Dr. B.N. Singh
Fish Nutrition and Fish Feed Technology Section
Freshwater Aquaculture Research and Training Centre
(CIFRI)
Dhauli, Kausalyagang, Bhubaneswar, Orissa
I. INTRODUCTION
Scientific fish culture in India aims at high rate of survival and production of healthy fish seed (fry and fingerlings) and table-sized fish. The natural food (phytoplankton, zooplankton and benthos etc.) is highly proteinous. About 50% of plankters dry weight is protein. In the absence of artificial feed, part of this protein may be utilized for meeting maintenance energy requirements of fish resulting in reduced rate of growth. Addition of energetic feeds such as grain and grain by-products corrects the protein carbohydrate ratio and favours utilization of natural protein for growth, thus increasing fish production. It is clear that growth effect of carbohydrate-rich feedstuffs will continue as long as protein in natural food is sufficient for fish growth. Density of spawn, fry and fingerlings etc. per unit area being high in intensive fish culture, protein and certain other essential nutrients may become growth limiting factors. This is overcome by supplementary feeding with carbohydrate and quality protein-rich diets balanced with vitamins and/or minerals and trace elements etc. provided the diet is of right composition, easily digestible and has high assimilation and conversion efficiencies. Therefore, not only the feed should be balanced and a complete diet but also that it should be formulated according to nutritional requirements of carps at various stages in their life.
Most of the early fish nutrition studies were carried out on cold water fishes like salmonid fishes (Trout and Salmon). However, during the last 2 decades or so, much attention has been paid to the nutritional requirements of few warmwater species viz. common carp (Cyprinus carpio) and Channel catfish, (Ictalurus punctatus). Warmwater fishes and shell fishes are those freshwater, brackishwater and marine species that have optimum growing temperatures of 25–30°C. Recently, nutritional and feed formulation studies on cultivable Indian carps and exotic carps have received attention of Indian Scientists also, and such works have been intensified during the last 10 years or so. To meet these research objectives fish nutrition and feed formulation laboratories have been established at Freshwater Aquaculture Research and Training Centre, Dhauli, Bhubaneswar and both basic and applied aspects of nutritional studies are undertaken.
A. NUTRITIONAL REQUIREMENTS
Nutrients required by the fish are broadly classified into the following categories:
Energy requirements of fish is also an important aspect of nutrition. Like other animals carps also require energy for maintenance and growth. Growth of fish is possible only after their maintenance energy requirements are met. The nutrient requirements vary according to species, genetic strain, sex and sexual maturity which affect growth rate, feed conversion and carcass composition finally affecting fish yield and fish flesh quality. The required dietary Level of nutrien ts will also be influenced by the following:
1. Protein and amino acids
Proteins are large, complex, organic compounds made up of amino acids. The amino acids are linked together through peptide bonds and cross-linked between chains by sulfhydryl bonds, hydrogen bonds and Van der waals forces. Although, altogether 23 amino acids have been isolated from natural proteins, most proteins are made up of 20 major amino acids.
Protein is the most important nutrient for growth performing a central role in the structure and functioning of all living organisms. Carps, like other animals must consume protein to maintain a continuous supply of amino acids. The consumed protein is digested or hydrolysed to release free amino acids that are absorbed from the intestinal tract of the animal and distributed by the blood to various organs and tissues. These amino acids are then used to synthesize new proteins. Therefore, a regular supply of optimum amount of quality protein is essential either to build new tissues (for growth and reproduction) or to repair worn out tissues.
(i) Gross protein requirements
In general, gross protein requirements are highest for larval stages but it decreases as fish grows in size. Protein requirement of carps is also influenced by water temperature, natural food availability (in ponds), the amount of non-protein energy in the diet, dietary protein quality, sex of fish and stocking density. Protein requirements of carps and certain other fishes are given in Table-I. It incidates that the protein requirements of carps is high and it may vary according to strain (see common carp) or environmental conditions also.
It has been observed that water temperature plays a significant role in protein assimilation and growth (Singh et al., 1979). The growth rate of rohu fingerlings feeding on artificial feeds having iso-protein levels (29.5–30.8%) was more than doubled (250–450.5 mg weight gain/day; and 5.4–12.2% feed conversion efficiency) at 26–29°C as compared to water temperatures of 18–22.5°C (90–220.5 mg weight gain/day; and 2.9–7.7% feed conversion efficiency). Singh et al. (1977 and 1979) found more than 100% increase in growth rate of rohu fingerlings if they were fed with 50% fish meal + 50% wheat powder diet (crude protein 29.5%) as compared to the diet of 50% groundnut cil cake + 50% rice bran. Protein quality also has a very significant effect on growth of carps. Protein quality refers to the nutritional value of proteins which is based on amino acid Sequence of protein source specially essential amino acids content and the biological availability of amino acids from the protein. When viewed from the point of essential amino acid content of the above diets, it is seen that the former diet has methionine + cystine content of 1.71% at 29.5% crude protein content (chemical score 107.3) as compared to 0.51% methionine + cystine content in latter at 30.75% crude protein (chemical score 30.18).
(ii) Amino Acids Requirements
About 23 amino acids have been isolated from natural proteins and 10 of these have been found to be indispensable (essential) for certain fishes including common carp, and others are grouped under dispensable (non-essential) amino acids. The essential amino acids (E.A.A.) are those which can not be synthesized at all in the body of the animal or they are not synthesized in sufficient quantity to support maximum growth. The 10 essential amino acids are arginine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan and valine. However, two non-essential amino acids (i.e. cystine and tyrosine have been found to have some sparing effect on E.A.A. i.e. methionine (cystine-methionine) and phenylalanine (tyrosine-phenylalanine combinations).
