TOXICOLOGY
Dimethenamid-P is the ISO approved common name for S-2-chloro-N-(2,4-dimethyl-3-thienyl)-N-(2-methoxy-1-methylethyl)-acetamide. This compound belongs to the chemical family of chloroacetamides and is used as a pre-emergent or early post-emergent herbicide with a broad spectrum of activity against most annual grasses and some important broad leaf weeds. It is taken up through the coleoptiles (grass seedlings) or the roots and emerging shoots (dicotyledonous seedlings) and reduces cell division and plant growth.
Dimethenamid is a racemic mixture of the M (or R) and P (or S) stereoisomers. When this compound was originally registered in various countries, all studies of toxicity were conducted with the racemic mixture. Later, it was discovered that only the P (or S) enantiomer has useful herbicidal activity. Dimethenamid-P and racemic dimethenamid have not been evaluated previously by the JMPR.
All critical studies complied with GLP.
Biochemical aspects
Racemic dimethenamid was slowly but well absorbed after oral administration and was extensively metabolized by rats. In rats given racemic dimethenamid by gavage, there was no significant difference in the degree of absorption (> 90%) at a low dose of 10 mg/kg bw and a high dose of 1000 mg/kg bw, or between single and multiple doses at 10 mg/kg bw per day. Maximum concentrations in blood were not achieved until about 72 h. Excretion was rapid and primarily via bile, between 45% and 64% of the oral dose being excreted within 7 h by this route; however, biliary elimination appeared to be saturated at 1000 mg/kg bw, because elimination in the urine was increased at this high dose. By 168 h after treatment, an average of 90% of the administered dose was eliminated. In rats, the concentration of radioactivity in blood decreased more slowly than in tissues and was associated with specific binding to globin; however, similar specific binding to blood components did not occur in human blood. Levels in other tissue after 168 h were low regardless of the dose or frequency of dosing. Consequently, there was no evidence of bioaccumulation. There was no significant difference in absorption, distribution and elimination between sexes.
Studies of dermal penetration in vivo in rats demonstrated that dermal penetration of racemic dimethenamid and dimethenamid-P at 24 h was approximately 26%. Based on the results of comparisons of penetration in human and rat skin in vitro, it was concluded that the rate of dermal penetration was lower in humans than in rats.
Metabolism was primarily via the glutathione conjugation pathway, but racemic dimethenamid was also metabolized by cytochrome P450 enzymes via reductive dechlorination, oxidation, hydroxylation, O-demethylation, and cyclization pathways, as well as conjugation with glucuronic acid. Unchanged dimethenamid in excreta accounted for only 1-2% of the administered dose, more than 40 metabolites having been detected. At least 20 of these metabolites were structurally identified by mass spectrometry and nuclear magnetic resonance, and confirmed by reference to synthesized standards. There was no significant difference in metabolism between the sexes.
Toxicological data
Although many of the critical studies of toxicity were conducted only with the racemic mixture, some studies were performed with both dimethenamid-P and racemic dimethenamid. These include studies of acute oral toxicity (LD50) in rats, dermal toxicity (LD50) in rats, acute toxicity after inhalation (LC50) in rats, dermal irritation in rabbits, eye irritation in rabbits, dermal sensitization in guinea-pigs, 90-day studies of oral toxicity in rats, prenatal developmental toxicity and teratogenicity in rats, mutagenicity in bacteria and Chinese hamster ovary cells in vitro, chromosome aberrations in Chinese hamster ovary cells in vitro, assays for unscheduled DNA synthesis in rat hepatocytes in vitro, and assays for micronucleus induction in bone-marrow cells in mice in vivo.
The acute toxicities of dimethenamid-P and the racemic mixture are characterized as moderate after oral administration and low after dermal or inhalation administration. The oral LD50 values in rats were: dimethenamid-P, 429 mg/kg bw (males) and 531 mg/kg bw (females); racemic dimethenamid, 371 mg/kg bw (males) and 427 mg/kg bw (females). Both substances produced only mild reversible skin and eye irritation. Skin sensitization was produced by dimethenamid-P in guinea-pigs in the Buehler test and by racemic dimethenamid in the Magnusson & Kligman test.
Overall, in short-term studies with racemic dimethenamid, the signs of toxicity observed in mice, rats and dogs were similar, with reduced body-weight gain and liver enlargement being common features. Dimethenamid-P and racemic dimethenamid produced very similar effects in the liver of rats. The Meeting concluded that increased liver weights were indicative of an adaptive response to exposure. Histopathology confirmed the liver as a target organ with observation of hypertrophy of hepatocytes, although this too is indicative of an adaptive response and was accompanied by the induction of several hepatic microsomal enzymes. These hepatic enzyme changes were resolved upon removal from treatment. In addition, however, vacuolization of hepatocytes and dilatation of liver sinusoids occurred in dogs.
The NOAELs for the short-term dietary studies were for dimethenamid-P and racemic dimethenamid, respectively: 90-day study in rats, 500 ppm (equal to 39 mg/kg bw per day) and 500 ppm (equal to 34 mg/kg bw per day); and, for racemic dimethenamid alone: 90-day dietary study in mice, 2000 ppm (equal to 301 mg/kg bw per day); 90-day study in dogs, 92 ppm (equal to 4.6 mg/kg bw per day); 12-month study in dogs, 250 ppm (equal to 10 mg/kg bw per day). In a 3-week study of dermal toxicity with racemic dimethenamid in rabbits, no substance-related systemic findings were detected at 1000 mg/kg bw per day, the highest dose tested.
Long-term feeding studies with racemic dimethenamid in rats and mice demonstrated that the primary target organ was the liver. There was no evidence for a carcinogenic potential in these studies. The NOAELs obtained in long-term studies were: rats, 100 ppm (equal to 7 mg/kg bw per day, on the basis of bile-duct hyperplasia and reduced body-weight gain in females); and mice, 300 ppm (equal to 40 mg/kg bw per day, on the basis of decreased body-weight gain and hepatocellular hypertrophy).
Dimethenamid-P and racemic dimethenamid were tested for genotoxicity in an adequate range of assays, both in vitro and in vivo. No evidence for genotoxicity was observed in any test with dimethenamid-P. Apart from an equivocal result in one of three assays for unscheduled DNA synthesis in vitro with racemic dimethenamid, none of the assays gave any indication that racemic dimethenamid might be genotoxic. The Meeting concluded that both dimethenamid-P and racemic dimethenamid are unlikely to be genotoxic.
In the absence of genotoxicity and any evidence of carcinogenicity in rodents, the Meeting concluded that dimethenamid-P and racemic dimethenamid are unlikely to pose a carcinogenic risk to humans.
The reproductive toxicity of racemic dimethenamid was investigated in a two-generation study of reproduction in rats and in a study of developmental toxicity in rabbits. The developmental toxicity of both dimethenamid-P and racemic dimethenamid was studied in rats.
Reproductive function was not affected in rats in the two-generation study of racemic dimethenamid and the NOAEL for reproductive function was 2000 ppm (equal to 175 mg/kg bw per day), the highest dose tested. The NOAEL for systemic toxicity in the parental animals in the two-generation study was 500 ppm (equal to 45 mg/kg bw per day). The only effect on pups noted was a decreased body-weight gain during lactation at the highest dose. The NOAEL for developmental toxicity in the F1 and F2 litters was 500 ppm (equal to 45 mg/kg bw per day).
In a study of developmental toxicity, rats were given dimethenamid-P at doses of up to 300 mg/kg bw per day. Both maternal and developmental toxicity were observed. There was an increased incidence of clinical signs of toxicity in the group receiving the highest dose. The effects on development included increases in delayed ossifications, but further evaluation demonstrated that these were attributable to unusually low control values and were not related to treatment. The NOAEL for maternal toxicity was 25 mg/kg bw per day on the basis of decreased body-weight increment, and the NOAEL for developmental toxicity was 300 mg/kg bw, the highest dose tested.
In a study of developmental toxicity, rats were given racemic dimethenamid at doses of up to 425 mg/kg bw per day. Signs of maternal toxicity that were recorded included excess salivation at 215 mg/kg bw per day and 425 mg/kg bw per day, and urine-stained abdominal fur at 425 mg/kg bw per day. Fetal body weights were reduced and the frequency of early deaths was increased at doses of 215 mg/kg bw per day and 425 mg/kg bw. The NOAELs for both maternal toxicity and developmental toxicity were 50 mg/kg bw per day.
In a study of developmental toxicity in rabbits given racemic dimethenamid at doses of up to 150 mg/kg bw per day, significant maternal toxicity (body-weight loss preceded by reduced food consumption and associated with dry faeces) was observed at the highest dose and less severe effects were noted at 75 mg/kg bw per day. Abortions in two rabbits at 150 mg/kg bw per day were considered to be treatment-related, but secondary to the clear maternal toxicity. The NOAEL for maternal toxicity was 37.5 mg/kg bw per day and the NOAEL for developmental toxicity was 75 mg/kg bw per day.
No evidence of neurotoxicity was noted in any studies.
The plant and soil oxalamide (M23) and sulfonate (M27) metabolites of racemic dimethenamid, which also occur as products of metabolism in rats, were tested in studies of acute oral toxicity, assays for mutagenicity in bacteria and for micronucleus formation in bone-marrow cells of mice. Both compounds had low acute oral toxicity with LD50 values of > 5000 mg/kg bw. Neither compound was mutagenic in bacteria or induced micronucleus formation in bone-marrow cells of mice.
Interviews with and written surveys of 50 people handling racemic dimethenamid and its formulated products over 7 years have been conducted. There were no reported cases of skin irritation or other adverse health effects.
Comparison of racemic dimethenamid with dimethenamid-P has been possible for a number of types of study. These have shown that there is little difference in the toxicological profile or, where appropriate, the NOAELs for these materials. Consequently, the Meeting concluded that data derived from assays with the racemic mixture could be used to supplement data from assays with dimethenamid-P. In the following tables, the actual material tested was identified.
The Meeting concluded that the existing database was adequate to characterize the potential hazards to fetuses, infants and children.
