Colin A.
Simpfendorfer
Center for Shark
Research, Mote Marine Laboratory
1600 Ken Thompson
Parkway
Sarasota, Florida,
34236, USA
<[email protected]>
9.1 INTRODUCTION
Information on the status of shark populations and how they respond to increases in mortality (e.g. from fishing, predation, disease), is critical to making management decisions about fished or endangered species. It is no surprise then that a considerable part of the fish and fisheries literature is devoted to this type of research. In the ideal situation long-term series of information about a population - catches, fishing effort, change in abundance -exist. In this situation dynamic fishery models can be applied to derive extensive management related information. However, in many situations the data required to support these types of management models do not exist. This is often the case with shark populations, where the collection of these data has been uneconomical or overlooked. In this situation population models that rely primarily on life history parameters can provide some useful information for management. Such models are normally referred to as demographic models. These demographic analyses became popular for shark stocks in the 1990s and are the most widely used form of population model for this group of fishes (Hoenig and Gruber, 1990; Cailliet, 1992; Cortes, 1995, 1998, 1999, 2002; Cortes and Parsons, 1996; Smith, Au and Show, 1998; Simpfendorfer, 1999a,b; Brewster-Geisz and Miller, 2000; Mollet and Cailliet, 2002).
The main parameter estimated by demographic analysis is the intrinsic rate of population increase (r), the measure of potential for growth rate in a population. There are two different techniques for estimating r -life tables and matrix models. Life tables are based on the Euler-Lotka equation:
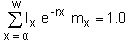 | (9.1) |
where lx is the survival to age x, mx is the fecundity at age x (female pups per female), a is the age at maturity, and w is the maximum reproductive age. The life table is a way of keeping track of the age-specific mortality and reproductive rates, and estimating r.
The second technique uses matrix algebra to estimate the finite rate of population increase (λ) from reproductive and mortality data. The finite rate of population growth and the intrinsic rate of population growth are related via the simple relationship
| λ = er | (9.2) |
Matrix methods can be applied to age-structured and stage-based data.
It is interesting to note that both life tables and matrix models were introduced to ecologists by the same person -P.H. Leslie (after whom the age-structured matrix model is named) -in the 1940s (Caswell, 2001). Life tables immediately became popular and were used extensively. However, matrix models did not gain favour with ecologists until the 1970s and have since become extremely popular. The slow rise in popularity of matrix models was probably the result of the need for an understanding of matrix algebra and the extra computational requirements. The increased availability of computers enabled researchers to overcome these drawbacks and embrace this powerful technique.
In this Section I review the use of life table and matrix approaches in modeling shark populations. I restrict this consideration to static assessments of populations. Both life table and matrix approaches can be used to develop dynamic models of populations, but in the shark literature they have been largely restricted to static assessments due to the lack of time-series data. For more general overviews of life table methods several general ecology books provide a more thorough consideration (Krebs, 1985), and for matrix models the revised “Matrix Population Models”(Caswell, 2001) is the authoritative text. In addition to the simple life table approach, I also describe a method developed by Au and Smith (1997) that estimates the rebound potential of a population. This method is based on the life table approach and is covered as a special case in that section.
9.2 LIFE TABLES
9.2.1 General life table approaches
Life tables were originally developed by life insurance companies as a means of determining life expectancies of humans. Ecologists, however, have adapted them for use in answering biological questions. As described in the introduction, the life table approach is based on the Euler-Lotka equation (9.1). The life table is a simple way of laying out the reproductive and mortality schedule of a population to aid in the solving of this key equation. The classic construction of the life table is shown in Table 9.1. The columns making up the life table can be simply derived from life history studies. Age data are essential to the construction of the table, both for maximum age as well as age at first reproduction. Methods of age determination are covered in Section 6 of this manual. The proportion of the population surviving at the beginning of each age class can be derived from estimates of natural and, or total mortality rates:
| lx = lx-1 e-Z | (9.3) |
Techniques for estimating mortality rates are covered in Section 8. The initial value of lxis normally set to a value of one, making it a “per recruit”analysis that examines if the population will replace that single recruit. The final pieces of data required are the age-specific number of female pups per reproductive event (litter for viviparous species, total eggs laid for oviparous species) and the frequency of reproductive events. In studies of shark populations the number of female pups is used as they are the only group that produces offspring. Thus, in reality this type of life table only tracks the female portion of the population. Rates of female pup production can be derived from total litter size by multiplying by the proportion of female embryos and dividing by the number of years between litters.
