 tonnes (Figure 8) showing an increase of 156 percent, lower than global growth, equivalent to 207 percent (from 31.597 tonnes in 1976 to 96.957 tonnes in 2001).
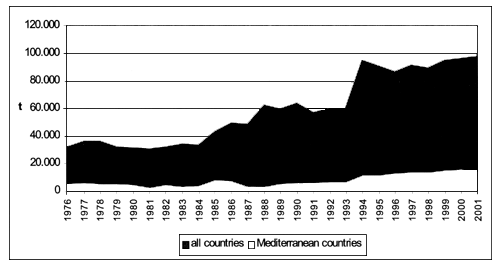
Figure 8. Global and Mediterranean exports of processed bivalve molluscs (FAO-Fishstat; 2001).
In the period 1976 - 2001 Mediterranean countries show a positive trend of fresh and frozen bivalve mollusc imports, with quantities always significantly higher than those referred to export. Furthermore, Mediterranean import trend appears significantly different from the one observed in non-Mediterranean countries (Figure 9). In 2001, reported fresh and frozen bivalve molluscs export was 59.449 tonnes, while import reached 87.937 tonnes, showing a gap of 28.488 tonnes (Table 4 and Table 6).
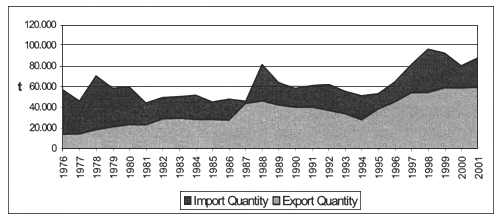
Figure 9. Import and export of fresh/frozen bivalve molluscs in Mediterranean area (FAO-Fishstat).
As shown in Table 4, in 2001 80 percent of imported fresh and frozen shellfish was represented by mussels, 11 percent by oysters and 9 percent by scallops.
Table 4. Mediterranean imports of fresh/frozen bivalve molluscs by species, quantity expressed in tonnes (FAO-Fishstat; 2001).
| Commodity | Trade Flow | 2001 | % |
| European flat oyster, shucked or not, fresh or chilled | Import Quantity | 4.598 | 5% |
| Mussels, fresh or chilled, nei | Import Quantity | 70.648 | 80% |
| Oysters, fresh or chilled, nei | Import Quantity | 5.165 | 6% |
| Scallops, shucked, fresh or chilled, nei | Import Quantity | 7.526 | 9% |
| Total | 87.937 | 100% |
The countries that have largely contributed to the import of these products are three: France, 60%; Italy, 28%; and Spain, 11% (Table 5).
Table 5. Importer Mediterranean countries of fresh/frozen bivalve molluscs, quantity expressed in tonnes (FAO-Fishstat; 2001).
| Country | Trade Flow | 2001 | % |
| Albania | Import Quantity | 25 | 0% |
| Algeria | Import Quantity | 8 | 0% |
| Croatia | Import Quantity | 7 | 0% |
| Cyprus | Import Quantity | 7 | 0% |
| France | Import Quantity | 52.910 | 60% |
| Greece | Import Quantity | 438 | 0% |
| Israel | Import Quantity | 36 | 0% |
| Italy | Import Quantity | 24.744 | 28% |
| Jordan | Import Quantity | 1 | 0% |
| Lebanon | Import Quantity | 67 | 0% |
| Malta | Import Quantity | 23 | 0% |
| Morocco | Import Quantity | 20 | 0% |
| Slovenia | Import Quantity | 28 | 0% |
| Spain | Import Quantity | 9.598 | 11% |
| Tunisia | Import Quantity | 25 | 0% |
| Total | 87.937 | 100% |
Referring to total fresh/frozen shellfish exports, mussels contribute with 79 percent, scallops with 11 percent and oysters with 10 percent (Table 6).