Toxicological evaluation
The Meeting concluded that the toxicology of the S enantiomer (dimethenamid-P) is not significantly different from that of the racemic mixture. For the purpose of dietary risk assessment, the residues of concern were defined as parent dimethenamid (R and S enantiomers); therefore the derivation of a separate ADI or ARfD for dimethenamid-P is not necessary.
An ADI of 0-0.07 mg/kg bw was established for dimethenamid-P and racemic dimethenamid based on the NOAEL of 7 mg/kg bw per day for bile-duct hyperplasia and reduced body-weight gain observed only in female rats in a 24-month study in rats given diets containing racemic dimethenamid, and a safety factor of 100.
The Meeting established an ARfD of 0.5 mg/kg bw for dimethenamid-P and racemic dimethenamid based on an overall NOAEL of 50 mg/kg bw for maternal clinical signs of toxicity and developmental toxicity (fetal body-weight deficits and increases in early deaths) in studies in rats, and a safety factor of 100.
A toxicological monograph was prepared.
Levels relevant to risk assessment
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
94-week study of toxicity and carcinogenicity with the racemic mixture |
Toxicity |
300 ppm, equal to 40 mg/kg bw per day |
1500 ppm, equal to 200 mg/kg bw per day |
|
Carcinogenicity |
3000 ppma, equal to 411mg/kg bw per day |
- |
||
|
Rat |
104-week study of toxicity and carcinogenicity with the racemic mixture |
Toxicity |
100 ppm, equal to 7 mg/kg bw per day |
700ppm, equal to 49 mg/kg bw per day |
|
Carcinogenicity |
1500 ppma, equal to 80 mg/kg bw per day |
- |
||
|
Two-generation study of reproductive toxicity with the racemic mixtureb |
Reproductive toxicity |
2000 ppma equal to 175 mg/kg bw per day |
- |
|
|
Parental toxicity |
500 ppm, equal to 45 mg/kg bw per day |
2000 ppma, equal to 175 mg/kg bw per day |
||
|
Offspring toxicity |
500 ppm, equal to 45 mg/kg bw per day |
2000 ppma, equivalent to 175 mg/kg bw per day |
||
|
Developmental toxicity with dimethenamid-Pc |
Maternal toxicity |
25 mg/kg bw per day |
150 mg/kg bw per day |
|
|
Embryo- and fetotoxicity |
300 mg/kg bwa per day |
- |
||
|
Developmental toxicity with the racemic mixturec |
Maternal toxicity |
50 mg/kg bw per day |
215 mg/kg bw per day |
|
|
Embryo- and fetotoxicity |
50 mg/kg bw per day |
215 mg/kg bw per day |
||
|
Rabbit |
Developmental toxicity with the racemic mixturec |
Maternal toxicity |
37.5 mg/kg bw per day |
75 mg/kg bw per day |
|
Embryo- and fetotoxicity |
75 mg/kg bw per day |
150 mg/kg bw per day |
||
|
Dog |
1-year study of toxicity with the racemic mixture |
Toxicity |
250 ppm, equal to 10 mg/kg bw per day |
1500 ppma, equal to 49 mg/kg bw per day |
a Highest dose tested
b Measurements of intake of the compound are the mean of the pre-mating phases for F0 and F1 females
c Gavage administration
Estimate of acceptable daily intake for humans
0-0.07 mg/kg bw
Estimate of acute reference dose
0.5 mg/kg bw
Information that would be useful for the continued evaluation of the compound
Results from epidemiological, occupational health and other such observational studies of human exposures
Critical end-points for setting guidance values for exposure to dimethenamid-P and racemic dimethenamid
|
Absorption, distribution, excretion and metabolism in mammals |
|
|
Rate and extent of oral absorption |
Low, plasma Tmax 7 h; high, > 90% absorbed in rats |
|
Dermal absorption |
> 20% (dimethenamid-P and racemic dimethenamid) in rats |
|
Distribution |
Distributed throughout the body; higher concentrations in adrenals, pancreas, kidney, liver, spleen and blood |
|
Potential for accumulation |
Very low |
|
Rate and extent of excretion |
High (determined by the slow absorption); essentially 100% excretion within 168 h |
|
Metabolism in animals |
Extensive, about 40 metabolites, little parent compound remaining |
|
Toxicologically significant compounds (animals, plants and environment) |
Parent |
|
Acute toxicity |
|
|
Rat LD50 oral |
429 mg/kg bw (dimethenamid-P); 371 mg/kg bw (racemic mixture) |
|
Rat LC50 inhalation |
> 2.2 mg/L (4h) (dimethenamid-P and racemic mixture) |
|
Rabbit LD50 dermal |
> 2000 mg/kg bw (dimethenamid-P and racemic mixture) |
|
Rabbit, skin irritation |
Slightly irritating (dimethenamid-P and racemic mixture) |
|
Rabbit, eye irritation |
Not irritating (dimethenamid-P and racemic mixture) |
|
Skin sensitization (test method used) |
Sensitizing (Buehler test) (dimethenamid-P) and Magnusson & Kligman (racemic mixture) |
|
Short-term studies of toxicity |
|
|
Target/critical effect |
Body-weight gain decrement, increased absolute and relative liver weight (dimethenamid-P and racemic mixture) |
|
Lowest relevant oral NOAEL |
10 mg/kg bw per day: (12-month study in dogs) (racemic mixture) |
|
Lowest relevant dermal NOAEL |
1000 mg/kg bw per day (21-day study in rabbits) (racemic mixture) |
|
Lowest relevant inhalation NOAEC |
No data available and not required |
|
Genotoxicity |
|
| |
Not genotoxic in vivo or in vitro (dimethenamid-P; racemic mixture) |
|
Long-term studies of toxicity and carcinogenicity |
|
|
Target/critical effect |
Liver, bile-duct hyperplasia (racemic mixture); body weight |
|
Lowest relevant NOAEL |
7 mg/kg bw per day (24-month study in rats) (racemic mixture) |
|
Carcinogenicity |
Dimethenamid-P and racemic dimethenamid are unlikely to pose a carcinogenic risk to humans |
|
Reproductive toxicity |
|
|
Reproductive target/critical effect |
None |
|
Lowest relevant reproductive NOAEL |
175 mg/kg bwa, b per day (racemic mixture) |
|
Developmental target/critical effect |
Not teratogenic; reduced fetal body weight (dimethenamid-P); not teratogenic; reduced fetal body weight and increased early deaths (racemic dimethenamid) |
|
Lowest relevant developmental NOAEL |
300 mg/kg bwa per day (rat) (dimethenamid-P) and 50 mg/kg bw per day (rat) (racemic mixture) |
|
Neurotoxicity/delayed neurotoxicity |
|
| |
No signs of neurotoxicity |
|
Other toxicological studies |
|
| |
Liver xenobiotic metabolizing enzyme induction. Strong binding to haemoglobin in rats, but this has no relevance to humans |
|
Medical data |
|
| |
There have been no reports of toxicity in workers exposed during manufacture or use |
|
Summary |
|||
| |
Value |
Study |
Safety factor |
|
ADI |
0-0.07 mg/kg bw |
Rat, 2-year study of toxicity and carcinogenicity (racemic mixture) |
100 |
|
ARfD |
0.5 mg/kg bw |
Rat, study of developmental toxicity (racemic mixture) |
100 |
a Highest dose tested
b Measurements of intake of the compound are the mean of the pre-mating phases for P and F1 females
RESIDUE AND ANALYTICAL ASPECTS
Residue and analytical aspects of the herbicide dimethenamid-P (S-dimethenamid) were considered for the first time by the present Meeting. Dimethenamid-P is one of the enantiomers in dimethenamid, the other being the herbicidally inactive dimethenamid-M (R-dimethenamid). In this report, the term 'dimethenamid' refers to the 50:50 mixture of R-dimethenamid and S-dimethenamid while the term 'dimethenamid-P' refers to the herbicidally active S-dimethenamid, containing up to 10% of the inactive enantiomer.
When applied as pre-plant, pre-emergent or early post-emergent treatments, this chloroacetamide herbicide is active against germinating broad-leaf and grass weeds, being taken up through the coleoptiles (grass seedlings) or the roots and emerging shoots (dicotyledonous seedlings) and reducing cell division and growth.
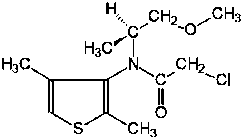
Chemical name:
|
IUPAC: |
S-2-chloro-N-(2,4-dimethyl-3-thienyl)-N-(2-methoxy-1-methyl-ethyl)acetamide |
|
CAS: |
(S)-2-chloro-N-(2,4-dimethyl-3-thienyl)-N-[-2-methoxy-1-methyl-ethyl]acetamide |
The manufacturer submitted studies on metabolism, analytical methods, supervised field trials, processing, freezer storage stability and rotational crop residues. Most of these studies involved the racemic mixture (dimethenamid) with supporting or bridging studies with dimethenamid-P also being provided. Information on GAP was submitted by the Netherlands.
The following abbreviations are used for the metabolites discussed below:
|
M7 |
2-chloro-N-(2,4-dimethyl-3-thienyl)-N-(2-hydroxy-1-methylethyl) acetamide |
|
M23 (oxalamide) |
2,2'-dithiobis(N-(2,4-dimethyl-3-thienyl)-N-(2-methoxy-1-methylethyl) acetamide) |
|
M25 |
2-[N-(2,4-dimethyl-3-thienyl)-N- (2-methoxy-1-methylethyl) amino]-2-oxoethyl-cysteine |
|
M27 (sulfonate) |
2-[N-(2,4-dimethyl-3-thienyl)-N- (2-methoxy-1-methylethyl)-amino]-2-oxoethyl-sulfonic acid |
|
M28 |
2-[N-(2,4-dimethyl-3-thienyl)-N- (2-methoxy-1-methylethyl) amino]-2-oxoethyl-sulfonic acid |
|
M29 |
sulfoxide of 2-[N-(2,4-dimethyl-3-thienyl)-N-(2-methoxy-1-methylethyl) amino]-2-oxoethyl-N-malonyl cysteine |
|
M30 |
sulfoxide of 2-[N-(2,4-dimethyl-3-thienyl)-N-(2-methoxy-1-methylethyl) amino]-2-oxoethyl thiolactic acid |
|
M31 |
sulfoxide of 2-[N-(2,4-dimethyl-3-thienyl)-N-(2-methoxy-1-methylethyl) amino]-2-oxoethyl thioglycolic acid |
Animal metabolism
The Meeting received animal metabolism studies for dimethenamid on lactating goats and laying hens. Comparison of racemic dimethenamid with dimethenamid-P toxicology has been possible for a number of types of study. These have shown that there is little difference in the toxicological profile or, where appropriate, the NOAEL values of these materials. Consequently, the Meeting concluded that the metabolism studies involving racemic dimethenamid could also apply to dimethenamid-P.