The quantitative essential amino acid requirements of common carp and certain other fishes is given in table-IIa. Studies on E.A.A. requirement of Indian major carps are already in progress using synthetic diets. Singh (unpublished observations) observed that maximum total E.A.A. level required in the synthetic diet for rohu fry was 27.4% having 1.58% methionine + cystine and 3.83% lysine. These are very similar to that used by Nose (1978) for common carp i.e. total maximum E.A.A. 28.95% having methionine and cystine 1.76% and 3.57% lysine. This indicated that E.A.A. requirement of rohu might be very similar to common carp. Table-II b clearly shows the importance of optimum requirement of E.A.A. such as methionine in diets of rohu. It further indicates that the optimum methionine + cystine requirement of rohu fingerlings is high (3.1% per 100 gm protein or 1.045% per 34.2% crude protein in the diets) which compares well with that of common carp (Table-IIa).
2. Carbohydrates
Carbohydrates are major source of dietary energy which can be used to meet the entire metabolic energy requirements leaving proteins for growth. Carbohydrates range from simple sugars glucose and fructose (monosaccharides), sucrose and maltose (disaccarides) to complex starches and cellulose (polysaccharides). Carbohydrates are present in small quantities in the animal body as glycogen, sugars and their derivatives. The metabolizable energy for fish ranges from near zero for cellulose to about 3.8 Kcal/g for easily digested sugars. Metabolizable energy (M.E.) of raw starch ranges from 1.2 to 2.0 Kcal/gm. Cocking of starch can in crease its ME to 3.2 Kcal/g. Feedstuffs of plant origin are good sources of carbohydrates and it is the cheapest nutrient in fish feeds.
Carps make good use of carbohydrates. Requirement of carbohydrates by the fishes is highly variable (10–45% of the diet). The optimum carbohydrate (as dextrin) requirement of common carp and rohu has been shown to be 26% (Sen et al. 1978) while for mrigal fingerlings it was 28% (Singh & Sinha 1981) in synthetic diets. Although common carp can utilize higher levels of carbohydrate, diets containing over 40% dextrin retarded growth and lowered feed efficiency (Furuichi and Yone, 1980). Various enzymes taking part in carbohydrate metabolism involving major pathways such as gluconeogenesis and glycogen synthesis, glycolysis, tricarboxylic acid cycle and pentose phosphate shunt have been studied for common carp also (Shimeno, 1982).
3. Lipids
Lipids are broadly classified as i) fats, ii) phospholipids, iii) Sphin gomyelins, iv) Waxes, and v) Sterols, Fats are the fatty acid esters of glycerol and are the primary energy depots of animals. The metabolizable energy of fat is estimated to be 8–9 Kcal/g. Dietary lipids help in absorption of fat-soluble vitamins. Phospho-lipids and sterol esters play a vital role in the structure of biological membranes both at cellular and subcellular levels. Triglycerides (neutral fats) are often the storage lipids and reflect the fatty acid composition of the feed than do the phospholipids. Lipids are also important in textural and flavour properties of fish. Many of fish fats contain numerous unsaturated double bonds (Polyunsaturated fatty Acids, PUFA) in the fatty acid structures. The fatty acid composition of fishes is affected by environmental factors such as temperature and salienity, seasonal variations, maturity and also diet composition. Fats are easily digested (digestibility about 90% or higher) and assimilated by fishes.
Fat requirements of common carp is met by 5% fats as Serytean oil (Sin, 1973). He also found some sparing action of fats for proteins in common carp. This carp has an essential fatty acid (F.F.A.) requirements for both W3 and W6 fatty acids. The best weight gains and feed conversions are obtained in common carp receiving a diet containing both 1%, 18:2W6 and 1 percent 18:3 W3 (the first number identifies the number of carbons; the second number, the number of double bonds; and the last number, the position of double bonds) fatty acids. However, PUFA 20:5n-3 and 22: 6n-3 are more efficient than 18:2n-6 or 18 : 3n-3 (Takeuchi et al., 1979) in common carp. Moreover, the hatchability of eggs from common carp is greatly reduced when the 22: 6n-3 of the egg lipids is less than 10 percent (Shimma et al., 1977). However, requirement of the n-3 series of fatty acid by carp creates problem with respect to feed storage since these type of fatty acids are very prone to oxidation. The products of lipid oxidation may react with other nutrients such as proteins, vitamins etc., and reduce the available dietary levels or the oxidation products may be toxic (Halver 1980).
Moreover, the fat content of carp diets should generally be about 5–10% particularly providing PUFA sources having n-3 and n-6 series. Sen et al. (1979) recorded maximum feed efficiency using 7% oil in the synthetic casein diets on fry and fingerlings of rohu and common carp. Singh et al. (1981) and Singh (1983) recorded maximum rate of growth of mrigal fry with 6–9% fat.
4. Vitamins
Vitamins are organic compounds required in trace amounts in the function of most forms of life but which some organisms are unable to synthesize. Like other fishes, carps meet their vitamin requirements from natural food in ponds. Since vitamins play a major role in maintaining most of the physiological and metabolic processes of fishes, these are to be supplied in artificial complete feed and also supplementary feeds. Vitamins are of two categories (i) water-soluble vitamins (Vitamin B Complex and Vitamin C) and (ii) fat-soluble vitamins (Vitamin A,D,E and K). Water soluble vitamins have unique coenzyme functions in cell metabolism with the exception of two water-soluble growth factors (Choline and inosital) and ascorbic acid. Vitamin A is involved in calcium transport across some membranes, in reproduction and embryonic development, and in cellular and subcellular integrity. Vitamin D stimulates absorption of calcium from the intestine. Vitamin E also helps in membrane permeability and normal development of vascularized areas such as gonad. Also it is said that Vitamin E protects highly unsaturated fatty acids in lipids of biological membranes from oxidation in the presence of molecular oxygen.