The first five columns in the table containing the life history data are then used to calculate the value of r. The calculation of r is iterative and is started by selecting a value of r and calculating the values for the two columns on the right-hand side of the life table. It can be seen that the summation of the final column is identical to the left side of equation 9.1. Thus when the final column is summed it will total 1.0 if the correct value of r has been selected. If the value does not equal 1.0, then a new value of r is picked and the process is repeated until the summation of column 7 equals 1.0. This may seem time-consuming and arduous, however, the process is almost instantaneous with the use of a non-linear optimization routine in a modern spreadsheet. The most commonly used of these routines is the “Solver”add-in that comes standard with Microsoft Excel. The life table can be entered into the spreadsheet and a cell containing the starting value of r added. This cell is then used in the formulae of columns 6 and 7 to represent r. The solver can then be started and the value of the sum of the final column set to equal 1.0 by changing the value of the cell containing r.
Once the life table has been constructed a number of other statistics can be calculated from the life table. The net reproductive rate (R0) is the total number of female offspring produced per individual in a single cohort:
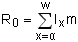 | (9.4) |
The mean generation length (G) is the mean period between birth of a parent and the birth of their offspring:
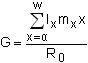 | (9.5) |
Krebs (1985) also demonstrated that it is possible to calculate a value related to r -the innate capacity for increase for the particular environmental conditions (rm). This statistic is calculated as:
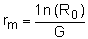 | (9.6) |
The value of rm, however, is not equivalent to r and should not be used as a substitute for it. The value of rm is a useful starting value for the iterative process of estimating r. The population doubling rate can also be simply calculated:
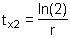 | (9.7) |
This statistic is handy for clearly showing differences between populations, or different mortality, or reproductive, scenarios within a population. The stable age distribution of the population (the proportion of individuals in each age class, Cx) can be calculated using the equation:
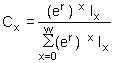 | (9.8) |
It is an assumption of the life table method that the age structure of the population is stable. In many situations, especially when a population is exploited, this assumption may be violated causing bias in the results. The static nature of life tables also means that they may underestimate the growth rate of a population as they do not include compensatory effects (e.g. decreases in mortality, increases in reproductive rate, decreases in reproductive age, etc. when population size is decreased). In the next section (9.2.2) a life table method is described that attempts to overcome this problem of not including compensation.
Initial use of the life table typically involves using age-specific survival values based only on natural mortality. However, age-specific values of fishing mortality (F) can easily be included by basing survival on total mortality. Several studies of shark populations have used this type of approach to investigate if current (or past) fishing mortality rates were sustainable (Simpfendorfer, 1999b) or at what level of fishing mortality r = 0 (i.e. the population will start to decline) (Simpfendorfer, 1999a). This type of information can be useful to resource managers. However, it is often difficult to translate a value of fishing mortality into a catch level without other information (i.e. catch and abundance data). Due to the age-structured nature of life tables it is possible to investigate other management measures. For example, the impact of nursery area closures can be studied by removing fishing mortality from the 0+ age class, or the impact of size regulations can be studied by applying fishing mortality to specific age classes.
Example -Australian sharpnose shark
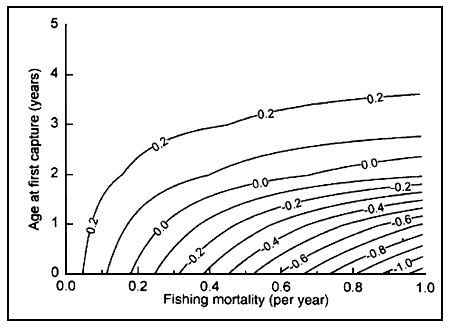
Simpfendorfer (1999a) produced life tables for the Australian sharpnose shark (Rhizoprionodon taylori) in northern Australian waters. One of these life tables (Table 9.1) was constructed using a natural mortality value estimated from a catch curve (for females only, M = 0.56 year-1). Based on these data the value of r was 0.271 year-1, the population doubling time (tx2) was 2.554 years, the generation time 2.304 years and the net reproductive rate, 1.758. Simpfendorfer (1999a) calculated the fishing mortality at which the population growth rate would be zero (Fc) to be 0.179 year-1. This was achieved by searching for the value of F that produces r = 0. Finally, a contour plot of r was produced for different values of age at first capture and fishing mortality (Figure 9.1) by constructing a large number of life tables. The use of spreadsheet software (e.g. Microsoft Excel) helps to speed the calculation of parameters when multiple life tables are required, with a simple macro being able to construct multiple life tables in almost no time.