Rats
Dimethenamid was well absorbed and extensively metabolized by rats, with about 90% of the administered dose being eliminated within 168 h and only 1-2% of unchanged dimethenamid was detected in excreta. About 40 metabolites were found in organic extracts using thin layer chromatography (TLC) analysis with 20 of these being identified.
Goats
A lactating goat was orally administered [3-14C-thienyl]dimethenamid for four consecutive days at a dose equivalent to 223 ppm in the diet. In this study, 36% of the administered dose was excreted in either urine or faeces and less than 2.3% TRR remained in animal tissues (0.02% in milk). In milk, residues reached a plateau after 3 days, with a maximum of 0.98 mg/kg dimethenamid equivalents reported 7 h after the third dose. Concentrations in kidney, fat, muscle and liver were 9.9, 1.0, 0.97 and 17 mg/kg, respectively. No residues of the parent compound were found, and metabolites reported at levels higher than 1.0 mg/kg were in kidney (M7 at 2.4 mg/kg) and in liver (M25 at 1.2 mg/kg, M22 at 1.0 mg/kg).
Because of the low recovery rate in this study, partly explained by the loss of a urine sample and reduced faecal production and the exhibition of toxicity symptoms (loss of appetite and decrease in body weight), a supplementary material balance study was also conducted, where a single goat was dosed once with [3-14C-thienyl]dimethenamid (equivalent to 250 ppm in the diet) and radioactivity measured in urine, faeces and milk over the subsequent 5 days. In this second study, more than 59% (urine) and 28% (faeces) of the TRR was excreted by the end of the 5-day study, with 0.09% TRR being measured in milk.
Hens
Laying hens (3) were fed with [3-14C-thienyl]dimethenamid for four days at a dose rate equivalent to 167 ppm in the diet. Elimination of the C14 was rapid, with more than 77 % of the total applied dose being found in the excreta, less than 0.5% in liver, between 0.3% and 0.4% in muscle, 0.07% in fat and 0.02% or less in eggs. Radiolabel concentrations in egg white increased from 0.19 mg/kg to 0.3 mg/kg dimethenamid equivalents over the four day period with the related egg yolk residues increasing from 0.01 mg/kg to 0.62 mg/kg over the same period. Residue levels in fat, muscle (breast), muscle (thigh) and liver were 0.29, 0.45, 0.58 and 8.33 mg/kg TRR, respectively.
Residues of dimethenamid were identified in fat (0.1 mg/kg or 36% of the fat radiolabel), with the major identified metabolites being M3 (0.43 mg/kg or 5% liver TRR) and M8 (0.65 mg/kg or 7.8% liver TRR). Up to 21 other metabolites were detected in tissues and eggs, all at less than 10% of the TRR, but these were not identified.
Dimethenamid was extensively metabolized by rats, goats and hens with 1.2% (hens) and 2.3% (goats) of the applied dose remaining in tissues after 4-5 days and 0.02% being found in milk and eggs. The proposed metabolic pathway was via glutathione conjugation, the formation of cysteine, mercapturate thioglycolic sulfoxide conjugates, with other pathways involving demethylation and reductive dechlorination. No residues of the parent compound were reported in milk or any animal tissues except in fat of hens, where dimethenamid residues of about 0.1 mg/kg were reported.
Plant metabolism
The Meeting received plant metabolism studies for dimethenamid in soya beans, maize and sugar beet. While these studies were conducted using dimethenamid, the Meeting considered that dimethenamid-P would exhibit the same metabolic profile and agreed that the plant metabolism studies involving dimethenamid could apply to dimethenamid-P.
Soya beans
In a metabolism study in soya beans treated with radiolabelled dimethenamid to simulate pre-emergence broadcast application (1.68 kg ai/ha and 3.36 kg ai/ha), dimethenamid was rapidly metabolized to a number of polar metabolites (20-30), most being present at low levels (< 0.01 mg/kg or < 3% TRR). No parent compound was detected in any of the samples, even at the 2× treatment rate. Metabolites present at levels higher than 10% TRR were M23 (17% in forage), M27 (11% in hay) and M30/M31 (12% in mature seeds).
Maize
The metabolic fate of dimethenamid was studied in maize plants, where radiolabelled dimethenamid was applied as a pre-emergence broadcast spray (1.68 kg ai/ha and 4.4 kg ai/ha). Translocation of radiocarbon to grain was minimal. Dimethenamid was rapidly metabolized to several weak acids and other highly polar residues, with many individual fractions present in very small amounts. No dimethenamid residues were found in any of the forage, silage, grain or straw samples, even at the exaggerated (4.4 kg ai/ha) application rate and no metabolites were present at levels greater than 0.05 mg/kg or 10% TRR. The most common metabolites found in foliage were M23, M27 and M30/M31
Sugar beet
In a sugar beet metabolism study, labelled dimethenamid was applied three times to sugar beet plants at a rate equivalent to 0.45 kg ai/ha per treatment. Levels of 14C in roots were about 3.5 times lower than in the tops. No parent residues were detected in any samples, with the major identified metabolites being M23, M27, M28 and M29 in the roots and M27, M29 and M30 in the tops. Numerous polar metabolites were also characterized. All the identified metabolites were present at levels below 10% of the TRR or < 0.01 mg/kg dimethenamid equivalents.
Dimethenamid is rapidly metabolized in plants and metabolism occurs through similar pathways in the three crops studied. The proposed metabolic pathway in plants involves conjugation of dimethenamid with glutathione and hydrolysis to the cysteine conjugate, both being considered transient intermediates undergoing rapid oxidation, deamination and/or decarboxylation to form many relatively polar metabolites, all of which are generally present at levels of < 0.05 mg/kg or less than 10% of the TRR. Bound radiocarbon increased with time, indicating incorporation of residues into the plant matrix. No parent compound was detected in any of the plant tissues at any sampling interval.
Environmental fate
Dimethenamid-P is stable in aqueous buffered solutions at pH 5, 7 and 9 (25°C in the absence of light) for at least 31 days. No information was provided on the formation of hydrolysis products, but it is not expected that hydrolytic processes will be a significant factor in the environmental degradation of dimethenamid-P and dimethenamid.
The Meeting received information on the comparative behaviour and fate of dimethenamid-P and dimethenamid in aerobic soil. No significant differences were observed in the degradation rates of dimethenamid and dimethenamid-P when the soil was mixed with dimethenamid or dimethenamid-P at a concentration of about 2 mg ai/kg (to simulate the concentration within the top 5 cm of soil following a pre-emergence broadcast field application at 1.68 kg ai/ha) and incubated under aerobic conditions at 23°C for 182 days. The calculated DT50 value for the aerobic degradation of both compounds in clay loam soil at 23°C was 10 days. After the 182 day incubation period, 14CO2 accounted for 28-29% TRR for both treatments. Non-extractable residues were found to increase to 40% TRR.
Soil metabolites, identified following exaggerated rate incubations (21 days, 9.5 mg/kg dry soil), were similar for both dimethenamid and dimethenamid-P, and none of these exceeded 9% of the TRR.
In a confined rotational crop study, labelled dimethenamid was applied to maize and soya bean crops as simulated pre-emergence treatments. The rotational crops used in this study were winter wheat (planted 141 DAT), spring wheat (planted 322 DAT), lettuce and carrots (planted 332 DAT).
The TRRs for all rotational crop samples from plots treated at a rate equivalent to 1.68 kg ai/ha were between 0.01 mg/kg and 0.06 mg/kg in carrot roots, carrot tops, lettuce leaves, wheat grain and immature wheat plants, with residues of 0.12 mg/kg and 0.17 mg/kg being reported in summer and winter wheat straw respectively. Total radioactive residues in the soya bean samples from the higher (2 ×) treatment rates were generally twice the above levels while in the high rate (2.6 ×) maize plots, samples generally contained residues two to three times higher than the above.
Metabolites M23, M27 and M30 were identified in the rotational crops, but all at levels below 0.01 mg/kg. Unidentified metabolites were also < 0.01 mg/kg and residues of dimethenamid were not detected in any samples.
These results indicate that the potential exposure of consumers to residues of dimethenamid from rotational crops is insignificant.
While the above crop rotation study was conducted using dimethenamid, the Meeting considered that dimethenamid-P should exhibit the same metabolic profile as the racemic mixture, and agreed that the results of these crop rotation studies could be applied to dimethenamid-P.
Methods of analysis
The Meeting received information on methods for the analysis of dimethenamid and two metabolites (M23 and M27) in plant and animal tissues. The methods developed for dimethenamid do not differentiate between the isomers and are therefore applicable for analysis of matrices treated with either dimethenamid or dimethenamid-P.
Most of the methods reported to the Meeting and used in the supervised residue trials were based on methanol:water extraction and clean-up using reversed phase C18 solid phase extraction columns, partitioning the aqueous eluate with toluene and silica gel column chromatography with ethyl acetate:cyclohexane elution. Analysis in the earlier studies was by CG equipped with thermioinic detector (TSD) and in the later studies, by GC-MS. In animal matrices and most plant matrices, the reported limit of quantification was 0.01 mg/kg, with mean recovery rates of 75% to 105%.
Several earlier methods, designed to measure both the parent compound and the M23 (oxalamide) metabolite also included an additional step to methylate the M23 metabolite by adding diazomethane, but the variable recovery rates in validation studies and in field trials resulted in these methods being discontinued. Supervised residue trials using these methods were not considered in this appraisal.
