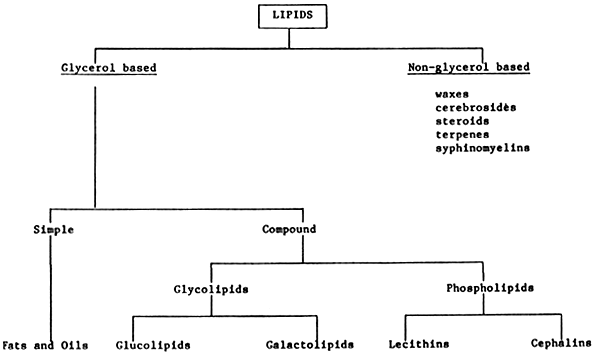
The lipids are a heterogenous group of substances found in plant and animal tissues, which share the property of being relatively insoluble in water, and soluble in organic solvents, such as ether, choloroform and benzene.
Lipids may be classified into two basic groups, according the presence or not of the alcohol glycerol:
Lipids are important sources of metabolic energy (ATP). In fact, the lipids are the most energy rich of all classes of nutrients: gross energy value of
| Lipid | 9.5 kcal/g |
| Protein | 5.6 kcal/g |
| Carbohydrate | 4.1 kcal/g |
In this respect, dietary lipids may be used to spare the more valuable protein for growth. In particular, free fatty acids derived from triglycerides (fats and oils) are the major aerobic fuel source for energy metabolism of fish muscle.
Lipids are essential components of all cellular and subcellular mambranes (lipid classes that are involved include the polyunsaturated fatty acid containing phospholipids, and sterol esters).
Lipids serve as biological carriers for the absorption of the fat soluble vitamins A, D, E and K.
Lipids are a source of essential fatty acids, which in turn are essential for the maintenance and integrity of cellular mambranes, are required for optimal lipid transport (bound to phospholipids as emulsifying agents), and are precursors of the prostaglandin hormones.
Lipids are believed to play a role as a mechanical cushion/support for the vital body organs, and aid in the maintenance of neutral buoyancy.
Lipids are a source of essential steroids, which in turn perform a wide range of important biological functions (ie. the sterol cholesterol is involved in the maintenance of membrane systems, for lipid transport, and as a precursor of vitamin D3, the bile acids, and the steroid hormones - androgens, estrogens, adrenal hormones, and corticosteroids).
From a feed technology viewpoint, lipids act as lubricants for the passage of feed through pellet dies, as substances which reduce the dustiness of feeds, and play a role in feed palatability.
Fuel or energy can be stored in plants as starch, and in animals as glycogen, but it can also be stored in both plants and animals in a more compact form as fats or oils. In plants, fats and oils are formed from carbohydrates (e.g. as plants seeds ripen, their starch content falls as their fat content increases). In animals fats can be formed also from carbohydrate (i.e. fattening a pig with food largely composed of carbohydrate). However, unlike plants, animals can also deposit fat in their body from fat ingested. The only difference between fats and oils is that the latter are liquid at room temperature, whereas fats are semi-solid at room temperature.
Fats and oils normally occur in foodstuffs and in the fat deposits of most animals in the form of triglycerides, which are esters of fatty acids and glycerol.
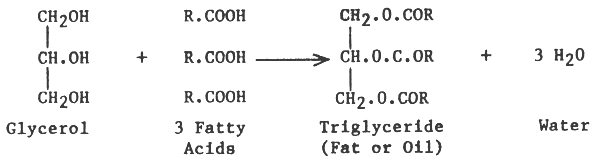
Naturally occurring fats and oils are composed of mixed triglycerides where glycerol is esterified with different types of fatty acids, i.e. fatty acid R1, R2 and R3, thus:
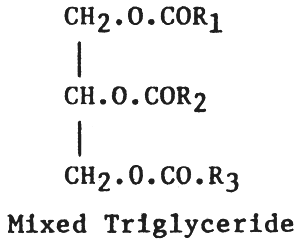
No naturally occurring fat or oil found in nature consists of single triglycerides. It can be seen that the basic unit and variable of all triglycerides is the fatty acid component, which in turn will effect the physical and chemical property of the fat or oil.