TABLE 9.1
Life table for the
Australian sharpnose shark, Rhizoprionodon taylori, from northern Australia
based on data from Simpfendorfer (1999a).
| Age (x) | Proportion surviving(lx) | Female pups(mx) | Reproductive rate(lxmx) | lxmxx | e-rx | lxmx.e-rx |
| 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| 1 | 0.570638 | 1.982785 | 0.64565 | 0.64565 | 0.762338 | 0.492204 |
| 2 | 0.325628 | 2.588733 | 0.481027 | 0.962054 | 0.581159 | 0.279554 |
| 3 | 0.185816 | 2.80877 | 0.297824 | 0.893471 | 0.44304 | 0.131948 |
| 4 | 0.106034 | 2.888671 | 0.174784 | 0.699137 | 0.337746 | 0.059033 |
| 5 | 0.060507 | 2.917685 | 0.10074 | 0.503702 | 0.257477 | 0.025938 |
| 6 | 0.034527 | 2.928221 | 0.057694 | 0.346163 | 0.196284 | 0.011324 |
| 7 | 0.019703 | 2.932047 | 0.032965 | 0.230757 | 0.149635 | 0.004933 |
| 8 | 0.011243 | 2.933437 | 0.01882 | 0.150561 | 0.114073 | 0.002147 |
| 9 | 0.006416 | 2.933941 | 0.010741 | 0.096672 | 0.086962 | 0.000934 |
| 10 | 0.003661 | 0 | 0 | 0 | 0.066294 | 0 |
9.2.2 Rebound potential
Au and Smith (1997) described a modification of the life table approach to estimate what they termed “rebound potential”(r2M). The rebound potential (or rebound rate) is a measure of how fast a population will recover after fishing mortality has been removed from a population. The technique is described and then some potential modifications, assumptions and nuances considered. The description of the technique will be relatively cursory due to space limitations. Those wishing to find more detail on this technique should consult Au and Smith (1997) and Smith, Au and Show (1998).
Au and Smith (1997) began by reformulating the Euler-Lotka equation (9.1) by introducing parameters describing the survival to the mean age at maturity (lá) and average number of female pups per litter (b). This allows equation 9.1 to be rewritten as:
| e-(Z+r) = lαbe-rα[1 - e-(Z+r)(w-α+1)] = 1.0 | (9.9) |
The value of Z (total mortality) is substituted for lx (survival to age x) in equation 9.1. This reformulation allows r to be estimated more simply than the traditional iterative method. However, it requires several assumptions about the mortality and reproductive schedule (see below). Smith, Au and Show (1998) noted that a similar formulation was described by Hoenig and Gruber (1990) in terms of the survival in the first year after birth.
FIGURE 9.1
Contour plot of intrinsic rate of population increase r as
a function of fishing mortality (F) and age at first capture (AAFC) for
Rhizoprionodon taylori from northern Australia. Estimates are based on a life
table where natural mortality was calculated by a catch curve. Fishing is
sustainable at values of r > 0. From Simpfendorfer (1999a).
The second step of the technique involves assuming that the maximum sustainable yield (MSY) is achieved at Z = 2M, and that at this level r = 0. They also assumed that all of the compensation in the population growth rate occurs as a result of increased survival to age at maturity (lα). Thus by substituting r = 0 and Z = 2M into equation 9.9 the increased value of lα(lα,2M) can be calculated. Finally, the value of rebound potential (r2M) is calculated by removing the fishing pressure from the population (i.e. Z = M) but retaining the increased value of lα,2M
Au and Smith (1997) also considered that in situations where fecundity varied with age the rebounding population is likely to have a different value of b than the fished population. This would occur because the average age of mature animals would decrease as more animals recruited after fishing was stopped. To investigate the impact of these types of changes Au and Smith (1997) and Smith, Au and Show (1998) used sensitivity tests with 1.0b, 1.25b and 1.5b when solving for r2M. Au and Smith (1997) showed that for the leopard shark (Triakis semifasciata) that increased values of b resulted in significant changes in r2M.