Vitamin requirements of fishes are affected by the size, age and growth rate of fishes, environmental factors and also by nutrient in terrelationships. Table-III gives the quantitative requirements of vitamins in common carp. This table also gives the major vitamin deficiency signs in common carp which throws light on the functions of various vitamins. It has been observed that both water-soluble and fat-soluble vitamins are required for healthy growth of mrigal and rohu (Singh & Sinha, 1981). The growth rate of rohu fingerlings is significantly in creased on addition of water-soluble and fat-soluble vitamins (Singh et al., 1984). Ascorbic acid deficiency causes scoliosis and lordosis (deformities of spinal column) in rohu and mrigal particularly if spawn is deprived of vitamin C from the beginning of the rearing period, Scoliosis and lordosis have also been observed in catle, silver carp and grass carp (Singh and Radheyshyam, MS). Vitamin C (ascorbic acid) requirement of mrigal in the early part of life has been found to be 65–75 mg/100 g diet (Mahajan and Agrawal, 1979) which appears to be high compared to other fishes. Singh and Radheyshyam (MS) observed that addition of vitamin C @ 200 mg/kg diet prevents occurrence of scoliosis and lordosis in rohu and mrigal spawn and fry.
5. MINERALS
Minerals are important nutrients as these are required for normal bone, tissue, blood plasma and hemoglobin formations and also for many enzymatic reactions. Calcium (Ca) and Phosphorus (P) are required for the formation of skeletal tissues. The ratio of calcium to phosphorus in scales and bones of common carp ranges from about 1.5 to 2.1. Calcium is also essential for blood clotting, muscle function, proper nerve impulse transmission, osmoregulation and for serving as a cofactor during various enzymatic processess. Phosphorus is also involved in energy transformations, cellular membrane permeability, genetic coding and general control of reproduction and growth. Fishes absorb a good quantity of calcium from water and rest from diet but most of phosphorus should be provided in the diet. For common carp, the minimum requirement of calcium in diet is about 0.028% and that of phosphorus is 0.6–0.7%. Other minerals required by fish are magnesium, sodium, chlorine potassium and chromium etc.
Trace elements required in traces are growth stimulants. Elements like manganese, copper, iron, cobalt, iodine and zinc are required in minute quantities in balanced ration (Table-VI) mainly for improving protein assimilation and survival rates. Cobalt chloridc and manganese @ 0.01 mg/day/fish gave higher rates of survival and growth of spawn, fry and fingerlings of Catla catla, Labeo rohita and Cirrhinus mrigala (Sen and Chatterjee, 1979).
B) CARP DIGESTIVE PHYSIOLOGY
The digestive physiology in fish concerns particularly with digestibility of feedstuffs, the digestive enzymes, absorption and nutrients availability from feeds to the fish.
1. Digestibility and Absorption:
Digestibility of feed in fishes is most often expressed in terms of digestible energy (DE), DE is defined as the energy in the food consumed, or in take energy (IE), minus the energy present in the faeces(FE).
DE = IE - FE
Metabolizable energy (ME) is defined as DE minus the energy excreted in the urine (UE) and that excreted through the gills (ZE), primarily in the form of ammonia:
ME = DE-(UE + ZE)
Only some information on ME and DE values in feedstuffs fed to fishes are available. This makes diet formulations, specially for warm-water fishes, very difficult since formulations should be done on the basis of DE requirement of the species concerned and not based on information on unrelated animals. This is particularly so, since fish have lower energy requirements than warm-blooded animals because of the following:
As the energy content of food regulates the amount of food eaten by the fish, therefore, it is important to provide optimal level of energy with optimal protein content of the diet. Based on preliminary observations, a digestible energy (DE) requirement of 8–9 Kcal/g protein is recommended for common carp.
2. Digestive enzymes:
Enzymes responsible for the digestion of carbohydrates (amylases), proteins (proteases), and fats (lipases) occur in pyloric caecae and intestinal mucosa of fishes. However, the initiation of digestion is in stomach in species that possess true stomach, where hydrochloric acid secreted leads to the activation of pepsin. The maximum reaction usually takes place within the pH ranges of 2.0 to 2.2. However, some fish such as the common carp, silver carp, grass carp, rohu, mrigal and catla etc. do not possess a true stomach and maintain a digestive tract pH that is much less acidic than fishes with stomachs. The distribution of pH in the intestine of certain carps is given in Table-IV.
Warmwater fishes have adaptations that assist in chewing or grinding food. For example, grass carp have strong pharyngeal teeth that aid in grinding macrophytic vegetation. Tilapia has rows of fine pharyngeal teeth that are used to break up food particles prior to digestion.
Carbohydrate digesting enzymes that occur at highest levels in the intestine of common carp are maltase and amylase. These are accompanied by lower levels of sucrase, lactase and related enzymes (Kawai and Ikeda, 1971). Enzymes that digest carbohydrate have been reported from common carp within 7 to 10 days of hatching (Kawai and Ikeda, 1971). However, amylases are not restricted to herbivorous species, they have also been found in such carnivores as red sea-bream.
The ability of fish to convert dietary protein into body flesh is dependent on its ability to hydrolyze dietary protein into digestible peptides and amino acids. Peptides and amino acids are then absorbed from the gut and synthesized into tissue protein. The most important protein digestive enzymes involved are the endopeptidases, chymotrypsin and trypsin; and the exopeptidases, aminopeptidase and carboxypeptidase. However, pepsin is also an important enzyme in true stomach possessing fishes. The distribution of proteases in certain carps is given in Table-V.
Tryptic and chymotryptic levels were highest in the first half of the intestine of the grass carp than in the second half. Hepatopancreas of grass carp has been found to be the primary site of tryptic, chymotryptic and carboxypeptidase secretion (Lane, 1973). Thus, it has been shown that the major organs of protein digestion in grass carp is hepatopancreas. In grass carp, it has been found that proteolytic activity is greatest in mid-intestine, lipase is most active in the anterior intestine and amylase is most active in the gall bladder and mid-intestine.