Table 6. Mediterranean exports of fresh/frozen bivalve molluscs by species, quantity expressed in tonnes (FAO-Fishstat; 2001).
| Commodity | Trade Flow | 2001 | % |
| European flat oyster, shucked or not, fresh or chilled | Export Quantity | 5.831 | 10% |
| Mussels, fresh or chilled, nei | Export Quantity | 46.877 | 79% |
| Oysters, fresh or chilled, nei | Export Quantity | 155 | 0% |
| Scallops, shucked, fresh or chilled, nei | Export Quantity | 6.586 | 11% |
| Total | 59.449 | 100% |
Among the Mediterranean countries listed in Table 7, Spain is the main exporter accounting for 35 percent of total product, followed by France and Greece 23 percent, and Italy 18 percent. The values referred to Spain and France are inclusive of both Mediterranean and Atlantic products origin.
Table 7. Exporter Mediterranean countries of fresh/frozen bivalve molluscs, quantity expressed in tonnes (FAO-Fishstat; 2001).
| Country | Trade Flow | 2001 | % |
| Albania | Export Quantity | 6 | 0% |
| Croatia | Export Quantity | 1 | 0% |
| Cyprus | Export Quantity | 3 | 0% |
| Egypt | Export Quantity | 35 | 0% |
| France | Export Quantity | 13.815 | 23% |
| Greece | Export Quantity | 13.526 | 23% |
| Italy | Export Quantity | 10.777 | 18% |
| Morocco | Export Quantity | 75 | 0% |
| Spain | Export Quantity | 21.047 | 35% |
| Tunisia | Export Quantity | 10 | 0% |
| Turkey | Export Quantity | 152 | 0% |
| Yugoslavia, Fed. Rep. of | Export Quantity | 2 | 0% |
| Total | 59.449 | 100% |
Processed bivalve molluscs production registered a considerable increase in Mediterranean countries in the period 1976–2001, increasing from 17.627 tonnes to 55.117 tonnes, which is equivalent to 280 percent growth (Figure 10).
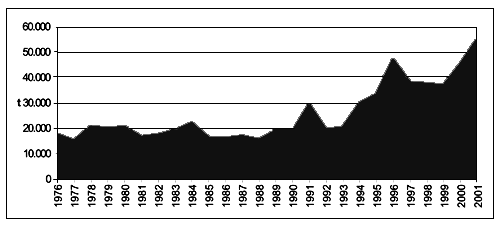
Figure 10. Processed bivalve molluscs production in Mediterranean area (FAO-Fishstat).
In 2001, 71 percent of total processed bivalve mollusc production is represented by processed mussels, 17 percent by frozen shellfish and 11 percent by clams (Table 8). 95 percent of the production, equivalent to 83.000 tonnes, is concentrated in Spain and the remaining quantity is distributed between Italy (4%) and France (1%) (Table 9).
Table 8. Mediterranean production of processed bivalve molluscs by species (FAO-Fishstat; 2001).
| Commodity | Trade Flow | 2001 | % |
| Bivalves, frozen | Production | 9.508 | 17% |
| Clam meat, canned | Production | 5.987 | 11% |
| Mussel meat, canned | Production | 38.964 | 71% |
| Scallop meat, canned | Production | 658 | 1% |
| Total | 55.117 | 100% |
Table 9. Processed bivalve molluscs production in Mediterranean countries (FAO-Fishstat; 2001).
| Country | Trade Flow | 2001 | % |
| France | Production | 658 | 1% |
| Italy | Production | 2.400 | 4% |
| Spain | Production | 52.059 | 95% |
| Total | 55.117 | 100% |
Both processed bivalve molluscs import and export in the period 1976–2001 exhibit a positive trend. Import grew from 8.037 tonnes in 1976 to 47.786 tonnes in 2001, whereas export increased from 5.706 tonnes in 1976 to 14.597 tonnes in 2001. Throughout this period imports have been significantly higher than exports, reaching a gap of 33.189 tonnes in 2001, similarly to what has been observed for fresh and frozen shellfish products.
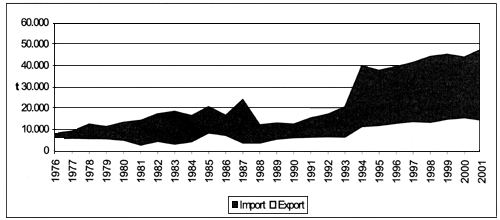
Figure 11. Import and export of processed bivalve molluscs in Mediterranean countries (FAO-Fishstat).