When using this method, researchers need to be aware of the assumptions and restrictions on its use. In reformulating the Euler-Lotka equation much of the ability to include age-specific rates of reproduction and mortality was lost. The sensitivity of the results to changes in the value of b indicates the limitations of such an approach. The assumption that MSY occurs at Z = 2M also needs to be considered. Shark populations are known to have limited ability to sustain fishing pressure (Holden, 1977; Musick, 1999) due to their low fecundity and late age at maturity. As such MSY may occur at lower levels of Z than 2M. In fact, a value of Z = 1.5M may be a more appropriate level for MSY. This change can easily be included in the technique to estimate r1.5M. As more research is undertaken on shark populations a clearer understanding of the mortality rates that produce MSY will be gained. As this information becomes available, it may be necessary to address the value of Z used in this technique.
9.3 MATRIX MODELS
9.3.1 Application
Matrix population models are commonly used by researchers in studying the demography of a population. They provide a versatile method that can be used in a wide range of situations. It is not possible here to cover the whole suite of matrix models and how to use them. In this section, two forms of static matrix models will be considered: age-structured and stage-based. In both cases we will only consider static formulations of those models that are equivalent to the life tables discussed above. Matrix models are quickly and easily adapted to produce dynamic population models, but these fall outside the scope of this Section. For a thorough coverage of all issues related to matrix population models consult Caswell (2001) or Caswell (1989). As with life tables, static matrix models only require life history information. The math involved in producing the estimates of the finite rate of population growth (λ= er) is more complex and requires an understanding of matrix algebra. However, the need for such an understanding can be largely overcome by the use of software developed specifically for use with matrix models. A good example of this type of software is POPTOOLS, a Microsoft Excel add-in that is available free on the internet (http://www.cse.csiro.au/poptools/).
9.3.2 Age-structured models (Leslie Matrix)
Static age-structured matrix models, also know as Leslie Matrices after the scientist who first described their use, have been less commonly used in the assessment of shark populations that have life tables. Hoenig and Gruber (1990) were the first to publish a paper that used a Leslie Matrix to estimate λ for a shark population (lemon shark, Negaprion brevirostris). The basis for the Leslie Matrix is:
| Nt+1= ANt | (9.10) |
where N is a vector describing the age composition of the population (either at time it t or t+1) and A is the transition matrix:
 | (9.11) |
For consistency with the life table section, the same notation has been used: mxis the number of female pups per female in age class x and lx is the survival to the end of age x. It is the transition matrix A that is normally referred to as the Leslie Matrix. The matrix columns represent the age classes. The value of λ is determined by finding the dominant eigenvalue of A by using matrix algebra. When the dominant eigenvalue is determined, two vectors (the right and left eigenvectors) also can be calculated. The first of these represents the age-specific reproductive values (v, the left eigenvector), and the second is the stable age/stage structure (w, the right eigenvector). These sets of values are functionally equivalent to the lxmx and cx values in life table models.
As for life tables, a Leslie Matrix can be adapted to include information on fishing mortality at specific ages, or changes in the reproductive schedule. In addition, the static nature of the simple Leslie Matrix does not include compensatory effects for a population that is being fished.
Example -Australian sharpnose shark
As a direct comparison to the example given in the life table section, a Leslie matrix was constructed from the data provided by Simpfendorfer (1999a):
| 0.00 | 1.98 | 2.59 | 2.81 | 2.89 | 2.92 | 2.93 | 2.93 | 2.93 | 2.93 | 0.00 | (9.12) |
| 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.00 | 0.57 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.00 | 0.00 | 0.33 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.00 | 0.00 | 0.00 | 0.19 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.00 | 0.00 | 0.00 | 0.00 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | |
| 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | |
| 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | |
| 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.006 | 0.00 | |
| 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.004 |
This matrix was analysed using the Microsoft Excel Add-In POPTOOLS. The dominant eigenvalue (λ) was 1.257 (r = 0.229 year-1) and the population doubling time was 2.37 years. These values are similar to those produced by the life table analysis, with the population doubling time different by approximately 0.2 years. The left and right eigenvectors (v and w) are given in Table 9.2.