3. Digestibility determinations:
The determination of digestibility involves measuring the amount of specific nutrient ingested and substracting the level present in the feces following digestion. This gives apparent digestibility of nutrients. However, endogenous material such as secretions from within the intestinal tract, sloughed epithelial cells and other materials of metabolic origin may also occur in the feces. If, the values of endogenous materials is deducted from apparent digestibility values, true digestibility is obtained. But from a practical standpoint, determination of endegenous fecal losses is not considered to be significant and in most of the digestibility studies, the apparent digestibility of nutrients has been determined. However, leaching of nutrients from voided fecal matter in the ambient water tends to increase the digestibility values in feces (Smith and Lovell, 1971).
The method presently used by most fish nutritionists to determine digestibility of feeds, feed ingredients is by using chromic oxide (Cr2O3) as an indigestible marker at 0.5 to 1.0 percent in test diets (Furukawa and Tsukahara, 1966). By analysing the feed and feces for their various components (Protein, lipid, carbohydrate and energy etc.) the digestibility of each nutrient can be determined from the following formula:
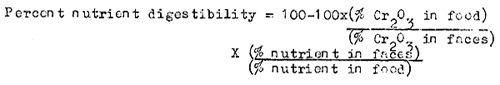
However, a general idea of digestibility can be obtained by measuring total digestibility simply by monitoring the difference between the total amount of feed fed to the fish and the amount of fecal matter voided. This will allow the preliminary screening of a large number of local ingredients for studies using precise and modern chromic oxide methodology. Such studies have shown that carps have good total digestibility and nutrient absorption for several locally available feed ingredients and their admixtures (Table-VI).
Digestibility values based on DE and ME measurements for several feedstuffs have been obtained from fishes other than carps (see values below) but recently DE studies for carps have been taken up at FARTC.
| Digestible Energy (Kcal/kg) | |||
| Feed | Channel catfish | Tilapia | |
| 1. | Corn, yellow grain non-heated | 1,100 | 2,460 |
| 2. | Corn (heated) | 2,530 | 3,020 |
| 3. | Fish meal (Menhaden) | 3,900 | 4,040 |
| 4. | Wheat, hard red grain | 2,550 | 2,890 |
| 5. | Oil, Safflower | 8,830 | - |
| 6. | Oil, Sunflower | 8,930 | - |
A general idea of total digestibility of feeds and feedstuffs can be obtained by studying the total amount of nutrients fed.
The crude protein digestibility of phytoplankton by silver carp in polyculture ponds was as follows (Data-Manadhar, 1977)
| Phytoplankton | Protein Digestibility (%) |
| Dictiosphaerium | 68.0 |
| Scenedesmus | 74.9 |
| Sphaerocystis | 64.2 |
| Oscillatoria | 75.9 |
| Merismopedia | 71.6 |
| Spirulina | 83.9 |
| Nannochlorus | 65.8 |
| Mixed algae (green+blue green) | 71.3 |
| Group A | |
| (i) Blue green algae | 74.8 |
| (ii) Green algae | 68.2 |
| Group B | |
| (i) Phytoplankton with mucilage sheath | 67.9 |
| (ii) Phytoplankton with no mucilage sheath | 75.1 |
4. Factors affecting digestibility
Digestibility is affected by enzymes present in the gut, meal size, frequency of feeding, the proportion of nutrients in the diet and also by the texture of the feed and oxygen content in ambient waters (NRC, 1985 and Singh et al. 1981). It has been observed that several fishes have decreased absorption efficiency as meal size increases. However, contrary to general belief, digestibility of nutrients remains the same over the range of temperatures encountered in normal aquaculture practices (NRC, 1981).
5. Absorption of nutrients from the gut
As regards the abscrption sites in the gut, it has been found that fat is most intensively absorbed in upper two thirds of the intestine. In grass carp, the absorption of protein takes place in the anterior 40–50% of the gut and the middle in testinal segment has the ability to absorb macromolecules of protein by pinocytosis. A similar situation appears to exist in silver carp, which shows strong alkaline phosphatase activity in the anterior intestine (Singh and Grizzle, unpublished Observation -- work done as visiting scientist at Auburn University, Alabama, U.S.A.).
Stroband and Van der Veen (1981) observed that almost all essential amino acids are absorbed rapidly in the anterior 40–50% portion of the intestine of grass carp. They further observed that macromolecules of protein such as ferritin is absorbed in the middle segment of the intestine by pinocytosis, i.e. engulfing of macromolecules of protein by the enterocytes. Such studies are very much wanting for silver carp, Indian major carps and other carps.
6. Carbohydrate metabolism in fish
Properties, distribution, activity and temperature optimd of the following six engymes involved in carbohydrate metabolism in common carp (an omnivore) and Yellow tail, Seriola quinqueradiata (a carnivore) along with certain other fishes have been studied in detail by Shimeno (1982).
He observed that the activities of all engymes of carbohydrate metabolism showed a decrease during starvation. However, both in carp and yellowtail the regulating mechanism of carbohydrate metabolism during starvation can be defined as the maintenance of blood sugar value. The glycogen content is regulated by the balance between glycolysis and gluconeogenesis.
Further, it has been found that when starch is added to the diet growth rate, feed efficiency and calorie efficiency are reduced as the amount of starch is increased beyond 40% in common carp diets. On the contrary, protein efficiency increased with an increase of starch in the diet. Thus, although the addition of starch or fat in the diet affects growth and feed efficiency in fish, the possibilities of lowering protein levels in the diet on the basis of sparing action of starch or fat should nonetheless be explored. Such studies will be very important in least-cost feed formulations as protein is the most expensive in feeds.
REFERENCES
A. Nutritional Requirements
Dabrowski, K. 1977. Protein requirements of grass carp fry (Ctenopharyngodon idella). Aquaculture-12 : 63–73.