Over 40 different fatty acids are known to occur in nature. They all can be represented by the general formula:
CH3 (CH3)n COOH
| where | n = 0 in Acetic acid |
| n = 1 in Propionic acid | |
| n = 2 in Butyric acid, etc. up to n = 24 (where n is usually an even number). Most naturally occurring fatty acids contain a single COOH group and a straight unbranched carbon (C) chain, which may in turn contain no double bond (saturated fatty acid), a single double bond (mono-unsaturated fatty acid), or more than one double bond (polyunsaturated fatty acids, PUFA). It follows that the degree of unsaturation will greatly influence the physical properties of the constituent fats, as in general unsaturated fatty acids are more chemically reactive and have lower melting points than the corresponding saturated fatty acids. Examples of saturated and unsaturated fatty acids are given below: |
| Fatty acid | Structure | Shorthand abreviation1 |
| Saturated | ||
| Butyric acid | CH3(CH2)2 COOH | 4 : 0 |
| Caproic | CH3(CH2)4 COOH | 6 : 0 |
| Capric acid | CH3(CH2)8 COOH | 10 : 0 |
| Lauric acid | CH3(CH2)10COOH | 12 : 0 |
| Myristic acid | CH3(CH2)12COOH | 14 : 0 |
| Palmitic acid | CH3(CH2)14COOH | 16 : 0 |
| Stearic acid | CH3(CH2)16COOH | 18 : 0 |
| Unsaturated2 | ||
| Palmitoleic acid | CH3(CH2)5CH=CH(CH2)7COOH | 16 : 1n- 7 |
| Oleic acid | CH3(CH2)7CH=CH(CH2)7COOH | 18 : 1n- 9 |
| Linoleic acid | CH3(CH2)4CH=CH(CH2)7COOH | 18 : 2n- 6 |
| Linolenic acid | CH3CH2CH=CHCH2CH=CHCH2CH=CH(CH2)7COOH | 18 : 3n- 3 |
| Arachidonic acid | CH3(CH2)4CH=CHCH2CH=CHCH2CH=CHCH2CH=CH(CH2)3COOH | 20 : 4n- 6 |
| Eicosapentaenoic acid | CH3CH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CH(CH2)3COOH | 20 : 5n- 3 |
| Docosahexaenoic acid | CH3CH2CH=CHCH2CH=CHCH2CH=CHCH2CH=CHCH2CH =CHCH2CH=CH(CH2)2COOH | 22 : 6n- 3 |
On the basis of the above classification polyunsaturated fatty acids (PUFA) may be divided into three major families; the oleic (n-9) series, the linoleic (n-6) series, and the linolenic (n-3) series; the family names representing the shortest chain member of the group, with other family members being derived from these three basic groups
With the exception of the land snail (Cepaea nemoralis) animals are incapable of de novo synthesis of fatty acids with double bonds in the n-6 (linoleic series) and n-3 (linolenic series) positions; only plants are able to synthesize these fatty acids de novo. However, most animals are able to synthesize even chain saturated fatty acids from acetate, or of adding two carbon units to the carboxyl end of a fatty acid and adding more double bonds on the carboxyl side of existing double bonds but not on the methyl end (Castell et. al., 1986). The biochemical pathways of PUFA biosynthesis in fish and shrimp can be summarised as follows:
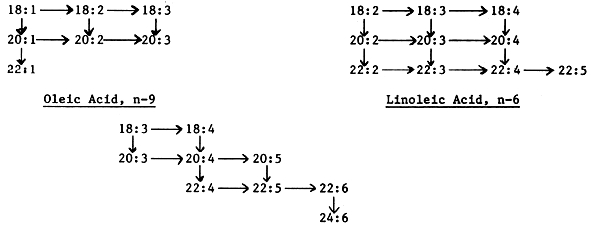
* Vertical arrows show chain elongation reactions. Horizontal arrows show desaturation reactions.
In view of the inability of animals to synthesize de novo fatty acids of the n-6 and n-3 series, these fatty acids must be supplied in a ready made form within the diet. For land animals, the linoleic (n-6) series has been found to have the highest essential fatty acid (EFA) activity, with the linolenic (n-3) series having only partial EFA activity. It follows, therefore, that the predominant fatty acids (PUFA) in the tissues of land animals belong to the linoleic series, namely 18:2n-6 (linoleic acid) and 20:4 n-6 (Arachidonic acid).