TABLE 9.2
Age specific reproductive value (w) and stable age
distributions (v) (proportional) of the Australian
sharpnose shark, Rhizoprionodon taylori, estimated
using a Leslie Matrix (equation 9.12).
| Age | w % | v % |
| 0 | 62.9 | 4.5 |
| 1 | 28.5 | 9.9 |
| 2 | 7.4 | 10.9 |
| 3 | 1.1 | 11.0 |
| 4 | 0.1 | 10.8 |
| 5 | 0.0 | 10.7 |
| 6 | 0.0 | 10.6 |
| 7 | 0.0 | 10.6 |
| 8 | 0.0 | 10.5 |
| 9 | 0.0 | 10.5 |
| 10 | 0.0 | 0.0 |
9.3.3 Stage-based models
In some situations the life history of a species can be divided into discrete segments or stages (e.g. neonate, juvenile, sub-adult, breeding adult, non-breeding adult, etc.). In this case a stage-base matrix model can be applied. This type of model can be useful if there is only limited age information for a species or the time spent in stages is variable. In long-lived species, stage-based models can also simplify the math involved in the calculations. The formulation of the static stage-based transition matrix is similar to that of the Leslie matrix, but the columns represent stages rather than ages, and the survival values are divided between the probability of an individual surviving and moving from one stage to the next (Gi) and the probability of an individual surviving and remaining in the same stage (Pi). There are several approaches to calculating these parameters. In a study of sandbar sharks Cortes (1999) applied a method that used the duration of each stage (dj) and the stage-specific survival probabilities (pi):
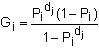 | (9.13) |
and
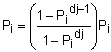 | (9.14) |
If the stage-specific survival value is not known, it can be estimated as the mean of the age-specific values (survival or mortality, with S = e-Z) in each stage. Other authors (e.g. Brewster-Geisz and Miller, 2000; Mollet and Cailliet, 2002) have taken a slightly different approach using the probability of survival of an individual in a stage (σi) and the fraction of individuals in a stage that move to the next stage (γi):
| Gi= σiγi | (9.15) |
and
| Pi= σi(1 - γi) | (9.16) |
This method requires an iterative approach to the estimation of the matrix parameters, and more detail can be found in Brewster-Geisz and Miller (2001) or Mollet and Cailliet (2002).
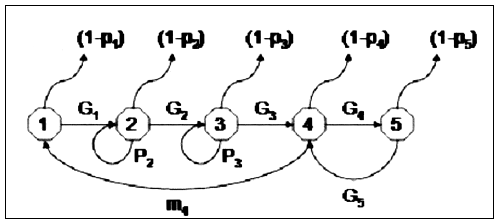
The staged-based transition matrix can take many forms depending on how the stages selected for the population are related. The best way to understand the elements of the stage-based transition matrix is via a life cycle graph. Figure 9.2 shows a life cycle graph for the sandbar shark (Carcharhinus plumbeus) with five stages (neonates, juveniles, sub-adults, pregnant adults and resting adults). This life cycle was used by Brewster-Geisz and Miller (2000). The transition matrix for this stage classification as specified by Brewster-Geisz and Miller (2000) is:
 | (9.17) |
Only stage four animals produce young (hence only m4) on the top line, the transition to all stages up to pregnant adult are one-way, but animals alternate between pregnant and resting stage adults on an annual basis (hence the lack of P4and P5since they will always move to the other group if the time step is annual), and finally neonates become juveniles after one year (hence there is no P1value). Such a transition matrix could be used for many shark populations, but would need to be modified if reproduction was annual, or if the resting adult stage lasted longer than one year. It is not possible to specify all possible combinations of matrices here. Caswell (2001) provides a thorough coverage of how to develop a life cycle graph (which maps out the stages) and the resulting transition matrix.
FIGURE 9.2
Life cycle graph of the sandbar shark, Carcharhinus plumbeus, used to
construct the matrix in equation 9.17. Compartments 1–5 represent the
different life stages (1 -neonate; 2 -juveniles; 3 -sub-adults; 4 -pregnant
adults; 5 -resting adults). Parameter values shown correspond to those in
equation 9.17. Based on information in Brewster-Geisz and Miller (2000).
As with a Leslie matrix the value of γ of the stage-based model is estimated by determining the eigenvalue of the matrix. Similarly, the eigenvectors produce information on the reproductive value and stable age structure, but they are stage-specific rather than stage-specific.