Garling, D.L. Jr., and R.P. Wilson, 1976. Optimum dietary protein to energy ratio for channel catfish fingerlings, Ictalurus punctatus J.Nutr. 106 : 1368–1375.
Halver, J.E., 1980. Proteins and amino acids. In Fish Feed Technology, Aquaculture Development and Coordination programme of UNDP-FAO, Rome, 1980, ACDP/REP/80/11, pp. 31–40.
Jauncy, K. 1982. The effects of varying dietary protein level on the growth, food conversion, protein utilization and body composition of juvenile tilapias (Sarotherodon mossambicus), Aquaculture, 27: 43–54.
Mahajan, C.L. and N.K. Agrawal, 1979. Nutritional requirement of vitamin C in Cirrhinus mrigala during early growth. Abstr. Symp. In land Aquaculture, CIFRI, Barrackpore, 116.
Mazid, M.A., Y. Tanaka, T. Katayama, M.A. Rahman, K.L. Simpson and C.O. Chichester, 1979. Growth response of Tilapia zilli fingerlings fed isocaloric diets with variable protein levels. Aquaculture, 18 : 115–122.
National Research Council, 1981. Nutrient requirements of cold water fishes. National Academy Press, Washington, D.C., 1981, Number 16, pp 3–21.
National Research Council, 1983. Nutrient requirements of warmwater fishes and shellfishes. National Academy Press, Washington, D.C. 1983. Revised Edition, pp. 2–29.
Nose, T.S. Arai, D.Lee and Y.Hashimoto, 1974. A note on amino acids essential for growth of young carp. Bull.Japn.Soc.Sci.Fish. 40: 903–908.
Ogino, C. and K.Saito, 1970. Protein nutrition in fish.I.The utilization of dietary protein by young carp. Bull.Japn.Soc.Sci.Fish. 36: 250–254.
Sen, P.R. and D.K. Chatterjee, 1979. Enhancing production of major Indian carp fry by addition of growth promoting substances. Proc. Edited by J.E.Halver and K.Tiews. World Symp.Fin Fish Nutrition, Fish Feed Technology, Hamburg, West Germany 20–23 June, 1979, Vol.II, pp 189–195.
Sen, P.R., N.G.S.Rao, S.R. Ghosh and M.Rout. 1979. Observations on the protein and carbohydrate requirements of carps. Aquaculture, 13: 121–129.
Sin, A.W. 1973. The dietary protein requirements for growth of young carp (Cyprinus carpio). Hong Kong Fish.Bull.3: 77–87.
Shimeno, S., 1982. Studies on Carbohydrate Metabolism in Fish. Publisher, A.A.Balkema, Rotterdam. 123 pp.
Singh,B.N., 1982. Nutritional requirements and natural and supplementary food of cultivated fishes with special reference to spawn, fry and fingerlings. Souvenir Workshop on the Development of Inland Fisheries in Orissa through institutional finance, FFDA, Balasoro (Orissa), 6–8 March, 1982, pp 121–129.
Singh,B.N. 1983. Nutritional requirements of carps. In Lecture on Composite Fish Culture and its Extension in India. The 1983–84 Session of the training course for Senior Aquaculturists in Asia. NACA/TR/83/7, Sept. 1983, 9 p. (Mimeo).
Singh,B.N. 1984. Nutrition and Feed formulation for carps. Souvenir, Fourth Advisory Committee Meeting of NACA(FAO/UNDP Project), 3–6 December, 1984, Bhubaneswar, Orissa, India pp. 53–67.
Singh,B.N. and Radheyshyam(MS). Poor Management of nursery ponds as one of the primary causes for spinal deformities in fry, fingerlings and table sized carp fishes due to nutritional deficiency.
Singh,B.N. and V.R.P. Sinha, 1981. Observations on the nutrition of Indian major carp. Cirrhinus mrigala (Ham.). Abstr. All India Seminar on Fish Biology, Bihar University, Muzaffarpur, Nov. 26–28, 1981, p. 21.
Singh, B.N., V.R.P. Sinha and D.P. Chakraborty, 1977. Growth and conversion efficiency of rohu, Labeo rohita fingerlings fed on artificial feeds during winter months. Abstr. Proc. 27th Internal. Union Physiol. Sci. Paris, Vol. XIII, p. 700.
Singh,B.N., V.R.P. Sinha and D.P. Chakraborty, 1979. Effects of protein quality and temperature on the growth of fingerlings of rohu, Labeo rohita (Ham.). Proc. World Symp. Fin Fish Nutrition and Fish Feed Technology, Hamburg, W. Germany, 20–23 June, 1978. Vol. II: 303–311.
Singh, B.N., V.R.P. Sinha and D.P. Chakraborty, 1980. Feed in take absorption, conversion and growth of fry and fingerlings of rohu, Labeo rohita (Ham.), Indian J. Fish, 27(No.1&2); 193–200.
Singh, B.N., V.R.P. Sinha, K.Kumar and D.N. Swamy, 1984. Observations on the feed formulations and fortification of conventional fish feed for rohu, Labeo rohita and mrigal, Cirrhinus mrigala fry and fingerlings. Abstr. Proc. 5th All India Seminar on Ichthyology, 13–17, Oct. 1984, Mhow, M.P., p. 48.
Takeuchi, T.T. Watanabe and C. Ogino, 1979. Optimum ratio of dietary energy to protein for carp. Bull. Jpn. Soc. Sci. Fish. 45: 983–987.
Wilson, R.P., D.E. Harding and D.L. Garling, Jr. 1977. Effect of dietary pH on amino acid utilization and the lysine requirement of fingerling channel catfish. J. Nutr. 107, 166–170.
Winfree, R.A. and R.R. Stickney, 1981. Effects of dietary protein and energy on growth, feed conversion efficiency and body composition of Tilapia aurea. J. Nutr. 111: 1001–1012.