By contrast, the predominant PUFA in the tissues of fish and shrimp belong to the linolenic (n-3) series, and this applies to freshwater and marine fish alike. The concentration of n-6 PUFA in the tissues of fish is generally low, although higher levels are reported in freshwater fish species. This is perhaps not surprising if one considers that the diet of freshwater fish contains a component derived from terrestrial sources, and consequently rich in n-6 series fatty acids. It is generally believed that the n-3 series fatty acids permit a greater degree of unsaturation - a requirement for greater membrane fluidity, flexibility and permeability at low temperatures. In fact, it is genrally believed that the dietary requirement (preferential) of fish for n-3 series EFA, over n-6 series, is fundamentally due to the low water temperature of their aquatic environment (as compared with mammals). In fact, the lower the water temperature, the greater the incorporation of n-3 series PUFA in the tissues. Apart form the differences in n-6 PUFA content of the tissues of freshwater and marine fish species, freshwater fish also generally have higher tissue concentrations of the shorter chain PUFA n-3 series.
With the exception of strict carnivorous fish species, fish are able to chain elongate and further desaturate 18:2 n-6 or 18:3 n-3 (depending on the fish species) to the corresponding highly unsaturated fatty acid (HUFA): 20:4 n-6 in the case of the n-6 series, and 20:5 n-3 or 22:6 n-3 in the case of the n-3 series. It is generally believed that these HUFA are responsible for the key metabolic functions ascribed to the EFA. In fact, for most fish species, HUFA have greater EFA activity than the corresponding basic unit (18:2 n-6 or 18:3 n-3).
In general, cold water freshwater fish have an exclusive requirement for n-3 series PUFA (18:3 n-3, 20:5 n-3, 22:6 n-3) in their diet (i.e. salmonids, Ayu), while warm freshwater fish have either a requirement for both the n-3 series and n-6 series PUFA (i.e. carps, eel, and possibly channel catfish), or for the n-6 series alone (i.e. Tilapias, and possibly the snakehead Channa micropeltes; for review see Kanazawa, 1985). In the case of marine carnivorous fish species (i.e. redseabream, black sea bream Mylio macrocephalus, opaleye Girella nigricans, puffer fish Fogu rubripens, yellow tail Seriola quinqueradiata, plaice Pleuronectes platessa, gilthead bream Sparus auratus, turbot Scophthalmus maximus), since the food organisms consumed are rich in 22:6 n-3 and 20:5 n-3, they have lost ability to chain elongate and further desaturate 18:3 n-3 to the corresponding HUFA. Marine carnivorous fish must, therefore, be supplied with 22:6 n-3 or 22:5 n-3 in a ready made form (Kanazawa, 1985). The dietary EFA requirement of fish are summarised in Table 7.
On a general basis, the dietary EFA requirement of fish have been found to increase with increasing dietary lipid level and/or with decreasing water temperature (Castell et. al., 1986).
| Fish | Requirement | Reference |
| Coolwater - freshwater | ||
| Rainbow trout | 1%18:3n-3 or 1%HUFAn-3 | Castell et.al., (1972); |
| Watanabe et.al., (1974); | ||
| Yu & Sinnhuber (1972); | ||
| Takeuchi & Watanable (1977) | ||
| Coho salmon | 1%18:3n-3 | Yu & Sinnhuber (1979) |
| Chum salmon | 1%18:3n-3+1%18:2n-6 or 1%HUFAn-3 | Takeuchi, Watanabe & Nose (1979) |
| Takeuchi & Watanabe (1982) | ||
| Ayu | 1%18:3n-3 or 1%20:5n-3 | Kanazawa (1985) |
| Warmwater-freshwater | ||
| Common carp | 1%18:3n-3+1%18:2n-6 or 0.5–1.0%HUFAn-3 | Takeuchi & Watanabe (1977a) |
| Channel catfish | <1%18:3n-3 | Robinson & Lovell (1984) |
| Tilapia zillii | 1%18:2n-6 or 1%20:4n-6 | Kanazawa et.al., (1980a) |
| Tilapia nilotica | 0.5–1%18:2n-6 or 1%20:4n-6 | Teshima, Kanazawa & Sakamoto(1982) |
| Takeuchi, Satoh & Watanabe (1983) | ||
| Eel | 0.5%18:2n-6+0.5%18:3n-3 | Takeuchi et. al., (1980) |
| Marine fish | ||
| Turbot | 0.6–1%HUFAn-3 | Gatesoupe et. al., (1977, 1977a); Leger et. al., (1979) |
| Redseabream | 0.5–2.0%HUFAn-3 | Yone et. al., (1978) |
At present there is no firm quantitative information on the dietary EFA requirement of marine shrimp or freshwater prawns; the information available at present being suggestive rather than conclusive. However, as with fish, it is believed that n-3 series fatty acids have a higher EFA activity than n-6 series fatty acids in shrimp and prawns (Castell et. al., 1986; NRC, 1983; Sandifer & Joseph, 1976).