A multi-residue method, based on the DFG Method S 19 has been developed, involving acetone:water (2:1) extraction, ethyl acetate:cyclohexane (1:1) partitioning, gel permeation and mini silica gel column cleanups and GC-MS analysis. The modification used in this method was the use of ethyl acetate:cyclohexane rather than dichloromethane in the clean-up partitioning step. The reported limit of quantification for this method was 0.01 mg/kg and mean recovery rates were 76-79%.
Stability of pesticide residues in stored analytical samples
The Meeting received information on the stability of dimethenamid and dimethenamid-P in various commodities under freezer storage (-16 to -20 °C). Residue degradation of dimethenamid during storage was less than 20% in maize forage, grain and fodder stored for 21 months, less than 10% in soya bean forage and beans stored for 16 months and no degradation was reported in onion bulbs stored for 9 months. Dimethenamid-P residues did not degrade in spring onion samples stored at -16 °C for 56 weeks.
Definition of the residue
Metabolism studies in animals (goats and hens) and plants (maize, soya beans) indicate that dimethenamid is rapidly and extensively metabolized, with a number of polar metabolites being produced, all at low levels (less than 10% TRR). The metabolic pathway is similar in the crops investigated. Residues of the parent compound were only found at a low level in poultry fat following administration of a highly exaggerated dose rate.
Based on the available comparative animal and soil metabolism studies and noting that the only difference between dimethenamid and dimethenamid-P was in the enantiomer ratio (50:50 vs 90:10), the residue profile and metabolic behaviour of dimethenamid-P is expected to be the same as for dimethenamid.
The available analytical methods to measure dimethenamid residues are also suitable for measuring dimethenamid-P residues, but they do not differentiate between the enantiomers.
The Meeting noted that national residue definitions for dimethenamid and/or dimethenamid-P included:
"dimethenamid, applied as either the 90:10 or 50:50 S:R isomers" (USA)
"dimethenamid-P including other mixtures of constituent isomers (sum of isomers)" (EU)
The Meeting concluded that for both animal and plant commodities, the definition of the residue for compliance with MRLs and estimation of dietary intake should be 'dimethenamid-P and its enantiomer' and noted that this residue definition could apply to residues arising from the use of either dimethenamid-P or dimethenamid.
Results of supervised trials on crops
The Meeting received supervised trials involving dimethenamid on onions (bulb), sweetcorn, beans (dry), soya beans, sugar beet, maize, sorghum and peanuts and trials with dimethenamid-P were also provided for spring onions, potato, sugar beet, maize and grass seed crops.
The Meeting agreed that because dimethenamid-P exhibited the same metabolic behaviour as dimethenamid, the results of trials involving dimethenamid could be applied to dimethenamid-P.
The Meeting also agreed that in trials involving pre-plant or pre-emergence applications and where the mature commodities were sampled at normal commercial harvest, the results could be used to support recommendations for MRLs, irrespective of the PHI used in the trials, since the label claims for these treatment methods were more related to crop growth stages (i.e. crop emergence and harvest) than to the number of days between treatment and harvest. In addition, the Meeting agreed that where the reported residues were below the limits of quantification in trials involving application rates higher than GAP and in the case of post-emergence applications where the PHIs were shorter than GAP, these results could be used to support recommendations for MRLs at the limit of quantification.
For commodities where the supporting trials used in the estimation of maximum residue levels all reported residues below the limit of quantification, even at exaggerated rates, the Meeting, taking into account the results of the plant metabolism studies, agreed to estimate STMRs, median residue levels, HRs and highest residue levels of 0 mg/kg, indicating that residues are not expected.
Onion, bulb
Field trials involving single post-emergence treatments with dimethenamid were made available to the Meeting from the USA. In all trials, residues were below the limit of quantification (0.01 mg/kg).
GAP in USA is for post-emergence use (max 1.1 kg ai/ha, PHI 30 days) and while there were no trials available that matched the USA GAP, the Meeting agreed to use the results from 8 dimethenamid trials from the USA with PHIs matching the USA PHI (30 days) but at higher application rates (1.68 kg ai/ha), since these all reported residues of < 0.01 mg/kg. The combined results were < 0.01 (8) mg/kg.
The Meeting estimated an STMR value of 0 mg/kg and a maximum residue level of 0.01 mg/kg (*) for onion, bulb. The HR was 0 mg/kg.
Garlic
The Meeting noted that GAP existed for dimethenamid-P in USA. This GAP is the same as that established for onion, bulb, and the Meeting agreed that the available residue data for onion, bulb could be extrapolated to garlic.
The Meeting estimated an STMR value of 0 mg/kg and a maximum residue level of 0.01 mg/kg (*) for garlic. The HR was 0 mg/kg.
Shallot
The Meeting noted that GAP existed for dimethenamid-P in USA. This GAP is the same as that established for onion, bulb, and the Meeting agreed that the available residue data for onion, bulb could be extrapolated to shallot.
The Meeting estimated an STMR value of 0 mg/kg and a maximum residue level of 0.01 mg/kg (*) for shallot. The HR was 0 mg/kg.
Spring onion
Field trials (6) involving single post-emergence treatments of dimethenamid-P were provided from Canada and USA, all reporting < 0.01 mg/kg, but no matching GAP information was available for dimethenamid-P.
The Meeting agreed not to estimate a maximum residue level, STMR or HR for spring onion.
Sweet corn
Field trials involving single pre-emergence and post-emergence treatments with dimethenamid were made available to the Meeting from Canada (4), France (2) and USA (14). In all trials, residues were below the limit of quantification in sweetcorn cobs (i.e. kernels plus cobs, without husks).
GAP in USA is for use as either a pre-plant, pre-emergence or post-emergence treatment (max 1.1 kg ai/ha, PHI 50 days). GAP in France and Germany is for pre-emergence use (max 1.0 kg ai/ha, PHI 60 days - France).
While there were no trials available that matched the USA pre-emergence GAP for dimethenamid-P, the Meeting agreed to use the results from 14 trials in USA involving dimethenamid with higher application rates (1.68 kg ai/ha) and with PHIs ranging from 70-98 days, since these all reflected residues in mature corn at harvest and reported residues of < 0.01 mg/kg.
Seven early post-emergence trials in USA involving dimethenamid, matching the USA PHI for dimethenamid-P (50 days), but at rates higher than the USA maximum rate for dimethenamid-P (1.1 kg ai/ha) also reported residues of < 0.01 (7) mg/kg.
The combined results from these pre- and post-emergence trials were < 0.01 (21).
The Meeting estimated an STMR value of 0 mg/kg and a maximum residue level of 0.01 mg/kg (*) for sweet corn (corn-on-the-cob). The HR was 0 mg/kg.
Beans, dry
Field trials involving single pre-plant, pre-emergence and post-emergence treatments with Dimethenamid were made available to the Meeting from Canada and USA. In all trials, residues in dry beans were below the limits of quantification (0.01 mg/kg in the USA trials and 0.02 mg/kg in the Canadian trials).
GAP in USA is for use as either a pre-plant, pre-emergence or early post-emergence treatment, up to 1.1 kg ai/ha, PHI 70 days.
While there were no dimethenamid-P trials available that matched the USA GAP for pre-plant or pre-emergence use, the Meeting agreed to use the results from 22 dimethenamid trials with higher application rates and with PHIs ranging from 76-133 days, since these all reflected residues in beans at harvest and reported residues were all below the limits of quantification. Results of these trials were: < 0.01 (14), < 0.02 (8) mg/kg.
While there were no dimethenamid-P trials available that matched the USA GAP for post-emergent use, the Meeting agreed to use the results from post-emergent dimethenamid trials with higher application rates and PHIs that matched the USA PHI (9 trials) as these were all below the limits of quantification. Reported residues in these trials were < 0.01 (9) mg/kg.
The combined results from these pre-plant, pre-emergence and post-emergence trials were < 0.01 (23), < 0.02 (8) mg/kg.
The Meeting estimated an STMR value of 0 mg/kg and a maximum residue level of 0.01 mg/kg (*) for beans, dry. The high residue was 0 mg/kg.
Soya bean, dry
Field trials involving single pre-plant, pre-emergence and post-emergence treatments with dimethenamid were made available to the Meeting from Canada and USA. In all trials, residues in dry beans were below the limit of quantification (0.01 mg/kg).
GAP in USA is for use as either a pre-plant, pre-emergence or post-emergence treatment, up to 1.1 kg ai/ha (applied from 1st to 3rd trifoliate leaf stage BBCH 12-14).
While there were no trials available that matched the USA GAPs for pre-plant or pre-emergence use, the Meeting agreed to use 18 pre-plant trials and 22 pre-emergence trials from Canada and USA involving dimethenamid at higher application rates of 1.68-3.0 kg ai/ha as these were all below the limits of quantification.
The combined results from these pre-plant and pre-emergence trials were < 0.01 (36), < 0.02 (4) mg/kg.
While there were no trials available that matched the USA GAP for post-emergence use, the Meeting agreed to use the results from 22 post-emergence dimethenamid trials with higher application rates, applied at the 2-4 leaf stage as these were all below the limits of quantification. Reported residues in these trials were < 0.01 (22) mg/kg.
The combined results from these pre-plant, pre-emergence and post-emergence trials were < 0.01 (58), < 0.02 (4) mg/kg.
The Meeting estimated an STMR value of 0 mg/kg and a maximum residue level of 0.01 mg/kg (*) for soya beans, dry. The high residue was 0 mg/kg.
Potato
Field trials involving single pre-plant, pre-emergence and post-emergence treatments with dimethenamid-P were made available to the Meeting from USA. In all trials, residues in tubers were below the limit of quantification (0.01 mg/kg).
GAP in USA is for use as a single pre-emergence treatment, up to 1.1 kg ai/ha with a PHI of 40 days.
One dimethenamid-P trial from USA matched the USA GAP (PHI 40 days) for the pre-emergence use, reporting a residue of < 0.01 mg/kg. Sixteen additional pre-emergence trials from USA, involving longer PHIs (62-128 days), reflecting commercial harvest intervals also reported residues of < 0.01 (16) mg/kg.