Processed products made of imported shellfish are reported in Table 10. Mussels are most represented as both frozen and canned products, equivalent to 54 percent of the total, followed by frozen scallops 42 percent, and processed clams 3 percent.
Table 10. Mediterranean production of processed imported product of bivalve molluscs by species, quantity expressed in tonnes (FAO-Fishstat; 2001).
| Commodity | Trade Flow | 2001 | % |
| Clam meat, canned | Import Quantity | 5 | 0% |
| Clam meat, frozen | Import Quantity | 1.565 | 3% |
| Mussel meat, canned | Import Quantity | 12.995 | 27% |
| Mussel meat, frozen | Import Quantity | 12.974 | 27% |
| Mussels, dried, salted or in brine | Import Quantity | 2 | 0% |
| Scallops meat, frozen | Import Quantity | 20.245 | 42% |
| Total | 47.786 | 100% |
The major importer countries for these types of products in 2001 were: France, 27.789 tonnes (or 57 percent of the total), Italy, 10.583 tonnes (22%) and Spain, 8.531 tonnes (8%). Import of other Mediterranean countries was not significant, with only Greece reaching a relevant quantity of 2 percent of total import (Table 11).
Table 11. Mediterranean countries importer of processed bivalve mollusc (quantity expressed in tonnes, FAO-Fishstat; 2001).
| Country | Trade Flow | 2001 | % |
| Algeria | Import Quantity | 20 | 0% |
| Croatia | Import Quantity | 154 | 0% |
| Cyprus | Import Quantity | 144 | 0% |
| Egypt | Import Quantity | 7 | 0% |
| France | Import Quantity | 27.089 | 57% |
| Greece | Import Quantity | 789 | 2% |
| Israel | Import Quantity | 44 | 0% |
| Italy | Import Quantity | 10.583 | 22% |
| Lebanon | Import Quantity | 4 | 0% |
| Malta | Import Quantity | 264 | 1% |
| Slovenia | Import Quantity | 94 | 0% |
| Spain | Import Quantity | 8.531 | 18% |
| Tunisia | Import Quantity | 12 | 0% |
| Turkey | Import Quantity | 36 | 0% |
| Yugoslavia, Fed. Rep. of | Import Quantity | 15 | 0% |
| Total | 47.786 | 100% |
Out of the total amount of exported processed shellfish products, mussels constitute 61 percent (or 9.000 tonnes), frozen clams 26 percent (3.781 tonnes) and scallops 14 percent (2.000 tonnes) (Table 12)
Table 12. Mediterranean production of processed exported product of bivalve molluscs by species (quantity expressed in tonnes, FAO-Fishstat; 2001).
| Commodity | Trade Flow | 2001 | % |
| Clam meat, frozen | Export Quantity | 3.781 | 26% |
| Mussel meat, canned | Export Quantity | 3.481 | 24% |
| Mussel meat, frozen | Export Quantity | 5.342 | 37% |
| Scallops meat, frozen | Export Quantity | 1.993 | 14% |
| Total | 14.597 | 100% |
The main exporter Mediterranean countries are shown in Table 13. As well as for imports, Spain is the country that exports the highest quantity of product, 7.026 tonnes (48 percent of the total), followed by France with 3.252 tonnes (22%) and Italy with 2.176 tonnes (15%). Amounts exhibited by Turkey, 7%, Greece, 5%, and Morocco, 2%, are also significant.
Table 13. Exporter Mediterranean countries of processed bivalve molluscs (quantity expressed in tonnes, FAO-Fishstat; 2001).
| Country | Trade Flow | 2001 | % |
| Albania | Export Quantity | 66 | 0% |
| Croatia | Export Quantity | 3 | 0% |
| Cyprus | Export Quantity | 2 | 0% |
| Egypt | Export Quantity | 33 | 0% |
| France | Export Quantity | 3.252 | 22% |
| Greece | Export Quantity | 711 | 5% |
| Italy | Export Quantity | 2.176 | 15% |
| Morocco | Export Quantity | 231 | 2% |
| Slovenia | Export Quantity | 1 | 0% |
| Spain | Export Quantity | 7.026 | 48% |
| Tunisia | Export Quantity | 46 | 0% |
| Turkey | Export Quantity | 1.050 | 7% |
| Total | 14.597 | 100% |
5. Interactions between capture fisheries and shellfish culture
Bivalve molluscs production in the Adriatic is the result of both harvesting from natural beds and aquaculture. Aquaculture also includes the activities related to the management of productive areas obtained as proprieties, lease, or through authorization. The ordinary activities carried out in these areas are seedings, substrate cleaning and pruning operations.