In general, marine fish, shrimp and mollusc oils are rich dietary sources of the n-3 series EFA; oils whose 20:5 n-3 and 22:6 n-3 content constitutes over 20% of the total fatty acids present include cod liver oil, cuttlefish liver oil, shortnecked clam oil, sardine oil, skipjack oil, shrimp headoil and squid liver oil. By contrast, plant oils are rich dietary sources of 18:2n-6, and contain little or no n-3 series EFA (with the exception of soybean oil, rapeseed oil and particularly linseed oil whose 18:3n-3 content may exceed 8, 7 and 56% respectively of the total fatty acids present). Plant oils whose 18:2n-6 constitutes 50% or more of the total fatty acids present include cottonseed oil, corn oil, sunflower seed oil and soybean oil. Finally, trace quantities of 20:4n-6 (0.5–1.5%) have been detected in terrestrial animal oils (lard, tallow), liver meal, salmon oil, pollack liver oil, cuttle-fish liver oil, short-necked clam oil, sardine oil, skipjack oil, squid liver oil and herring oil (Figure 4).
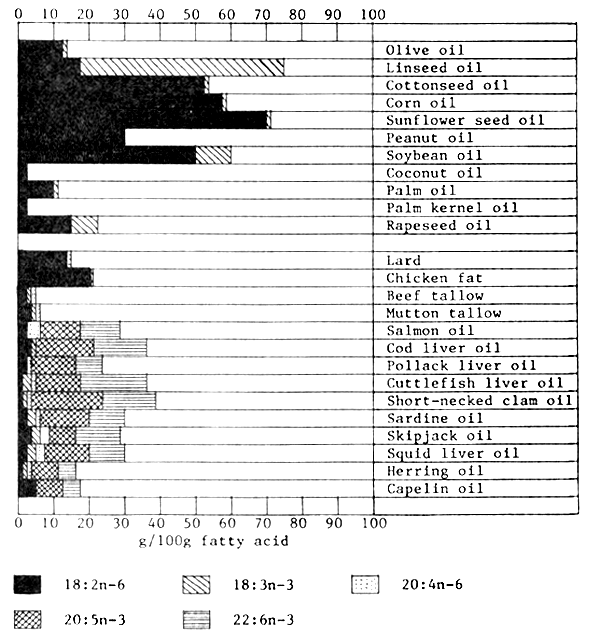
Figure 4. Essential fatty acid composition (g/100g fatty acid) of some common fats and oils
Within the animal body the phospholipids represent the second largest lipid component after the triglyceride fats and oils. All phospholipids are yellow greasy solids and share the property of being soluble in lipid solvents with the exception of acetone (this property allows them to be readily distinguished from fatty acids).