In addition, residues were all below the limit of quantification (0.01 mg/kg) in 17 pre-plant USA trials where treatments were made the same day as the above pre-emergence treatments (i.e. the day of planting) and in 34 post-emergence trials from the USA, where tubers were harvested 39-50 days after treatment. While not directly related to the USA GAP (pre-emergence use), the Meeting agreed that these results could be used as supporting data.
The combined results from these pre- and post-emergence trials were < 0.01 (68) mg/kg.
The Meeting estimated an STMR value of 0 mg/kg and a maximum residue level of 0.01 mg/kg (*) for potato. The HR was 0 mg/kg.
Sweet potato
The Meeting noted that GAP existed for dimethenamid-P in the USA. This GAP is the same as that established for potato, and the Meeting agreed that the available residue data for potato could be extrapolated to sweet potato.
The Meeting estimated an STMR value of 0 mg/kg and a maximum residue level of 0.01 mg/kg (*) for sweet potato. The high residue was 0 mg/kg.
Sugar beet
Field trials involving single post-emergent treatments with dimethenamid or dimethenamid-P were made available to the Meeting from France, Germany, Netherlands, Switzerland and USA. In all trials, residues in sugar beet roots were below the limit of quantification (0.01 mg/kg).
GAP in Germany is for a single post-emergent treatment (max 0.65 kg ai/ha), at the 6-8 leaf stage, GAP in Netherlands is for either a single post-emergent treatment (max 0.65 kg ai/ha) or 2-3 split post-emergence applications (max 0.65 kg ai/ha per season). In Belgium, GAP is also for either a single or split (3 applications) post-emergence treatments (max 0.72 kg ai/ha) up to the 8-leaf stage. GAP in USA is also for either a single or split (2 applications) post-emergence treatments (max 1.1 kg ai/ha per season, up to the 12-leaf stage - PHI 60 days).
Four trials in Germany, France and Netherlands, matching the single post-emergence application GAP of Belgium, Germany and Netherlands reported residues of < 0.01 (4) mg/kg and 12 USA post-emergence trials on sugar beet, matching the USA single-application GAP but with longer PHIs that reflect commercial harvest intervals (80-121 days) also reported residues below the limits of quantification. Combined residues in these trials were < 0.01 (16) mg/kg.
In addition, 5 single post-emergence dimethenamid trials from Germany, France and Switzerland with higher application rates but otherwise matching the Belgium GAP, reported residues of < 0.01 (5) and sixteen multiple-application dimethenamid trials in France, Germany and Switzerland, involving rates higher than the split-application Belgian GAP or with more than 3 treatments per season also reported residues of < 0.01 (16) mg/kg. The Meeting agreed to use the results from these post-emergence dimethenamid trials as residues were all below the limit of quantification and the combined results were < 0.01 (21) mg/kg.
The combined results from all the above post-emergence trials involving dimethenamid or dimethenamid-P were < 0.01 (37) mg/kg.
The Meeting estimated an STMR value of 0 mg/kg and a maximum residue level of 0.01 mg/kg (*) for sugar beet. The high residue was 0 mg/kg.
Beetroot
The Meeting noted that GAP existed for dimethenamid-P in beetroot in USA. This GAP is the same as that established for sugar beet, and the Meeting agreed that the available residue data for sugar beet could be extrapolated to beetroot.
The Meeting estimated an STMR value of 0 mg/kg and a maximum residue level of 0.01 mg/kg (*) for beet root. The high residue was 0 mg/kg.
Maize
Field trials involving single pre-plant, pre-emergence and post-emergence treatments with dimethenamid were made available to the Meeting from Belgium, Canada, France, Germany, Greece, Italy, Netherlands, Spain, Switzerland and USA. In all trials, residues in maize (grain) were below the limit of quantification (0.01 mg/kg).
GAP in USA is for use as a single pre-plant or pre-emergence treatment (max 1.1 kg ai/ha), or either a single or double (split-application) post-emergence treatment, with a maximum rate of 1.1 kg ai/ha per season (up to 30cm plant height). GAP in France is for pre-emergence use (max 1.1 kg ai/ha, PHI 90 days), in Germany, Netherlands and Spain GAP is for a single application, either pre-emergence or post-emergence (max 1.0 kg ai/ha) up to the 6-leaf stage, while the GAP in Belgium is for a post-emergence treatment (max 1.0 kg ai/ha) at the 3-4 leaf stage.
While there were no trials available that matched the GAP for pre-plant use in USA the Meeting agreed to use the results from the pre-plant dimethenamid trials (17) in USA and Canada with higher application rates (1.7-3.0 kg ai/ha), as these were all below the limits of quantification. Reported residues in these trials were < 0.01 (17) mg/kg.
While there were no trials available that matched the GAP for pre-emergence use in France, Germany, Netherlands, Spain and USA, the Meeting agreed to use the results of 11 dimethenamid pre-emergence trials from USA and 20 pre-emergence trials from Belgium, France, Germany, Greece, Italy and Netherlands, all involving higher rates than the respective GAPs in USA, Belgium and Italy, all reporting residues below the limit of quantification. Combined residues in these trials were < 0.01 (31) mg/kg.
Four post-emergence trials with dimethenamid-P in Germany, Italy and France, matching the GAP of Belgium, Germany, Netherlands and Spain reported residues of < 0.01 (4). The Meeting agreed to also use the results from 11 USA post-emergence dimethenamid trials involving higher rates but applied at the recommended USA GAP growth stage and 9 trials from Europe with higher application rates but applied at growth stages matching the GAP of Belgium, Germany or Spain as these were all below the limits of quantification. Reported residues in these trials were < 0.01 (20) mg/kg.
The combined results from all of the above pre-plant, pre-emergence and post-emergence trials with dimethenamid or dimethenamid-P were < 0.01 (72) mg/kg.
The Meeting estimated an STMR value of 0 mg/kg and a maximum residue level of 0.01 mg/kg (*) for maize. The high residue was 0 mg/kg.
Sorghum
Field trials involving single pre-emergence and post-emergence treatments with dimethenamid were made available to the Meeting from USA. In all trials, residues in sorghum grain were below the limit of quantification (0.01 mg/kg).
GAP in USA is for use as either a single pre-plant, pre-emergence or post-emergence treatment, or as split pre-plant/pre-emergence treatments, up to 1.1 kg ai/ha per season, PHI 80 days.
While there were no trials available that matched the USA GAP for pre-emergence use for dimethenamid-P, the Meeting agreed to use the results from pre-emergence dimethenamid trials (14) with higher application rates and longer PHIs (106-155 days), reflecting commercial harvest intervals, with the reported residues in these trials being < 0.01 (14) mg/kg.
While there were no trials available that matched the USA GAP for post-emergence use, the Meeting agreed to use the results from post-emergence dimethenamid trials with higher application rates that matched the USA GAP PHI (8 trials) but with higher application rates (1.68 kg ai/ha) as these were all below the limits of quantification. Reported residues in these trials were < 0.01 (8) mg/kg.
The combined results from the above pre-emergence and post-emergence trials with dimethenamid were < 0.01 (22) mg/kg.
The Meeting estimated an STMR value of 0 mg/kg and a maximum residue level of 0.01 mg/kg (*) for sorghum. The high residue was 0 mg/kg.
Peanut
Field trials involving single pre-emergence and post-emergence treatments with dimethenamid were made available to the Meeting from USA. In all trials, residues in peanut (nuts without shells) were below the limit of quantification (0.01 mg/kg).
GAP in the USA is for a single pre-plant, pre-emergence or post-emergence treatment, or as split pre-plant/pre-emergence treatments (max 1.1 kg ai/ha/season, PHI 80 days).
While there were no trials available that matched the USA GAP for pre-emergence use, the Meeting agreed to use the results from pre-emergence dimethenamid trials (14) with higher application rates and longer PHIs (121-145 days), reflecting commercial harvest intervals, with the reported residues in these trials being < 0.01 (14) mg/kg.
While there were no trials available that matched the USA GAP for post-emergence use, the Meeting agreed to use the results from 14 post-emergence dimethenamid trials with higher application rates that matched the USA GAP PHI, as these were all below the limits of quantification. Reported residues in these trials were < 0.01 (14) mg/kg.
The combined results from the above pre-emergence and post-emergence trials with dimethenamid were < 0.01 (28) mg/kg.
The Meeting estimated an STMR value of 0 mg/kg and a maximum residue level of 0.01 mg/kg (*) for peanut. The high residue was 0 mg/kg.
Animal feed commodities
Bean forage
Field trials involving single pre-plant, pre-emergence and post-emergence treatments with dimethenamid were made available to the Meeting from Canada and USA. Residues in bean forage were below the limits of quantification (0.01 mg/kg in the USA trials and 0.02 mg/kg in the Canadian trials) except in young plants (at the 6-8 leaf stage (BBCH16-18)) sampled 12-18 days after a late post-emergence treatment.
GAP in USA is for use as either a pre-plant, pre-emergence or post-emergence treatment (max 1.1 kg ai/ha). The PHI for beans is 70 days, with post-emergence use being from 1st to 3rd trifoliate leaf stage BBCH 13-14 Crop stage.
While there were no trials available that matched the USA GAP for pre-plant or pre-emergence use, the Meeting agreed to use the results from 5 pre-plant and 17 pre-emergence dimethenamid trials from Canada and USA with higher application rates (1.3-2.7 kg ai/ha) since the reported residues were all below the limits of quantification. Reported residues in these trials were < 0.01 (14), < 0.02 (8) mg/kg.
There were no trials available that matched the USA GAP for post-emergence use, and the Meeting agreed to use the results from 14 USA trials involving dimethenamid with higher application rates (1.68 kg ai/ha) as residues in mature bean forage (i.e. just before senescence) all reported residues below the limit of quantification. Residues in these trials were < 0.01 (14).
The combined results from these pre-plant, pre-emergence and post-emergence trials were < 0.01 (28), < 0.02 (8) mg/kg.