Example -sandbar sharks
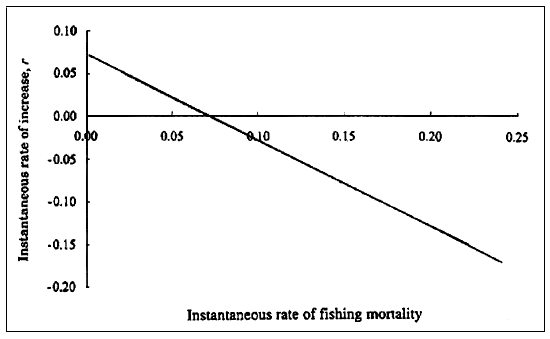
Brewster-Geisz and Miller (2000) used a stage-based matrix model to examine some management options for the sandbar shark (Carcharhinus plumbeus) in the western North Atlantic. The life cycle graph for this species is shown in Figure 9.2 and the matrix formulation is shown in equation 9.17. The analysis examined the results of five scenarios with varying amounts of fishing mortality on the five stages ranging from the current situation (in 1996) to total protection of the neonates and the pregnant females (including the unrealistic assumption of no natural mortality on neonates). They estimated that in the current situation r = -0.124 year -1, indicating that the population was over-fished and declining. The four other scenarios used to explore protection for different stages by eliminating fishing mortality also returned negative values of r. They examined the effect of fishing mortality on r (Figure9.3) and demonstrated that if fishing mortality at all stages was equal, r = 0 occurred at F = 0.071 year -1. This plot also demonstrates that when no fishing occurs the value of r is approximately 0.07 year -1.
FIGURE 9.3
The relation between the intrinsic rate of increase (r) and fishing
mortality (F). Fcriticalis reached at 0.071. If F is less than Fcritical, the population will increase. If F is greater than Fcritical, the population will decrease. From Brewster-Geisz and
Miller (2001).
9.3.4 Elasticities
One piece of information that can be useful in interpreting the results of matrix models is how much influence changes in vital rates (reproductive and mortality rates) can have on the population growth rate. In absolute terms this is known as the sensitivity, but is normally reported as the elasticity, which is the proportional change. Elasticity is calculated from the elements of the transition matrix (aij), the population growth rate (γ) and the elements of the right and left eigenvectors (vi, wi):
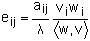 | (9.18) |
where (w,v) is the scalar product of the two vectors (i.e, (w,v)=v1w1+v2w2+… + vnwn). Since elasticities are proportions they sum to give one:
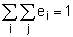 | (9.19) |
For each column of the matrix, which correspond to individual age or stage classes, elasticity values can be calculated by:
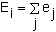 | (9.20) |
where Ei is the age or stage elasticity.
Since elasticity will identify the age or stage where the smallest changes in vital rates will produce the biggest change in population growth rate, the researcher has a powerful tool to find where management or conservation action might produce the greatest benefits to the population. For example, Cortes (2002) used elasticity values from a wide range of shark species to show that populations of large, slow-growing, long-lived species were most vulnerable to changes in the survival of the juveniles (as opposed to the adults). Such a result suggests that management arrangements that protect juveniles (e.g. nursery area closures) would provide greater benefit to the population than those that protect adults (e.g. maximum size limit). For a more detailed discussion of the calculation and interpretation of elasticity values for matrix models see Caswell (2001).
9.4 CONCLUSIONS AND ADVICE
The static modeling approaches outlined in this Section provide the researcher with methods to assess the status of a population based solely on life history data. This is particularly useful when there is little or no fishery information available for a population making more complex dynamic modeling approaches inappropriate. However, these simple approaches come with limitations, which must be kept in mind when interpreting the results and applying them to management or conservation. For example, a life table can provide good information on the intrinsic rate of increase for a population, or the fishing mortality rate at which the population will start to decline. However, it will not provide information on the abundance of the population, its level of population decline or the appropriate quota level to achieve a target biomass. These later types of information are more appropriately determined using the dynamic approaches described in Section 10.
The results of the static approaches should also be considered as conservative in their estimates of population growth rates. This is because both simple life tables and static matrix models do not allow for compensatory effects at low population sizes (e.g. increased growth, reproductive or survival rates). The rebound potential approach of Au and Smith (1997) described in the life table section is an attempt to overcome this limitations. However, the simple framework in which it is implemented means that a number of restrictive assumptions need to be made.
The choice between life tables or matrix models is largely a matter of personal preference. Each of the approaches will provide similar results if used in comparable ways. However, the trend in the fisheries and ecological literature is towards matrix models. Although the math involved in matrix models is more complex the development of software to quickly and easily do the analyses means that these approaches can be easily implemented on a personal computer. In addition the ability to easily calculate elasticity values, and their usefulness in determining management or conservation strategies, provides an incentive to take this approach.