B - Digestive Physiology
Anon, 1982–84. Annual Report of Central Inland Fisheries Research Institute, Barrackpore (ICAR). 1982 to 84.
Al-Hussaini, A.H., 1949. On the functional morphology of the alimentary tract of some fish in relation to differences in their feeding. Quart. J. Microsc. Sci. 90: 109–139.
Benuvalet, H. 1933. Etude de la digestion Chez les poissons Sans estomac. C.R. Soc. Biol. Paris, 112 : 640–641.
Bondi, A., Spandorf, A. and Calmi, R. 1957. The nutritive value of different feeds for carp. Bamidgeh, Vol. 9.
Furukawa, A. and Tsukahara, H., 1966. On the acid digestion method for the determination of chromic oxide as an index substance in the study of digestibility of fish feeds. Bull. Japn. Soc. Sci. Fish. 32 (6): 502–506.
Hickling, C.F. 1966. On the feeding process in white Amur, Ctenopharyn godon idella. J.Zool. 148: 408–419.
Jhingran, V.G. 1980. Fish and Fisheries of India. Revised 2nd Edition. Hindustan Publishing Corporation.
Kawai, S. and Ikeda, S. 1971. Studies on digestive enzymes of fishes. IV. Development of digestive enzymes of carp and black sea bream after hatching. Bull. Japn. Soc. Sci. Fish. 39 : 877–881.
Lane, W.L. 1973. Investigations on the proteolytic digestive enzymes of the channel catfish, Ictalurus punctatus (Rafinsque) and white Amur. Ctenopharyngodon idella (Valanciennes). M.S. Thesis. Auburn University, U.S.A. 34 pp.
Lovell, R.T. and B.N. Singh, 1984. Review of book entitled Studies on carbohydrate Metabolism in Fish. By S. Shimeno, Publisher, A.A. Balkema Rotterdam, 123 pp.
Magita, M. and Hashimo, J. 1949. On the digestion of higher carbohydrates by Ctenopharyngodon idella. Bull. Japan. Soc. Sci. Fish. 15(6): (abstrs.)
Manadhar, H.N. 1977. Digestibility of phytoplankton by silver carp (Hypophthalmichthys molitrix) and three Tilapias (Sarotherodon spp.) in polyculture with channel catfish (Ictalurus punctatus) M.S. thesis, Auburn University, Auburn, U.S.A. 45 pp.
National Research Council, 1981. Nutrient requirements of cold water fishes. National Academy Press, Washington D. C. 1981, Number 16, pp. 3–21.
National Research Council, 1983. Nutrient requirements of warmwater fishes and Shell fishes. National Academy Press, Washington, D. C. 1983, Revised Edition pp. 39–44.
Shimeno, S. 1982. Studies on Carbohydrate Metabolism in Fish. Publisher, A.A.Balkema, Rotterdam. 123 pp.
Singh, B.N. 1984. Nutrition and feed formulation for carps. Souvenir, Fourth Advisory Committee Meeting of NACA, FAO/UNDP Project 3–6 Dec. 1984, Bhubaneswar, Orissa, India, pp. 53–67.
Singh, B.N. and J.M. Grizzle, (MS). Histology of the intastine and localization of alkaline phosphatase in the intestine of silver carp, Hypophthalmichthys molitrix.
Singh,B.N.,V.R.P. Sinha and D.P. Chakraborty, 1980. Feed in take, absorption, conversion and growth of fry and fingerlings of rohu, Labeo rohita (Hamilton). Indian J. Fish. 27(1&2): 193–200.
Singh, B.N. and V.R.P. Sinha, 1981. Observations on the nutrition of an Indian major carp, Cirrhinus mrigala (Ham.). Abstr. All India Seminar on Fish Biology, Bihar University, Muzaffarapur, 26–28 Nov. 1981, p. 21.
Singh, B.N.,V.R.P. Sinha, K.Kumar and D.N. Swamy, 1984. Observations on the feed formulations and fortification of conventional feed for rohu, Labeo rohita and mrigal, Cirrhinus mrigala fry and fingerlings. Abstr. Proc. 5th All India Seminar on Ichthyology, 13–17 Oct. 1984, Mhow, M.P. p. 48.
Smith, B.W. and R.T.Lovell, 1971. Digestibility of nutrients in semipurified rations by channel catfish in stainless troughs. Proc. Annu. Conf. Southeast. Assoc. Game fish Comm. 25: 452–459.
Stroband, H.W.J. and F.H.Van der Veen, 1981. Localization of protein absorption during transport of food in the intestine of grass carp, Ctenopharyngodon idella (Val.) J. Exptl. Zool. 218: 149–156.
Table-1: Estimated dietary protein requirements of certain cultivable carps (Protein requirements of channel catfish and Tilapia are given for a comparision)
| Species | Estimated protein requirement (%) | Reference |
| Common carp (Cyprinus carpio) | 31–38 and 45 | Ogino & Saito'70 Takeuchi et al. '79 Sen et al., 1978 |
| Rohu (Labeo rohita) | 45 | Sen et al., 1978 |
| Mrigal (Cirrhinus mrigala) | 45 | Singh (1983) & Singh et al., (MS) |
| Grass carp (Ctenopharyngodon idella) | 41–43 | Dabrowski (1977) |
| Channel catfish (Ictalurus punctatus) | 32–36 | Garling & Wilson, 1976 |
| Tilapia | ||
| Tilapia aurea(fry) | 56 | Winfree and Stickney, 1981 |
| T. aurea | 34 | -do- |
| T. mossambica | 40 | Jauncey, 1982 |
| T. Zilli | 35 | Mazid, et al., 1979 |
Table-IIa. Essential amino acid (EAA) requirements of common carp (EAA of channel catfish and Chick is given for a comparision). Requirements are expressed as per cent of protein. In paren thesis, the numerators are requirements as percent of diet and the demominators are percent total protein in diet. Data for common carp from Nose et al. (1977), Channel cat fish from Wilson et al., (1977) and for Chick from NRC, 1981.