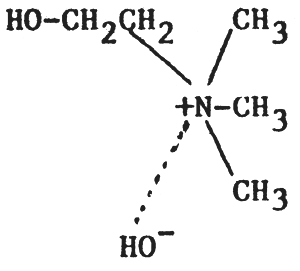
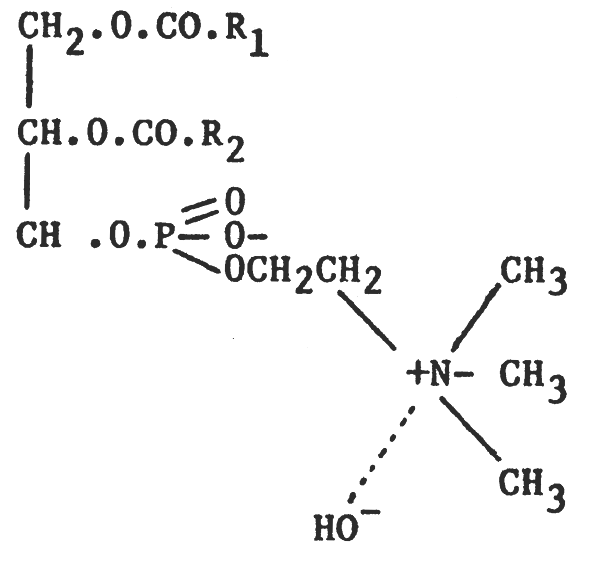
Like fats and oils, phospholipids are all esters of fatty acids and glycerol. However, whereas in simple fats and oils the trihydric alcohol glycerol is esterified with three fatty acids, in phospholipids only two of the alcohol groups of glycerol are esterified with fatty acids; the remaining group being esterified with phosphoric acid and a nitrogenous base. According to the nitrogenous base present, phospholipids may be divided into two groups: lecithins (nitrogenous base choline) and cephalins (nitrogenous base - ethanolamine). Their structures can be represented thus:
| Nitrogenous base* | Phospholipid |
| 1) Choline | Lecithin (phosphatidylcholine, PC) |
 |  |
| 2) Ethanolamine | Cephalin (phosphatidylethanolamine, PE) |
 | 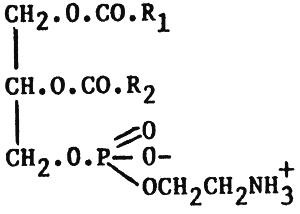 |
* Other nitrogenous bases may include serine and inositol.
From these structural formulae, it can be seen that the phospholipids, like fatty acids, have a polar region and a non-polar region. However, unlike the fatty acids, the ionic functions are greatly increased by the presence of phosphoric acid and the nitrogenous organic base; which therefore results in combining withing the same molecule, both hydrophilic and hydrophobic (fatty acid chain) sites. It is because of this unique surface active property that phospholipdis, in conjunction with proteins, form the basic lipoprotein structure of biological membranes. It is interesting to note here that the fatty acids contained within animal phospholipids (R1 R2) are much more unsturated than corresponding fatty acids from triglycerides (fats and oils). The increased unsaturation of the phospholipid acids is largely due to the increased levels of the C20 and C22 polyunsaturated fatty acids, which are almost exclusively bound at the 2 position. In particular, the fatty acids 20:5 n-3 and 22:6 n-3 (EFA) may account for 80% of the total fatty acid found at the 2 position. It follows, therefore, that during EFA deficiency, examination of the phospholipids in tissues shows the presence of high levels of polyunsaturated fatty acids derived from oleic and palmitoleic acid, in contrast to the usual situation where polyunsaturated fatty acids derived from linolenic acid predominate. Phospholipids also play important roles as emulsifying agents in biological systems and are particularly involved in the transportation of fats within the body. For example phospholipids may take part in the emulsification of dietary lipids in the digestive tract, and as constituents of high-density lipoproteins in the transport of lipids within the body (Kanazawa, Teshima & Sakamoto, 1985). Rich dietary sources of phospholipids include eggs and soybean oil.
Dietary phospholipids have been found to have a beneficial effect on the growth and survival of marine fish larvae (red sea bream: Kanazawa et. al., 1983; knife jaw Oplegnathus fasciatus: Kanazawa at. al., 1983; Ayu: Kanazawa et. al., 1983a) and marine shrimp (P. japonicus: Deshimaru, 1981; Kanazawa, Teshima & Sakamoto, 1985; Teshima et. al, 1982: P. monodon: Pascual, 1984) fed semi-synthetic test diets in which choline and the EFA were added separately at equivalent levels. Furthermore, the efficacy of phospholipid on growth and suvival has been shown to vary with the type and source of phospholipid used. For example, the efficacy of bonito-egg PC, soybean PC and soybean phosphatidylinositol (PI) has been found to be much higher than that of bonito-egg PE, ovine-brain PE, ovine-brain phosphatidylserine (PS) or chicken-egg PC in P. japonicus larvae (Kanazawa, Teshima & Sakamoto, 1985). These researchers also showed that the optimum level of dietary phospholipid for P. japonicus larvae varied with the dietary lipid sourced used; thus, a dietary soybean PC requirement of 6.0% and 3.5% was obtained when 18:1 n-9 and 1.0% highly unsaturated fatty acids, or pollack liver oil was used as the dietary lipid source, respectively. A 3% dietary requirement for soybean PC has also been reported for Ayu (Kanazawa et al.1983a) and P. japonicus larvae (Teshima et. al. 1982) when pollack liver oil was used as the basal lipid source. Kanazawa (1985) concluded that (1) phospholipids containing either choline or inositol exerted a positive effect on growth and survival, (2) phospholipids containing 18:2 n-6, 18:3 n-3, 20:5 n-3 and 22:6 n-3 in the molecule were the most effective in promoting growth and survival, and (3) the effectiveness of the phospholipids seemed to be dependent on the nature of the fatty acids in the α and β positions of the phospholipid molecule.