The Meeting estimated a median residue of 0 mg/kg and a high residue of 0 mg/kg for bean forage.
Bean fodder
Field trials involving single pre-plant, pre-emergence and post-emergence treatments with dimethenamid were made available to the Meeting from Canada and USA. Residues in bean fodder were below the limits of quantification (0.01 mg/kg in the USA trials and 0.02 mg/kg in the Canadian trials)
GAP in USA is for use as either a pre-plant, pre-emergence or post-emergence treatment, up to 1.1 kg ai/ha. The PHI for beans is 70 days, with post-emergence use being from 1st to 3rd trifoliate leaf stage.
While there were no trials available that matched the USA GAP for pre-plant or pre-emergence use, the Meeting agreed to use the results from 22 dimethenamid trials with higher application rates and with PHIs ranging from 76-133 days, since these all reflected residues in bean fodder at harvest and reported residues were all below the limits of quantification. Results of these trials were: < 0.01 (14), < 0.02 (8) mg/kg.
While there were no trials available that matched the USA GAP for post-emergence use, the Meeting agreed to use the results from post-emergence dimethenamid trials with higher application rates and PHIs that matched the USA GAP (14 trials) as these were all below the limits of quantification. Reported residues in these trials were < 0.01 (14) mg/kg.
The combined results from these pre-plant, pre-emergence and post-emergence trials were < 0.01 (28), < 0.02 (8) mg/kg.
The Meeting estimated a median residue of 0 mg/kg and a maximum residue level of 0.01 (*) mg/kg for bean fodder. The highest residue was 0 mg/kg.
Peanut forage
Field trials involving single pre-emergence and post-emergence treatments with dimethenamid were made available to the Meeting from USA. In all trials, residues in peanut forage were below the limit of quantification (0.01 mg/kg).
GAP in USA is for use as either a single pre-plant, pre-emergence or post-emergence treatment, or as split pre-plant/pre-emergence treatments, up to 1.1 kg ai/ha per season, PHI 80 days (hay or straw).
While there were no trials available that matched the USA GAP for pre-emergence use, the Meeting agreed to use the results from pre-emergence dimethenamid trials (14) with higher application rates and longer PHIs (121-145 days), reflecting commercial harvest intervals, with the reported residues in these trials being < 0.01 (14) mg/kg.
While there were no trials available that matched the USA GAP for post-emergence use, the Meeting agreed to use the results from 14 post-emergence dimethenamid trials with higher application rates that matched the USA GAP PHI, as these were all below the limits of quantification. Reported residues in these trials were < 0.01 (14) mg/kg.
The combined results from the pre-emergence and post-emergence trials with dimethenamid were < 0.01 (28) mg/kg.
The Meeting estimated a median residue of 0 mg/kg and a highest residue of 0 mg/kg for peanut forage.
Peanut fodder
Field trials involving single pre-emergence and post-emergence treatments with dimethenamid were made available to the Meeting from USA. In all trials, residues in peanut fodder were below the limit of quantification (0.01 mg/kg).
GAP in USA is for use as either a single pre-plant, pre-emergence or post-emergence treatment, or as split pre-plant/pre-emergence treatments, up to 1.1 kg ai/ha per season, PHI 80 days (hay or straw).
While there were no trials available that matched the USA GAP for pre-emergence use, the Meeting agreed to use the results from pre-emergence dimethenamid trials (14) with higher application rates and longer PHIs (121-145 days), reflecting commercial harvest intervals, with the reported residues in these trials being < 0.01 (14) mg/kg.
While there were no trials available that matched the USA GAP for post-emergence use, the Meeting agreed to use the results from 14 post-emergence dimethenamid trials with higher application rates that matched the USA GAP PHI, as these were all below the limits of quantification. Reported residues in these trials were < 0.01 (14) mg/kg.
The combined results from the pre-emergence and post-emergence trials with dimethenamid were < 0.01 (28) mg/kg.
The Meeting estimated an STMR value of 0 mg/kg and a maximum residue level of 0.01 mg/kg (*) for peanut fodder. The highest residue was 0 mg/kg.
Soya bean forage and fodder
Field trials involving single pre-plant, pre-emergence and post-emergence treatments with dimethenamid were made available to the Meeting from Canada and USA. GAP in USA is for use as either a pre-plant, pre-emergence or post-emergence treatment, up to 1.1 kg ai/ha but with a restriction that treated soya bean forage, hay or straw must not be fed to livestock.
The Meeting agreed not to estimate STMRs, maximum residue levels or highest residues for soya bean forage (green) or soya bean fodder.
Fodder beet
The Meeting noted that GAP existed for use on fodder beet in Belgium and Netherlands. These GAPs were the same as those established for sugar beet, and the Meeting agreed that the available residue data for sugar beet could be extrapolated to fodder beet.
The Meeting estimated an STMR value of 0 mg/kg and a maximum residue level of 0.01 mg/kg (*) for fodder beet. The highest residue was 0 mg/kg.
Hay or fodder (dry) of grasses
Field trials on perennial grass seed crops, involving single post-emergence treatments with dimethenamid-P were made available to the Meeting from the USA. GAP in the USA is for use as post-emergence treatment, up to 1.1 kg ai/ha but with a restriction that livestock must not be grazed on treated areas and that treated grasses, forage, hay, silage, straw, seed or seed screenings must not be fed to livestock.
The Meeting agreed not to estimate STMRs, maximum residue levels or highest residues for hay or fodder (dry) of grasses.
Maize forage
Field trials involving single pre-plant, pre-emergence and post-emergence treatments with dimethenamid (number) and dimethenamid-P (6) were made available to the Meeting from Belgium, Canada, France, Germany, Greece, Italy, Netherlands, Spain, Switzerland and USA. Residues in maize forage were below the limit of quantification (0.01 mg/kg) in all pre-plant and pre-emergence trials. Residues were detected in some post-emergence trials, ranging from 0.01 mg/kg to 0.04 mg/kg in samples taken 21-43 days after treatment.
GAP in USA is for use as a single pre-plant or pre-emergence treatment (max 1.1 kg ai/ha), or either a single or 2 split-applications post-emergence, with a maximum rate of 1.1 kg ai/ha per season, PHI 40 days. GAP in Germany, Netherlands and Spain is for a single application, either pre-emergence or post-emergence (max 1.0 kg ai/ha) while GAP in Belgium is for a post-emergence treatment, up to 1.0 kg ai/ha and GAP in France is for a pre-emergence use (max 1.1 kg ai/ha, PHI 90 days).
While there were no trials available that matched the USA GAPs for pre-plant and pre-emergence uses, the Meeting agreed to use the results from the pre-plant and pre-emergence dimethenamid trials with higher application rates, as these were all below the limits of quantification.
Trials with dimethenamid from Canada (6) and USA (11), involving higher pre-plant application rates of 1.68-3.0 kg ai/ha and longer PHIs (56-70 days) that reflected commercial forage intervals, reported residues of < 0.01 (17) mg/kg.
Sixteen pre-emergence trials from USA and Canada, involving dimethenamid application rates higher than the USA GAP and with longer PHIs (56-69 days) that reflected commercial forage harvest intervals reported residues of < 0.01 (16). Dimethenamid pre-emergence trials (14) in France, Italy, Spain and Switzerland using rates higher than the GAP of Germany, Netherlands, France and Spain and with PHIs that reflected commercial forage harvest intervals (of about 60-90 days), reported residues of < 0.01 (14) mg/kg.
Six post-emergence trials involving dimethenamid-P in Germany, Italy and France, matching the GAP of Belgium, Germany, Netherlands and Spain, with PHIs of 21-47 days, reported residues of < 0.01 (3), 0.02, 0.03 and 0.04 mg/kg.
The Meeting agreed to combined results from these pre-plant and pre-emergence trials with dimethenamid and the post-emergence trials with dimethenamid-P to give a residue data set of < 0.01 (50), 0.02, 0.03 and 0.04 mg/kg.
Based on a dry matter content of 40%, the Meeting estimated a median residue of 0.025 mg/kg and a highest residue of 0.1 mg/kg for maize forage.
Maize fodder
Field trials involving single pre-plant, pre-emergence and post-emergence treatments with dimethenamid and dimethenamid-P (6) were made available to the Meeting from Belgium, Canada, France, Germany, Greece, Italy, Netherlands, Spain, Switzerland and USA. Residues in maize fodder were below the limit of quantification (0.01 mg/kg) in all trials except one residue of 0.01 mg/kg in fodder treated with dimethenamid, 118 days after a post-emergence treatment (1.43 kg ai/ha) in Belgium.
GAP in USA is for use as a single pre-plant or pre-emergence treatment (max 1.1 kg ai/ha), or either a single or 2 split-applications post-emergence, with a maximum rate of 1.1 kg ai/ha per season, PHI 40 days. GAP in Germany, Netherlands and Spain is for a single application, either pre-emergence or post-emergence (max 1.0 kg ai/ha) while the GAP in Belgium is for a post-emergence treatment, up to 1.0 kg ai/ha and GAP in France is for a pre-emergence use (max 1.1 kg ai/ha, PHI 90 days).
While there were no trials available that matched the GAP for pre-plant use in USA, the Meeting agreed to use the results from the pre-plant dimethenamid trials (17) in USA and Canada with higher application rates (1.7-3.0 kg ai/ha), as these were all below the limits of quantification. Reported residues in these trials were < 0.01 (17) mg/kg.
While there were no trials available that matched the GAP for pre-emergence use in France, Germany, Netherlands, Spain and in USA, the Meeting agreed to use the results of 17 dimethenamid pre-emergence trials from USA and Canada and 8 trials from France, Germany and Switzerland, all involving higher rates than the respective GAPs in USA, Belgium and Italy and all below the limit of quantification. Reported residues in these trials were < 0.01 (25) mg/kg.
Six post-emergence trials in Belgium, Germany, Italy, Netherlands and France, matching the GAPs of Belgium, Germany, Netherlands and Spain, with PHIs of 78-114 days, reported residues of < 0.01 (6).