Some shellfish species production depends on both harvesting and aquaculture. For other species only one of the two systems contributes to the final yield. Mussel (Mytilus galloprovincialis), Japanese littleneck clam (Tapes semidecussatus) and less relevantly the European flat oyster (Ostrea edulis) and the Pacific oyster (Crassostrea gigas) belong to the first category. Shellfish species that are exclusively harvested are: stripped venus (Chamelea gallina), scallop (Pecten jacobaeus), queen scallops (Aequipecten opercularis and Proteopecten glaber), chequered carpet shell (Tapes decussatus), golden carpet shell (Paphia aureus), smooth callista (Callista chione), warty venus (Venus verrucosa), razor clams (Solen spp, Ensis spp).
Interactions between shellfish culture and both bivalve molluscs harvesting and capture fisheries can be identified in different sectors: political-management, environmental, economical and social.
5.1 Political-management interactions
From a political point of view it is important to pursue an integrated development of fisheries and aquaculture contrasting the effects of competitive allocation of resources to these two productive sectors. Among the planning and legislative tools that can be engaged to this purpose there is the identification of developing plans that recognise the value of territorial realities, both on a local and macro-regional scale, taking into account the necessity to consider aquaculture as a specific entity within a fishing area. The integration of fisheries and aquaculture should also be pursued through planning actions related to coastal management. The importance of clam farming, exclusively practiced in coastal lagoon areas, can be mentioned in this context. With regard to management of trans-boundary shared resources, bivalve molluscs beds (scallops and queen scallops), distributed far from the coast should be considered.
The availability of reliable statistical surveys, comparable to other countries' statistics, is necessary for the identification of a correct policy.
5.2 Ecological interactions
Ecological interactions between fishery and aquaculture are quite strong. One of the most important issues is the introduction of species or varieties of different geographical origin, either directly through voluntary introduction of allochthonous species or indirectly through the flora and fauna associated to the imported products.
The translocation of molluscs from different geographic areas can have the following side-effects:
In order to avoid undesirable effects it is necessary to regulate the introduction and culture of products of different geographic origin, even if the product is recognized as an autochthonous species, providing for periodical controls on culture farms and on the origin and traceability of the products. Moreover, the development of hatcheries where certificated indigenous spawners are used should be supported.
Spat imported from other geographical areas should always be certificated in order to avoid the possibility of it becoming a vector of unwanted species.
Particular attention should be given to adults' introductions, as associated flora and fauna species might be introduced with molluscs or with the culture ground. As an example, biocenosis represented by hydroids, sea-squirt, amphipods, algae etc could be carried through the mussels' mesh tubing.
Another impact that shellfish culture can have on fisheries is the enrichment of the environment with inorganic waste as a consequence of routine working operations in the farm, and the accumulation of organic waste or disperse material on the sea bottom under the farm structure. The release of tubing used for mussels' net-bags into the environment can cause problems to both gillnet and trawl fisheries, affecting the efficiency of the gear.
Accumulation of organic material in still water can cause anoxia with negative consequences on biological equilibrium in the surrounding area. On the other hand, in areas where currents favour water turnover, the increase of organic material can represent a positive factor causing an increase of the total biomass.
Several benefits for fisheries can be identified within a correct management framework of shellfish culture:
Shellfish farms, situated in near the coast, might function as protected areas: as nursery areas for juveniles of several fish species; as sheltering areas for both benthonic and nektonic species thanks to the tigmotrophic effect; providing for nutrients supply, organic substances and associated biocenosis.
The presence of a high number of spawners in shellfish farms might increase the abundance of populations of cultured species in the wild, determining a valuable restocking action. Due to the molluscs' life cycle, the effect can reach long distances.