The beneficial effect of dietary phospholipids on the growth and survival of marine fish larvae and crustaceans is particularly surprising bearing in mind the natural capacity of these animals for phospholipid biosynthesis from fatty acids and diglycerides (Lui, Sage and O'Connor, 1974). Although a true requirement for dietary phospholipids remains to be confirmed under practical farming conditions, it has been suggested that the dietary essentiality of phospholipids (if at all) is due to a specific requirement for phospholipids for fatty acid transport within the body and a slow rate of biosynthesis of phospholipids in relation to metabolic demand during the larval growth phase (Teshima, Kanazawa & Kakuta, 1986).
Glycolipids are similar to phospholipids in that they are glycerol based and have two of the alcohol groups present esterified by fatty acids, but differ from them in that the third group is linked to a sugar residue.
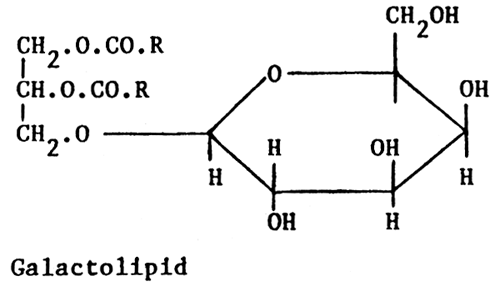
The lipids of grasses and clovers, which form the major part of the dietary fat of ruminants, are predominantly (60%) galactolipids. In general, about 95% of the fatty acid present is linoleic acid (18:2n-6).
The waxes are esters of fatty acids with high molecular monohydric alcohols. Like fats, natural waxes occur as mixtures of different esters, which are usually solid at room temperature. Waxes are widely distributed in both plants and animals, where they generally perform protective roles. For example, waxes often occur within the cuticle of leaves and fruit so as to minimise water losses due to transpiration; whereas in animals, wool and feathers are often protected against water by the hydrophobic nature of a wax coating. Among the better known animal waxes are lanolin (obtained from wool), beeswax (an insect secretion), and spermaceti from the sperm whale.
In some aquatic animals, waxes often replace the triglycerides to some extent. For example, in some whales and many crustaceans such as the copepod Calanus sp. wax esters form the major component of the depot fat. Although waxes are not readily hydrolysed by terrestrial animals, and so have no real nutritive value, certain aquatic animals such as marine fish (ie. sardines, herring, salmon) which prey upon wax rich animals (ie. copepods) do possess wax ester lipases which are able to cleave the wax esters and so make them available for digestion. However, since the fatty acid component of these wax esters is generally saturated, and so deficient in the long chain PUFA, they probably only serve as energy sources, rather than for structural purposes.
The steroids include a very important and widely distributed group of substances including the sterols, the bile acids, the adrenal hormones, and the sex hormones. Although these steroids have a very wide range of biological properties, they all have the basic structural unit of a phenanthrene nucleus linked to a cyclopentane rin
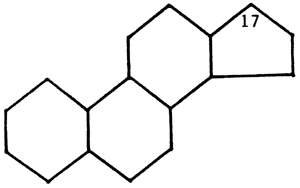
Cyclopentane ring
The individual compounds differ in the number and positions of their double bonds and in the nature of the side chain at carbon atom 17. Reference here will only be made to the zoosterol cholesterol and the bile acids.
Cholesterol is widely distributed in the animal body, being particularly abundant in the brain and nervous tissue, blood, bile, liver, and the skin. Within the body cholesterol may exist in its free state (ie. cholesterol is the chief component of gall stones) or in esterified form with fatty acids and other organic acids.