The Meeting agreed to combined results from these pre-plant and pre-emergence trials with dimethenamid and the post-emergence trials with dimethenamid-P to give a residue data set of < 0.01 (48) mg/kg.
The Meeting estimated an STMR value of 0 mg/kg and a maximum residue level of 0.01mg/kg (*) for maize fodder. The highest residue was 0 mg/kg.
Sorghum forage (green)
Field trials involving single pre-emergence and post-emergence treatments with dimethenamid were made available to the Meeting from USA. In all trials, residues in sorghum forage were below the limit of quantification (0.01 mg/kg).
GAP in USA is for use as either a single pre-plant, pre-emergence or post-emergence treatment, or as split pre-plant/pre-emergence treatments, up to 1.1 kg ai/ha per season, PHI 60 days.
While there were no trials available that matched the USA GAP for pre-emergence use, the Meeting agreed to use the results from pre-emergence dimethenamid trials (14) with higher application rates and longer PHIs (59-107 days), reflecting commercial harvest intervals, with the reported residues in these trials being < 0.01 (14) mg/kg.
While there were no trials available that matched the USA GAP for post-emergence use, the Meeting agreed to use the results from 11 post-emergence dimethenamid trials with higher application rates that matched the USA PHI (60 days), as these were all below the limits of quantification. Reported residues in these trials were < 0.01 (11) mg/kg.
The combined results from these pre-emergence and post-emergence trials with dimethenamid were < 0.01 (25) mg/kg.
The Meeting estimated a median residue of 0 mg/kg and a highest residue of 0 mg/kg for sorghum forage.
Sorghum straw and fodder, dry
Field trials involving single pre-emergence and post-emergence treatments with dimethenamid were made available to the Meeting from USA. In all trials, residues in sorghum fodder were below the limit of quantification (0.01 mg/kg).
GAP in USA is for use as either a single pre-plant, pre-emergence or post-emergence treatment, or as split pre-plant/pre-emergence treatments, up to 1.1 kg ai/ha per season, PHI 80 days.
While there were no trials available that matched the USA GAP for pre-emergence use, the Meeting agreed to use the results from pre-emergence dimethenamid trials (14) with higher application rates and longer PHIs (106-155 days), reflecting commercial harvest intervals, with the reported residues in these trials being < 0.01 (14) mg/kg.
While there were no trials available that matched the USA GAP for post-emergence use, the Meeting agreed to use the results from post-emergence dimethenamid trials with higher application rates that matched the USA GAP PHI as these were all below the limits of quantification. Reported residues in these trials were < 0.01 (8) mg/kg.
The combined results from these pre-emergence and post-emergence trials with dimethenamid were < 0.01 (22) mg/kg.
The Meeting estimated an STMR value of 0 mg/kg and a maximum residue level of 0.01 mg/kg (*) for sorghum fodder. The highest residue was 0 mg/kg.
Sugar beet leaves or tops
Sugar beet field trials involving single post-emergence treatments with dimethenamid or dimethenamid-P were made available to the Meeting from France, Germany, Netherlands, Switzerland and USA. In all trials, residues in sugar beet leaves or tops were below the limit of quantification (0.01 mg/kg) within 30 days after treatment.
GAP in Germany is for a single post-emergence treatment (max 0.65 kg ai/ha) at the 6-8 leaf stage (BBCH 16-18), GAP in Netherlands is for either a single post-emergence treatment (max 0.65 kg ai/ha) or 2-3 split post-emergence applications (max 0.65 kg ai/ha per season). In Belgium, GAP is also for either a single or split (3 applications) post-emergence treatments (max 0.72 kg ai/ha) up to the 8-leaf stage (BBCH 18). GAP in USA is also for either a single or split (2 applications) post-emergence treatments (max 1.1 kg ai/ha per season) up to the 12-leaf stage - PHI 60 days).
Four trials in Germany, France and Netherlands, matching the single post-emergence application GAP of Belgium, Germany and Netherlands reported residues of < 0.01 mg/kg and 12 USA post-emergence trials on sugar beet, matching the USA single-application GAP but with longer PHIs that reflect commercial harvest intervals (80-121 days) also reported residues below the limits of quantification. Combined residues in these trials were < 0.01 (16) mg/kg. In addition, six single-application dimethenamid trials from Germany, France and Switzerland with higher application rates but otherwise matching Belgian GAP, reported residues of < 0.01 (6) mg/kg.
Sixteen multiple-treatment post-emergence dimethenamid trials in France, Germany and Switzerland, involving rates higher than the split-application Belgian GAP or with more than 3 treatments per season also reported residues of < 0.01 mg/kg.
The Meeting agreed to use the results from these single and split-application post-emergence dimethenamid trials as residues were all below the limit of quantification and the combined results were < 0.01 (22) mg/kg.
The combined results from these single or split-application post-emergence trials with dimethenamid-P or dimethenamid were < 0.01 (38) mg/kg.
The Meeting estimated a median residue of 0 mg/kg and a highest residue of 0 mg/kg for sugar beet leaves or tops.
Fodder beet leaves or tops
The Meeting noted that GAP existed in Belgium and Netherlands for fodder beet at the same GAPs established for sugar beet, and agreed that the available residue data for sugar beet could be extrapolated to fodder beet.
The Meeting estimated a median residue of 0 mg/kg and a highest residue of 0 mg/kg for fodder beet leaves or tops.
Fate of residues in storage and during processing
The effect of processing on the level of residues of dimethenamid-P in potatoes and of dimethenamid in soya beans and maize were reported to the Meeting.
Potatoes from a USA field trial where dimethenamid-P was applied twice at an exaggerated (5x) rate of 3.5 kg ai/ha, pre-emergence and post-emergence (PHI 40 days), were processed into chips and flakes using procedures that reflected commercial practice. Dimethenamid residues were not found (LOQ 0.01 mg/kg) in either the initial tubers or in any of the processing fractions (wet peel, chips and flakes).
Soya beans from two USA field trials where dimethenamid was applied pre-emergence at an exaggerated (5×) rate of 8.4 kg ai/ha were processed into oil using procedures that reflected commercial practice. Dimethenamid residues were not found (LOQ 0.01 mg/kg) in either the unprocessed beans or in any of the processing fractions (including hulls, meal, soap stock, crude lecithin, crude oil and refined oil).
Maize from two USA field trials where dimethenamid was applied as either pre-plant or pre-emergence treatments at an exaggerated (5×) rate of 8.4 kg ai/ha was processed into flour, meal and oil using both dry and wet procedures that reflected commercial practice. Dimethenamid residues were not found (LOQ 0.01 mg/kg) in either the unprocessed grain or in any processing fractions (including dust, grits, meal, flour, press cake, soap stock, crude oil and refined oil).
Farm animal dietary burden
The Meeting estimated the dietary burden of dimethenamid-P residues in cattle and poultry on the basis of the diets listed in Appendix IX of the FAO Manual (FAO, 2002). Calculations from highest residues provide the levels in feed suitable for estimating animal commodity MRLs, while calculations from STMR or median residue values for feed are suitable for estimating STMRs.
Detectable residues were only reported in maize forage (median residue level of 0.01 mg/kg dry matter, highest residue level 0.1 mg/kg dry matter) and residues were below the limit of quantification (0.01 mg/kg) in all other animal feed commodities considered by the Meeting (STMRs or median residue levels of 0 mg/kg and highest residues of 0 mg/kg).
Estimated maximum dietary burden of farm animals
|
|
Diet content (%) |
Residue contribution, mg/kg |
|||||||||
|
Commodity |
Group |
Residue (mg/kg) |
Basis |
% DM |
Residue ¸ DM |
Beef cattle |
Dairy cows |
Poultry |
Beef cattle |
Dairy cows |
Poultry |
|
Maize forage |
AF |
0.04 |
Highest |
40 |
0.1 |
40 |
50 |
- |
0.04 |
0.05 |
- |
|
TOTAL |
|
|
|
|
|
40 |
50 |
0 |
0.04 |
0.05 |
0 |
Estimated median dietary burden of farm animals
|
|
Diet content (%) |
Residue contribution, mg/kg |
|||||||||
|
Commodity |
Group |
Residue (mg/kg) |
Basis |
% DM |
Residue ¸ DM |
Beef cattle |
Dairy cows |
Poultry |
Beef cattle |
Dairy cows |
Poultry |
|
Maize forage |
AF |
0.01 |
Median |
40 |
0.025 |
40 |
50 |
- |
0.01 |
0.013 |
- |
|
TOTAL |
|
|
|
|
|
40 |
50 |
0 |
0.01 |
0.013 |
0 |
The total dietary burdens for animal commodity MRL estimation (residue levels in animal feeds expressed on dry weight) are 0.04 ppm for beef cattle, 0.05 ppm for dairy cattle, and 0 ppm for poultry. The associated median dietary burden for STMR estimation are 0.01 ppm (beef cattle), 0.013 ppm (dairy cattle) and 0 ppm (poultry).
Animal commodity maximum residue levels
The Meeting noted that in the goat metabolism study, no residues of dimethenamid were found in milk, muscle, fat, liver or kidney of goats dosed for four days with the equivalent of 223 ppm dimethenamid in the diet. As this dosing level is more than 4000 times higher than the maximum estimated dietary burden (0.05 ppm) arising from the uses of dimethenamid-P, the Meeting agreed that residues would not be expected in livestock and estimated STMRs and HRs of 0 mg/kg for meat (from mammals other than marine mammals), edible offal, mammalian and milks.
The Meeting estimated maximum residue levels of 0.01 (*) mg/kg for meat (from mammals other than marine mammals); 0.01 (*) mg/kg for edible offal, mammalian and 0.01 (*) mg/kg for milks.
For poultry, the estimated dietary burden is 0 ppm and the Meeting estimated STMRs and HRs of 0 mg/kg for poultry meat, poultry, edible offal and eggs.
The Meeting estimated maximum residue levels of 0.01 (*) mg/kg for poultry meat; 0.01 (*) mg/kg for poultry edible offal of, and 0.01 (*) mg/kg for eggs.