Although clam culture relies on the exploitation of an introduced species (Tapes decussatus), and therefore implies all the problems related to the allochthonous species, it can represent a valid solution for wetlands management when recommendations on sustainable use of resources are observed.
The running operations carried out in the culture productive sites, such as pruning spat transfer, bottom cleaning, hydraulic vivification and monitoring of environmental phenomena, contribute to maintain balanced environmental conditions.
In shellfish culture the use of wild resources can be considered. In these cases synergic actions between the fisherman who provides the product and the shellfish farmer who follows the grow-out phase can develop. This worthy behaviour can be strengthened by the restocking actions determined by shellfish farms, and it could be usefully applied to species such as flat oyster (Ostrea edulis) or scallop (Pecten jacobaeus). However, regulated management of natural mollusc beds, establishing controls on access and gears, should follow these actions
5.3 Economical and commercial interactions
The increase of shellfish culture production observed during the last 20 years has strongly influenced the molluscs fishery sector, for the reason that both products are placed on the same market. Although naturally harvested mussels represent a product which is qualitatively different from cultured mussels, marketing competition favours farm production, so that areas traditionally bound to shellfish harvesting are progressively turning into molluscs farming. One of the main reasons for this is the competitive prize of cultured molluscs. Consumers traditionally keen on harvested shellfish consider this product qualitatively superior. However, other consumers regard cultured mussels and molluscs in general as safer products as far as hygienic and sanitary aspects are concerned. A similar issue has emerged after the introduction of Japanese littleneck clam, which in some markets have totally replaced the stripped venus (Chamelea gallina) harvested on natural beds; although it now seems that an equilibrium has been reached due to the decline of fishery resources.
The market subject is strongly connected to the one of product quality. In order to guarantee a right coexistence to both farm and harvest products, it is essential to proceed with the application of labelled certifications where the type of production processes and traceability are documented. It is therefore wise to support trade programmes based on the application of health marks, in order to ensure the product origin, the production process and the hygienic and sanitary safety.
5.4 Social interactions
As regards the fishery, shellfish culture activity has shown to be a social stabilizing element, representing a valuable opportunity for alternative or complementary employment. Notwithstanding the related environmental problems due to the introduction of Tapes semidecussatus, clam culture development has been an important employment opportunity for different social class workers devoted to small-scale fisheries in lagoons. The same can be said regarding the mussel culture, a sector which is absorbing that part of employees who are gradually being expelled from artisanal fishery due to fleet restructuring.
Although the idea of a positive influence of sustainable farming activities on fish resources is finding its way, there are still many conflicts between fishery and shellfish culture.
The large presence of culture products on the market, appreciated by many consumers for their sanitary and hygienic characteristics, might prove to be a driving force for consumption of fishery products too.
6. Legislative aspects and international regulations and actions on shellfish resources management and exploitation
6.1 Italian legislation of bivalve molluscs fisheries
Several aspects are considered by the laws that regulate resource management in both fishery and shellfish culture sectors:
Other important regulations are connected to these normatives on subjects such as hygienic and sanitary aspects of processing and trading of products, and on spat collection used for culture activities.
Clam (Chamelea gallina) fisheries regulation will be illustrated as one of the most represented bivalve mollusc fished along Italian and Adriatic coasts.
6.2 Regulation of bivalve mollusc Chamelea gallina fishery
In Italy clam (Chamelea gallina) fishery is practiced by authorized vessels equipped with a hydraulic dredge system. During the last years no new licenses have been issued, rather, license return has been financially subsidized/supported.
Management and conservation of bivalve molluscs has been partitioned among management consortiums (D.M. 12 January 1995), which, within a regulation framework can exert a restricted decision-making role.
Through D.M. July 4th 2003, the suspension Law February 11th 2003 concerned with the “Conservation and management of bivalve molluscs and new provisions for management consortiums” has been postponed.
Main regulations for rational resources management are reported in D.M. December 22nd 2000 “Regulation on fishing for bivalve molluscs”. These regulations are concerned with minimum size (25mm, Reg. 1639/68), daily catches (600 kg), limits of fishing activities within the area where vessel is registered, minimal fishing depth (3 m), technical characteristic of the vessel, fishing gear and selection gear.