| Amino Acid | Common carp | Channel catfish | Chick |
| Arginine | 4.2(1.6/38.5) | 4.3(1.03/24) | 5.6(1.00/18) |
| Histidine | 2.1(0.8/38.5) | 1.5(0.37/24) | 1.4(0.26/18) |
| Isoleucine | 2.3(0.9/38.5) | 2.6(0.62/24) | 3.3(0.60/18) |
| Leucine | 3.4(1.3/38.5) | 3.5(0.84/24) | 5.6(1.00/18) |
| Lysine | 5.7(2.2/38.5) | 5.0(1.5/30) | 4.7(0.85/18) |
| Methionine | 3.1(1.2/38.5) | 2.3(0.56/24) | 3.3(0.60/18) |
| Phenylalanine | 6.5(2.5/38.5) | 5.0(1.2/24) | 5.6(1.00/18) |
| Threonine | 3.9(1.5/38.5) | 2.0(0.53/24) | 3.1(0.56/18) |
| Tryptophan | 0.8(0.3/38.5) | 0.5(0.12/24) | 0.9(0.17/18) |
| Valine | 3.6(1.4/38.5) | 3.0(0.71/24) | 3.4(0.62/18) |
Table-IIb: Showing essentiality of high methionine+cystine content in the diet of rohu fingerlings. The chemical score is based on comparision with whole egg protein of the following composition (as percentage of protein): Cystine 2.2% and methionine 3.3%.
| Sl. No. | Composition of diet (% Ingredients) by weight | Water temp. (°C) | Crude Protein content (%) | Methionine + Cystine (%) | Chemical score of Methionine + Cystine | Growth rate (Daily increment in wt.for 100g fish in mg | Reference |
| 1. | Groundnut oil cake (GOC)+Rice bran (RB) (50:50) | 26–29 | 30.75 | 0.51 | 30.18* | 50.1*** | Singh et.al. 1979 |
| 2. | GOC+RB+CaHPO4+Salt +TM + Vit.mix. (65:33.2:1.5:0.3:0.1:0.1) | 25–30 | 34.2 | 0.50 | 26.54* | 34.5*** | Singh et.al. 1984 |
| 3. | GOC+Sesame oil cake+ RB+CaHPO4 +Salt +TM + Vit.mix (24.5:24.5:49:1.5:0.3:0.1:0.1) | 25–30 | 25.5 | 0.80 | 56.91 | 49.2** | Singh et al. 1984 |
| 4. | FM+GOC+WB+Yeast (20:40:35:5) | 19–27 | 34.2 | 1.045 | 55.54 | 310.9* | Singh et al 1980 |
| 5. | Fish meal (FM)+Wheat powder (Flour) (50:50) | 26–29 | 29.5 | 1.71 | 107.3 | 358.4 | Singh et al 1979 |
* Methionine + cystine chemical score shows seriously limiting value for growth.
** Lysine is limiting
*** Methionine + cystine and also lysine is limiting
+ None of the essential amino acids are limiting.
Table-III: Minimum levels of vitamins required in the diet to prevent signs of deficiency. Major vitamin deficiency signs in common carp and other carps are also given (From NRC 1983 and Singh 1984).
| Vitamin | Carp Cyprinus carpio (mg/kg diet) | Major vitamin deficiency signs in carps |
| Thiamin | 2–3 | Fin congestion, nervousness, fading of body colour. |
| Riboflavin | 7.0 | Skin and fin hemorrhages, mortality. |
| Pyridoxine | 5–6 | Nervous disorders |
| Pantothenic acid | 30–50 | Poor growth, anemia, skin hemorrhages, exophthalmia. |
| Nicotinic acid | 28 | Skin hemorrhages, mortality |
| Biotin | 1 | Poor growth |
| Folic acid | N | None detected |
| Vitamin B12 | N | None detected |
| Choline | 500–600 | Fatty liver |
| Inositol | 440 | Skin lesions |
| Ascorbic acid | 30–50 | Scoliosis and lordosis and impaired collagen formation |
| Vitamin A | 10,000 IU | Faded colour, exophthalmia, warped operculum, fin and skin hemorrhages |
| Vitamin D | N | Not tested |
| Vitamin E | 200–300 | Muscular dystrophy, mortality |
| Vitamin K | N | Not tested |
Note: Major deficiency signs exclude anorexia and poor growth unless those are the only signs observed. Mortality means high and rapid mortality and thus might be useful to distinguish some vitamin deficiencies N = No dietary requirement demonstrated under experimental conditions, NT = Not tested
Table IV: Showing change in pH of different sections of intestine of carps. Normally pH from Anterior, middle, posterior in testine and rectum were measured, but in mrigal pH was measured from various sections as indicated below:
| Species | Sections of intestine and its pH | |||||||
| Anterior (Proximal) Intestine | Middle in testine | Posterior (distal) Intestine 150cm before Rectal segment | Rectal segment Last 20 cm of rectum | Reference | ||||
| Intestinal bulb | 1st 30 cm behind the bulb | 2nd 30cm behind the bulb | Next 11 cm behind 2nd segment | |||||
| Mrigal(adult fish) | 6.0 | 6.0 | 5.6 | 5.8 | 7.0 | 6.8 | 6.8 | Singh & Kuljeet K.Bhanot (unpublished observation) |
| Rohu fingerling (Total length 14.2cm) | 6.4 | 6.6 | 6.6 | 6.8 | -do- | |||
| (about 10–14 cm behind in testinal bulb,i.e. 1st Section of in testine) | (about 15–19cm behind the 1st section of intestine) | (10–12 cm from rectal opening) | ||||||
| Grass carp | -----------7.4 to 8.5----------- | 7.2–7.6 | 6.8 | Hickling, 1966 | ||||
| Silver carp | -----------7.1 to 7.6----------- | 7.2–7.6 | 7.3–7.6 | Manadhar, 1977 | ||||
| Common carp | -------------7.7------------- | 6.0 | Al-Hussaini, 1943 | |||||
Table V : Showing distribution of digestive enzymes in carps gut. The primary sites of secretion of enzymes is also indicated in cases where known.