Cholesterol performs many important functions within the body:
It is an essential component of biomembranes systems in all eukaryotic species, together with the phospholipids and proteins. The bulk of the cholesterol in animal tissues is associated with membrane systems.
Many important sterols found within the body are synthesised from cholesterol. For example, cholesterol is a precursor of the bile acids, the steroid hormones (including the androgens, oestrogens, and corticosteroids), and of vitamin D3.
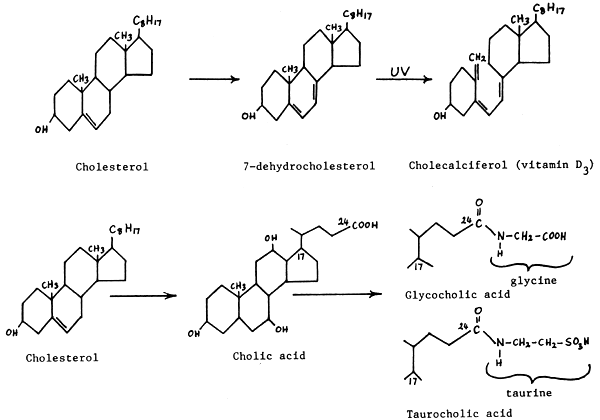
Cholesterol also plays an important role in the absorption of fatty acids from the intestine and in their consequent transportation in the blood or haemolymph. Here cholesterol combines with fatty acids to form cholesterol esters, which are more soluble and emulsifiable than the free fatty acid molecules,
In contrast to fish, crustaceans like other arthropods have been found to be incapable of synthesizing sterols de novo from acetate and mevalonate (Teshima and Kanazawa, 1971; Teshima, 1983). Cholesterol is therefore regarded as an essential dietary nutrient for marine shrimp and freshwater prawns. Based on laboratory studies conducted with P. japonicus, the optimum level of dietary cholesterol is reported to be 0.5–2.0% of the dry diet (Deshimaru, 1981; Kanazawa et al., 1971; Teshima et al., 1982; Teshima and Kanazawa, 1986). A rich source of dietary cholesterol is shrimp head oil.
These steroids are formed by the combination of the amino acids glycine or taurine with cholic acid (a derivative of cholesterol). The bile acids are formed and concentrated by the liver, and are excreted by the liver into the bile and pass to the gastro-intestinal tract (duodenum) via the bile duct, where they act as important biological emulsifiers. They help to solubilise fat globules from the food so that the water soluble enzymes or lipases can react with the fat molecules and split them to facilitate fat absorption. Bile acids also facilitate the major excretion route of cholesterol.
All fish and shrimp examined to date display reduced growth and survival, and poor food conversion efficiency when fed experimental diets deficient in the essential fatty acids (EFA). The following additional gross anatomical deficiency signs have been reported under laboratory conditions with juvenile fish or shrimp fed EFA deficient diets:
| Fish/shrimp species | EFA deficiency signs1 |
| Rainbow trout (S. gairdneri) | Increased mortality, increased susceptibility to caudal fin erosion by Flexebacterium sp., fainting or shock syndrome, decreased haemoglobin and erd blood cell volume (1); fatty infiltration/degeneration of liver, swollen pale liver (1,2); reduced spawning efficiency (low hatching/survival rate, 3) |
| Coho salomon (O. kisutch) | Swollen pale liver, increased hepatosomatic index (fatty liver), high mortality (2) |
| Chum salmon (O. keta) | Swollen pale liver, increased hepatosomatic index (fatty liver), high mortality (2) |
| Common carp (C. carpio) | Increased mortality (4); fatty liver (5) |
| Eel (A. japonica) | Increased mortality (6) |
| Tilapia (O. niloticus) | Swollen pale liver, fatty liver (7) |
| Red sea bream (C. major) | Reduced spawning efficiency (decreased hatching rate/survival, 3) |
| Turbot (S. maximus) | Increased mortality, reduced growth, degeneration of gill epithelium (8) |
Dietary EFA deficiencies generally result from poor feed formulation.