The hydraulic dredge is a rectangular steel cage capable of penetrating into the bottom with an adjustable blade and a number of jets running the full width of its lower leading edge; it must have the following characteristics:
The lower part of the cage must have a mesh or bar spacing to ensure the gear selectivity. Bar spacing can not be smaller than 12 mm, with less than 1 mm tolerance.
Minimum mesh sizes of the selective gear called vibro-vaglio must be at least 12 mm for bars with at least 21 mm diameter for perforated plates with round holes.
The trawling method is established by the consortium. Where there is no constituted consortium, or for unregistered vessels, the trawling must be carried out by hauling the anchor.
Two months of closed season (Fermo pesca) are compulsory: they are fixed in the period April 1st-October 31st, throughout the year the closure is obligatory also on weekends and holidays. In the period between April 1st and September 30th one working day chosen by the Consortium must be added.
Further obligations concerning statistical data collection are provided for this fishery method. The license holder has to fill a form within the 5th of each month. The form is to be sent to the Consortium. The Consortium will put together the statistics of all the vessels registered and will send a summary form to the Ministry within the 15th of the following months.
The D.M. December 22nd provides also that the Consortium draws up an annual management plan of seed stocking and other management measures.
6.3. Bivalve molluscs trade regulation in the EC countries, and relative issues in respect of third countries
The European Communities Council with a view to harmonize the relations among Member States, to bring about competition on equal terms while ensuring quality products for the consumer and to establish regulations apt to ensure the health of live bivalve molluscs placed on market, has issued the Council Directive 91/492 July 15th 1991. The normative contains the principles concerned with resources utilization and product trade, including products of non-EC countries' origin. A Community regulation framework has been established for imports, within which provisions of Chapter III must be implemented. These conditions must be at least equivalent to those applicable for trading within the Community.
Bivalve molluscs exports from a third country to an EC country must be authorized through inspections carried out on the spot by Community and Member States experts to ensure that production and trade can be deemed equivalent to those of the Community on the basis of the following conditions: the legislation in force in the third country; organization of the competent inspection authority; the effective implementation of sanitary controls, especially in the production areas; the rapidity of the information provided by the third country about the sanitary conditions of production areas; the assurance that a third country can give on the compliance with the standard sanitary controls on both fish and culture product.
Countries that comply with the provided conditions and are allowed to export to EC, are listed in the Annex to Decision 97/20/CE of December 17th 1996 (updated in Decision 2002/469/CE). Two lists are provided: the first includes the so called “harmonized” third countries, which are subject to a specific decision based on Directive 91/492. They can sell their product in EC countries with fewer restrictions and less control at the border inspection points. The second list includes countries that can be subject to temporary decisions according to Decision 95/408/CE June 22nd 1995 (prelisting condition). In the EC they are allowed to trade only with those countries with which they have a bilateral agreement, and do not benefit of inspection controls relief. For all countries included in either list, production areas and authorized establishments are listed. For countries in the list 1 the identified sites are valid within the entire Community, whereas for countries in the list 2 it depends on bilateral agreements between Member States.
Inspection procedures applied to bivalves at the border inspection points are laid down in the Directive 97/78/CE in accordance with Reg. CE 136/2004, which provides for the control of imports authorizations and veterinary certificates, the consistency between identification certificates and the product, the product itself through sampling and lab analysis. Sample control is the only step that can be derogated from inspection procedures of “harmonized” countries.
In order to limit the spreading of transferable diseases and to protect the zootechnical resources of the Member States, zootechnical health controls are also provided for in the trade product regulation. The classification of “recognized” zootechnical health sites and establishments included in Directive 91/67/CE, requires safeguarding and monitoring actions, as well as historical data acquisition referring to molluscs diseases. The fact that classification is valid as long as products are exclusively traded among “recognized” establishments, has so far represented a strong limit to the application of this rule.
7. References
Mattei, N., Pellizzato, M. (1997) Mollusc fisheries and aquaculture in Italy. In U.S. Dep. Commer., NOAA tech. Resp. NMFS 129, p 201–216.
Prioli, G. (2001) Censimento nazionale sulla molluschicoltura del Consorzio Unimar. Unimar Osservatorio tecnico-biologico. 97 pp.