Finally, which ever approach is chosen, it is important to remember that there is a degree of uncertainty and, or variation in the input parameters to any model. For this reason a good demographic analysis will always include a range of scenarios that consider different sets of life history parameters that reflect uncertainty or variation. There are two approaches to this. The first is to construct a number of life tables or matrices that reflect the potential ranges of values. The second approach is to construct a stochastic analysis such as that used by Cortes (1999) for sandbar sharks. With this approach probability distributions for the input parameters are constructed and several hundred random draws from the distributions are made and the life table or matrix solved. The result is probability distributions of the output parameters (e.g. r). The first approach is best suited to cases where there is uncertainty in the value of the parameters. The second approach is suited to the situation where there is variation in the value of the parameters.
9.5 LITERATURE CITED
Au, D.W. & Smith, S.E. 1997. A demographic method with population density compensation for estimating productivity and yield per recruit of the leopard shark (Triakis semifasciata). Canadian Journal of Fisheries and Aquatic Sciences, 54: 415–420.
Brewster-Geisz, K.K. & Miller, T.J. 2000. Management of the sandbar shark, Carcharhinus plumbeus: implications of a stage-based model. Fishery Bulletin, 98: 236–249.
Cailliet, G.M. 1992. Demography of the central Californian population of the leopard shark (Triakis semifasciata). Australian Journal of Marine and Freshwater Research, 43: 183–193.
Caswell, H. 1989. Matrix population models: construction, analysis and interpretation. Sinauer, Sunderland, MA. 328 pp.
Caswell, H. 2001. Matrix population models. Construction, analysis, and interpretation. 2nd Edition. Sinauer, Sunderland, MA.
Cortes, E. 1995. Demographic analysis of the Atlantic sharpnose shark, Rhizoprionodon terraenovae, in the Gulf of Mexico. Fishery Bulletin, 93: 57–66.
Cortes, E. 1998. Demographic analysis as an aid in shark stock assessment and management. Fisheries Research, 39: 199–208.
Cortes, E. 1999. A stochastic stage-based population model of the sandbar shark in the Western North Atlantic. In J.A. Musick (ed.). Life in the slow lane. Ecology and conservation of long-lived marine animals, pp. 115–136. American Fisheries Society Symposium 23, Bethesda, Maryland.
Cortes, E. 2002. Incorporating uncertainty into demographic modeling: application to shark populations and their conservation. Conservation Biology, 16: 1048–1062.
Cortes, E. & Parsons, G.R. 1996. Comparative demography of two populations of the bonnethead shark (Sphyrna tiburo). Canadian Journal of Fisheries and Aquatic Sciences, 53: 709–718.
Hoenig, J.M. & Gruber, S.H. 1990. Life history patterns in the elasmobranches: implications for fisheries management. In H.L. Pratt Jr, S.H. Gruber & T. Taniuchi (eds). Elasmobranchs as living resources: advances in the biology, ecology, systematics, and the status of fisheries, pp. 1–16. NOAA Technical Report NMFS 90.
Holden, M.J. 1977. Elasmobranchs. In J.A. Gulland (ed.). Fish population dynamics, pp.187–215. John Wiley & Sons, London.
Krebs, C.J. 1985. Ecology: the experimental analysis of distribution and abundance, 3rd ed. Harper and Row, New York. 67 pp.
Mollet, H.F. & Cailliet, G.M. 2002. Comparative population demography of elasmobranchs using life history tables, Leslie matrices and stage-based matrix models. Marine and Freshwater Research, 53: 503–516.
Musick, J.A. 1999. Ecology and conservation of long-lived marine animals. In J.A. Musick (ed.). Life in the slow lane. Ecology and conservation of long-lived marine animals, pp.1–10. American Fisheries Society Symposium 23, Bethesda, Maryland.
Simpfendorfer, C.A. 1999a. Mortality estimates and demographic analysis for the Australian sharpnose shark, Rhizoprionodon taylori, from northern Australia. Fishery Bulletin, 97: 978–986.
Simpfendorfer, C.A. 1999b. Demographic analysis of the dusky shark fishery in southwestern Australia. In J.A. Musick (ed.). Life in the slow lane. Ecology and conservation of long-lived marine animals, pp. 149–160. American Fisheries Society Symposium 23, Bethesda, Maryland.
Smith, S.E., Au, D.W. & Show, C. 1998. Intrinsic rebound potential of 26 species of Pacific sharks. Marine and Freshwater Research, 48: 663–678.