| Species | Digestive Enzymes | Lipase | Reference | ||||||||
| Carbohydrases | Proteases | ||||||||||
| Amylase | Maltase | Lactase | Sucrase | Pepsin | Trypsin | Chymotrypsin | Aminopeptidase | Carboxypeptidase | |||
| + + + | + + + | + | + | Not present | + + + | Kawai & Ikeda, 1973 | |||||
| Common carp | (in intestine) | (in intestine) | (By intestine & hepatopane creas) | ----- | + + + | + + + | |||||
| Grass carp | + + + | ----- | ----- | ----- | Absent | + + + | +++ | +++ | + + + | Hickling 1966 & Migita & Hashimo, 1949 | |
| (most active in gut bladder and Mid intestine) | (By hepatopancreas & intestine) | (By Hepato & intestine) | (By intestine) | (Primary site hepato pancreas and also secreted by intestine) | (anterior intestine) | ||||||
Table VI : Showing digestibility of some formulated feed, in rohu and common carp. Data from Singh et al. 1980,84, Singh 1984, Bondi Spandorf and Calmi 1957 and Jhingran, 1980 and Anon (CIFRI), 1982–84.
| Sl. No. | Feed (Ingredients with percent composition) | Crude Protein content (%) | Species | Total digestibility (%) |
| 1. | Wheat powder (Flour) | 11.7 | Rohu fry | 84.6 |
| 2. | Wheat powder + Yeast(5%) | 13.7 | -do- | 84.6 |
| 3. | Soybean meal (powder) | 33.0 | -do- | 79.5 |
| 37.8 | Common carp | 74.4 | ||
| 4. | Soyabean meal + 5% yeast | 35.2 | Rohu fry | 82.0 |
| 5. | Groundnut oil cake+Wheat bran (50 : 50) | 29.2 | -do- | 86.8 |
| 6. | GOC + RB (50:50) | 22.8 | Rohu fingerlings | 68.5 |
| 7. | FM+GOC+WB (10:40:50) | 28.2 | Rohu fry | 84.4 |
| 8. | FM+GOC+WB+Yeast(10:40:50:5) | 30.2 | -do- | 80.8 |
| 9. | FM+GOC+WB(20:40:35) | 33.2 | -do- | 83.6 |
| 10. | FM+GOC+WB+Yeast(20:40:35:5) | 34.2 | -do- | 86.9 |
| 11. | FM+GOC+WB+Yeast(25:65:5:5) | 45.4 | -do- | 85.2 |
| 12. | FM+GOC+WB+Yeast(25:65:5:5) | 45.4 | Rohu fingerlings | 84.4 |
| 13. | GOC+RB+CaHPO4+Salt+TM+Vit.mix. (49:49:1.5:0.3:0.1:0.1) | 23.5 | -do- | 78.3 |
| 14. | GOC+RB+CaHPO4+Salt+TM+Vit.mix. (65:33.2:1.5:0.3:0.1:0.1) | 34.2 | -do- | 53.5 |
| 15. | GOC+WB+CaHPO4+Salt+TM+Vit.mix. (60.73:37.86:1.5:0.3:0.1:0.1) | 25.5 | -do- | 56.5 |
| 16. | GOC+Sesame oil cake+RB+CaHPO4 +Salt+T.M.+Vit.mix. (24.5:24.5:49:1.5:0.3:0.1:0.1) | 25.5 | -do- | 52.3 |
| 17. | Sesame oil cake+RB+CaHPO4+ Salt+TM+Vit.mix. (77.0:21.0:1.5:0.3:0.1:0.1) | 25.5 | -do- | 43.3 |
| 18. | GOC+RB+CaHPO4+Salt+TM+Vit.mix (78.4:19.6:1.5:0.3:0.1:0.1) | 30 | 1. Rohu fry 2. Common carp fry | 63.3 65.5 |
| 19. | GOC+RB+Sal seed cake +CaHPO4 +Salt+TM+Vit.mix. (78.4:9.8:9.8:1.5:0.3:0.1:0.1) | 30 | 1. Rohu fry 2. Common carp fry | 64.2 71.3 |
| 20. | GOC+RB+Sal seed cake+CaHPO4 +Salt+TM+Vit.mix. (78.4:4.9:14.7:1.5:0.3:0.1:0.1) | 30 | 1. Rohu fry 2. Common carp fry | 53.5 64.0 |
| 21. | Chicken feed | 19.3 | Rohu fingerlings | 54.8 |
| 22. | Groundnut oil cake | 17.3–21.2 | Common carp | 67.5–69.8 |
| 23. | Rice bran | 9.2–13.5 | -do- | 79.4 |
| 24. | Maize (corn) | 6.2–9.6 | -do- | 77.9 |
| 25. | Maize (Fresh) | 8.8–9.9 | -do- | 74.9–75.1 |
| 26. | Fresh silk worm pupae | 17.1 | -do- | 34.3 |
| 27. | Dried silk worm pupae | 55.91–57.5 | -do- | 42.3 |
| 28. | Small shrimps | 55.5 | Common carp | 58.9 |
| 29. | Snail (Vivipara) | 14.1 | -do- | 12.9 |
| 30. | Cotton seed meal | 43.0 | -do- | 73.6 |
N.B.: GOC= Groundnut oil cake,
RB= Rice bran,
WB= Wheat bran,
FM= Fish meal