DIETARY RISK ASSESSMENT
The evaluation of dimethenamid-P has resulted in recommendations for MRLs at the limit of quantification with STMRs and HRs of 0 mg/kg for raw and processed commodities. The Meeting concluded that the long-term and short-term intake of residues of dimethenamid-P from uses that have been considered by the JMPR do not present a public health concern.
TOXICOLOGY
Ethoxyquin is the ISO approved name for 1,2-dihydro-6-ethoxy-2,2,4-trimethylquinoline. It is used primarily as an antioxidant preservative in animal feed and dehydrated storage forage crops and as an antiscald agent in pears and apples. It is also used as a colour preservative in spices and as an anti-degradation agent in rubber. Ethoxyquin was first evaluated by the Meeting in 1969, when an ADI of 0-0.06 mg/kg bw was established based on the NOAEL in a long-term feeding study in dogs and a study of reproductive toxicity in rats. It was re-evaluated in 1998 within the periodic review programme of CCPR, and an ADI of 0-0.005 mg/kg bw was established on the basis of the minimal-effect level of 2.5 mg/kg bw per day for clinical signs and deposition of pigments in liver in a multigeneration study in dogs, and a 500-fold safety factor to account for the lack of a NOAEL in this study and for the incompleteness of the database. The 1998 Meeting concluded there was no need to establish an ARfD for ethoxyquin. In 1999, the Joint Meeting reviewed the residue chemistry of ethoxyquin and concluded that the plant metabolites/degradation products, C-N and N-N dimers, demethylethoxyquin (DMEQ), methylethoxyquin (MEQ), dehydromethylethoxyquin (DHMEQ) and dihydroethoxyquin (DHEQ) were not formed in rats. In 2000, the Meeting recommended that information on the acute toxicity and genotoxicity of the plant metabolites/degradation products would be necessary to complete the evaluation of ethoxyquin.
The Meeting reviewed new data on the genotoxicity and acute toxicity of ethoxyquin and three of its plant metabolites/degradation products (MEQ, DHMEQ and DHEQ) in dogs, relevant data from previous evaluations and other information from the published literature. DMEQ was not sufficiently stable to permit its synthesis and study.
All the new studies submitted for consideration at the present Meeting complied with GLP.
Biochemical aspects
Previous evaluations have established that ethoxyquin is rapidly absorbed from the gastrointestinal tract of rats and mice, with peak blood concentrations occurring within 1 h. The highest tissue concentrations were found in liver, kidney and adipose tissue. Excretion is predominantly as metabolites via the urine and is rapid, with > 85% of doses of up to 25 mg/kg bw being eliminated within 24 h.
Toxicological data
Previous evaluations have reported that ethoxyquin has low acute toxicity when administered orally (LD50 = 1700 mg/kg bw), dermally (LD50 > 2000 mg/kg bw) or by inhalation(LC50 > 2 mg/L) in rats.
In studies in dogs given ethoxyquin and three plant metabolites/degradation products (MEQ, DHMEQ and DHEQ) as single oral doses, the main target for all four compounds was the liver. Dogs were used in preference to rats as previous studies had shown that dogs are more sensitive to the toxic effects of ethoxyquin.
Dogs were fed capsules containing ethoxyquin, MEQ, DHMEQ or DHEQ as single doses at 50 to 200 mg/kg bw. Ethoxyquin, MEQ and DHEQ caused increases in serum and urinary concentrations of bilirubin at all doses. DHEQ had marginal effects on bilirubin concentrations at the highest dose. Ethoxyquin and MEQ produced changes in the liver indicative of bile stasis and/or accumulation of bile pigment. Similar changes were reported previously in longer-term studies with ethoxyquin. During the 2-week recovery period (two dogs of each sex per group) elevations in serum enzymes for liver function (aspartate aminotransferase and alanine aminotransferase) were noted. After administration of the three metabolites/degradation products, clinical signs, including emesis and oral discharge, were noted. On the basis of the information available, the rank order of the toxic potency for the four compounds was MEQ > ethoxyquin > DHEQ > DHMEQ. The effects observed at 50 mg/kg were minimal to mild, and their toxicological significance is equivocal. The presence of dark-coloured urine at the lowest dose of the compounds was attributed to the presence of a chromophore in the compound or a derivative thereof. The Meeting did not consider that this was toxicologically significant. The Meeting concluded that the NOAEL for all four compounds was 50 mg/kg bw.
Ethoxyquin and the three plant metabolites/degradation products were evaluated for genotoxicity in an adequate range of tests in vitro and in vivo. All compounds gave negative results in tests for mutagenicity in bacteria, with and without metabolic activation, confirming previous published reports on ethoxyquin. In a test for chromosomal aberrations in Chinese hamster ovary cells, all four compounds gave positive results. Ethoxyquin also gave positive results in a published study in which it was tested for chromosomal aberrations in isolated human peripheral blood lymphocytes. Although there have been positive findings for clastogenicity in vitro, all four compounds gave negative results in a test for micronucleus formation in bone-marrow cells of mice in vivo. This confirms the results of a previous published study of macronucleus formation with ethoxyquin in bone marrow. It has also been reported in a published report that ethoxyquin gave negative results in tests for chromosomal aberrations and for sister chromatid exchange in vivo.
The Meeting concluded that ethoxyquin and the three plant metabolites/degradation products tested do not represent a genotoxic risk in vivo.
The 1969 and 1998 Meetings reviewed a number of published reports in which ethoxyquin had been administered to rodents for a prolonged period of time. These did not reveal any potential for ethoxyquin to produce a tumourigenic response.
In the absence of DNA reactivity and clastogenic effects in vivo and absence of tumours in rodents, the Meeting considered it unlikely that dietary exposures to this compound would pose any carcinogenic risk to humans.
The 1969 Meeting evaluated three studies of reproductive toxicity in which rats received diets containing ethoxyquin at concentrations of up to 1125 ppm. All had non-standard protocols, and the results were contradictory. Two of the studies, including the most extensive, showed no apparent effects on the end-points studied at up to the maximum concentration tested (equivalent to 56 mg/kg bw per day), while the other showed an increased incidence of stillbirths at 1126 ppm and decreased litter size at 375 ppm. The Meeting concluded that the design and reporting of these studies were inadequate.
The 1998 Meeting evaluated a two-generation study of reproductive toxicity in dogs given diets containing ethoxyquin at a concentration of 0, 100, or 225 ppm. There was no effect on reproductive parameters at up to the highest concentration tested (equivalent to 5.6 mg/kg bw per day). Clinical signs observed included dehydration and excess lachrymation. There was evidence of hepatic toxicity, particularly in the females. The effects were seen at 100 ppm, the lowest concentration tested, and were consistent with effects observed in short-term studies in dogs. The lowest concentration tested, 100 ppm (equivalent to 2.5 mg/kg bw per day) was considered to be a minimal-effect level for clinical signs of toxicity and liver effects.
A study of developmental toxicity in rats was evaluated by the 1998 JMPR. Rats were treated with ethoxyquin at doses of up to 350 mg/kg bw per day by gavage. Ethoxyquin was not fetotoxic or teratogenic at doses up to the highest tested. The NOAEL for maternal toxicity was 50 mg/kg bw per day on the basis of reduced body-weight gain at higher doses. No studies of developmental toxicity had been performed in other species.
Toxicological evaluation
The 1998 JMPR established an ADI of 0-0.005 mg/kg bw based on the minimal-effect level of 2.5 mg/kg bw per day for clinical signs in a multigeneration study in dogs and a safety factor of 500, because there was no NOAEL in this study and the database was incomplete owing to the lack of studies of genotoxicity and long-term studies of toxicity. No additional information was available to this Meeting on the long-term effects of ethoxyquin, although information on the genotoxicity of ethoxyquin and its three metabolites had been provided. It was concluded that these compounds were not genotoxic in vivo. The acute toxicity of the plant metabolites/degradation products DHEQ and DHMEQ was no greater than that of ethoxyquin. The toxicity of the plant metabolite/degradation product MEQ appeared to be slightly greater than that of ethoxyquin. However, the Meeting concluded that a safety factor of 500 would be sufficient to allow for this difference in toxicity. Hence the Meeting confirmed the ADI established by the 1998 JMPR and extended it to cover the three plant metabolites/degradation products, MEQ, DHMEQ and DHEQ.
On the basis of the acute effects of ethoxyquin and its plant metabolites/degradation products in dogs, the Meeting established an ARfD of 0.5 mg/kg bw based on a NOAEL of 50 mg/kg bw for effects on the hepatic biliary system and clinical signs at higher doses from a study in dogs given single doses, and a safety factor of 100. The studies of reproductive toxicity in rats were not considered to be an adequate basis for the derivation of an ARfD. The ARfD established applies to ethoxyquin and to the three plant metabolites/degradation products, MEQ, DHMEQ and DHEQ. It is applicable to the whole population.
An addendum to the toxicological monograph was prepared.
Levels relevant to risk assessment
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Rat |
Developmental toxicitya |
Maternal toxicity |
50 mg/kg bw per day |
150 mg/kg bw per day |
|
Fetotoxicity |
350 mg/kg bw per dayd |
- |
||
|
Dog |
1-year study of toxicitya |
General toxicity |
3 mg/kg bw per day |
10 mg/kg bw per day |
|
Two-generationb |
General toxicity |
- |
100 ppm equivalent 2.5 mg/kg bw per daye |
|
|
Reproductive performance |
225 ppm, equivalent 5.6 mg/kg bw per dayd |
- |
||
|
Single oral dosec study with parent and plant metabolites |
Toxicity |
50 mg/kg bw per day |
100 mg/kg bw per day |
a Gavage administration
b Dietary administration
c Capsule
d Highest dose tested
e Marginal effects of equivocal toxicological relevance on brain acetylcholinesterase activity
Estimate of acceptable daily intake for humans
0-0.005 mg/kg bw, applicable to ethoxyquin, MEQ, DHMEQ and DHEQ
Estimate of acute reference dose
0.5 mg/kg bw, applicable to ethoxyquin, MEQ, DHMEQ and DHEQ
Information that would be useful for the continued evaluation of the compound
Results from epidemiological, occupational health and other such observational studies of human exposures.