Under laboratory conditions it has been shown that a dietary excess of EFA may exert a negative effect on growth and feed efficiency (rainbow trout - Yu and Sinnhuber, 1976; Takeuchi and Watanabe, 1979; coho slamon - Yu and Sinnhuber, 1979; channel catfish - Robinson and Lovell, 1984; Lewis, Marks and Stickney, 1985; nile tilapia - Takeuchi, Satoh and Watanabe, 1983; Stickney and Wurts, 1986).
Cyclopropenoic acid is a toxic fatty acid found in the lipid of cottonseed products. Experimentally, cyclopropenoic acid has been shown to reduce growth rate in rainbow trout and to act as a potent synergist for the carcinogenity of aflatoxins (Lee and Sinnhuber, 1972; Hendricks et al., 1980). Other pathologies observed with trout include extreme liver damage (pale in colour) with increased glycogen deposition and decreased protein content, and a decrease in activity of several key enzymes (Roehm et al., 1970; Taylor, Mongomery and Lee, 1973).
In the absence of suitable antioxidant protection lipids rich in PUFA are highly prone to auto-oxidation on exposure to atmospheric oxygen. Under these conditions, the nutritional benefit of EFA in fact becomes deleterious to the health of the fish or shrimp. Feedstuffs rich in PUFA which are particularly susceptible to lipid oxidative damage (oxidative rancidity) include fish oils, fish meal, rice bran and expeller oil seed cakes containing little or no natural antioxidant activity. During the process of lipid auto-oxidation chemical degradation products are formed, including free radicals, peroxides, hydroperoxides, aldehydes and ketones, which in turn react with other dietary ingredients (vitamins, proteins and other lipids) reducing their biological value and availability during digestion. At present oxidative rancidity is believed to be one of the major deteriorative changes which occurs in stored feedstuffs (Cockerell, Francis and Halliday, 1972; Chow, 1980).
Numerous gross anatomical pathological signs have been reported in fish fed rations containing oxidized fish/plant oils with no antioxidant (vitamin E) protection:
| Fish species | Pathological effects of oxidized fish oil1 |
| Tilapia (O. niloticus) | Marked congestion, with some haemorrhage, in dermal vessels around snout and at bases of pectoral/ dorsal fins, lordosis, exopthalmia, abdominal swelling (oedema), cataract, orbital collapse, darkening of liver, marked distension of bile duct, steatitis of all abdominal fat bearing tissue, deposits of intracellular ceroid in liver, spleen, kidney and choroid, increased mortality (1) |
| Chinook salmon (O. tshawytscha) | Dark body colouring, anaemia, lethargy, brown-yellow pigmented liver (ceroid deposition), abnormal kidney and evidence of gill clubbing (2) |
| Common carp (C. carpio) | Poor growth, loss of appetite, muscular dystrophy, high mortality, reduced absorption of dietary lipids (3–5) |
| Channel catfish (I. punctatus) | Poor growth, poor food conversion efficiency, increased mortality, exudative diathesis, muscular dystrophy, depigmentation, fatty livers (6) |
| Yellow tail (S. quinqueradiata) | Reduced growth, swollen liver, decreased lipid deposition (7); anorexia, leaning of dorsal muscle, muscular dystrophy (8) |
| Rainbow trout (S. gairdneri) | Reduce growth (9,10); poor food conversion efficiency (9); microcytic anaemia (10,11); reduced haematocrit and haemoglobin content (9); liver lipoid degeneration (ceroid accu-mulation, 10,11); severe muscle damage (9); in-creased mortality and erythrocyte fragility (9,11,12) |
With the exception of the study of Soliman, Roberts and Jauncey (1983) with O. niloticus, the pathological effects of oxidized lipids have been shown to be prevented by dietary supplementation with dl-alpha-tocopherol acetate (vitamin E).
In the absence of suitable antioxidant protection the rate of lipid auto-oxidation in stored feedstuffs has been found to increase in the presence of lipoxidase (present in raw soybeans); haeme compounds (myoglobin/haemoglobin are pro-oxidants present in meat/fish meals); peroxides (product of lipid auto-oxidation); light (UV - formation of singlet oxygen and free radicals); increased temperature (reaction rate); and trace elements (Fe and Cu have been found to accelerate lipid oxidation by direct electron transfer in redox reactions, whereas Zn induces the breakdown of hydro-peroxides to free radicals (ADCP, 1983).