M. Marzalina[9], Z. N.
A. Nashatul & N. Jayanthi
Forest Research Institute Malaysia, Kuala Lumpur, Malaysia
Introduction
Forest genetic resources are the genetically heritable component of intra-specific variability that is of actual or potential use to man. In situ conservation is the principal method for conserving forest genetic resources. International perspectives, as Roche (1994) notes, are important to striking a balance between national ex situ and in situ conservation efforts. For economically important plant species, it is also necessary to consider the relationship between conservation and utilization, and to recognize that biotechnology can play an important role in conservation programmes.
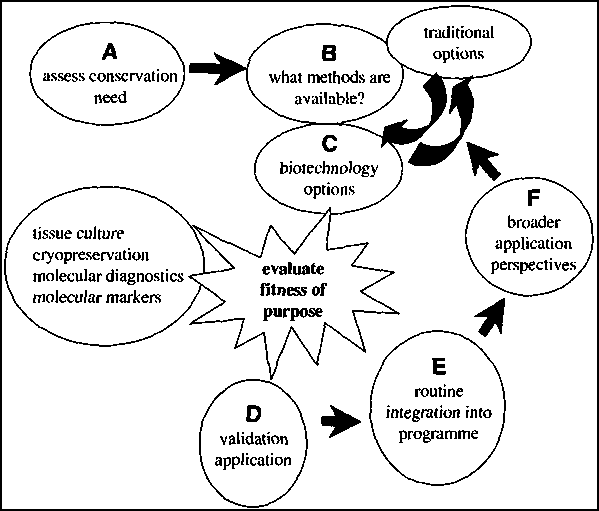
Benson (1999) outlines the key steps to follow when embarking on a conservation strategy that incorporates biotechnology applications (Figure 1). The feasibility of using biotechnology must be considered in terms of resources, expertise and specific training needs, cost and long-term maintenance. This paper addresses these issues in the context of ex situ conservation of Neobalanocarpus heimii, a highly endemic tree species confined to Peninsular Malaysia and Pattani in southern Thailand (Symington 1943; Ashton 1982). It is classified by IUCN as 'vulnerable' but not critically endangered (Chua 1999).
Figure 1. Integrating biotechnology into conservation projects. Source: Benson (1999).
Natural distribution
Descriptions of N. heimii, known locally as chengal, are given by Ashton (1982) in Flora Malesiana and Wong et al. (1994) in the Plant Resources of South-East Asia. The tree produces a heavy hardwood timber which is highly valued for its strength, durability and workability. It also produces a high-quality resin known as dammar. N. heimii is regarded by the timber trade as a 'primary hardwood', but the Malaysian government has banned its export in round log form. According to unpublished information of the Forest Department of Peninsular Malaysia, the largest specimen of N. heimii in Malaysia is in Pasir Raja Forest Reserve in Dungun, Terengganu. This tree is 65m tall, with a girth of 16.75m and a diameter at breast height of 5.33m.
According to Wong et al. (1994) and Chua (1998), N. heimii is extinct in Singapore and faces a high risk of extinction (or may already be extinct) in southernmost peninsular Thailand. In Peninsular Malaysia, N. heimii can still be found in protected areas in most states, except Perlis, Penang and Malacca (Figure 2). It is the second most dominant species in the Pasoh Forest Reserve in Negeri Sembilan, although it accounts for only 1% of all trees (Manokaran 1990). It is often found growing on undulating, well-drained areas with soils of average fertility (Ashton 1982; Wyatt-Smith 1987), but occurs less frequently at higher elevations. Symington (1943) reported that it was often found at low densities (fewer than five trees per hectare) in natural stands. Stands dominated by N. heimii usually have only a few small and intermediate trees. More recent surveys (Chin et al. 1997) suggest high endemism and localised but dense distribution of this species.
Figure 2. The distribution of N. heimii in Peninsular Malaysia and the location of the study sites. Source: L. G. Saw.
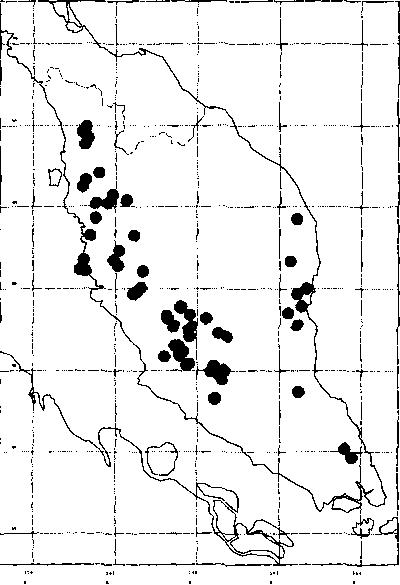
According to Wong et al. (1994), this species is much rarer now than it was early in the 20th century. Its endemism and limited distribution justify conservation measures to safeguard the remaining populations. In view of the demand for this and other timber species, the Malaysian government has taken steps to implement conservation measures and sustainable management practices in remaining forest areas. Most of areas populated by N. heimii in Malaysia's virgin jungle reserve system have been designated as research plots. The largest plot is in Balok Forest Reserve, Compartment 8 in Pahang (Saw & Raja Barizan 1991).
A great deal of experience in cultivating N. heimii has been gained since the era of gutta percha extraction around 1900-1913 (Ashton 1983; Appanah & Weinland 1994). This information is considered adequate for current planting efforts. The limited availability of seed sources, however, is still a constraint to conservation programmes for this species.
Genecology
The elucidation of population structures and patterns of gene distribution within ecosystems provides information that can be used to support in situ conservation efforts. A molecular knowledge of genetic diversity can facilitate germplasm collection and greatly assist in the decision-making processes of ex situ conservation (Benson 1999). An understanding of genetic structure and gene flow within plant populations in their natural distribution range is needed before any conservation plan can be prepared.
Konuma et al. (2000) found that the reproduction of N. heimii is mediated by a long-distance pollinator. They estimated a minimum average mating distance of 524m. According to Appanah (1979), bees from the genera Apis and Trigona are the main pollinators of N. heimii flowers. Any conservation efforts, therefore, should focus not only on the tree itself, but also on its mating and dispersal systems, including pollen and seed dispersal vectors. The disappearance of these vectors would reduce seed production and dispersal, and cause serious reproductive deficiencies.
Appanah (1979) also found that flower production in N. heimii peaks around the fourteenth to fifteenth day of flowering. He concluded that peak flowering events in N. heimii, as in Shorea, tend to occur in a single burst (see Phenology below). Such behaviour may maximize the opportunities for out-crossing and simultaneously increase the size of the potential pool for gene exchange (Chan & Appanah 1980). Owing to the fragmentation of forest areas, the establishment of seed production areas and seed orchards for N. heimii has long been mooted by tree improvement and tree breeder groups. Bringing together genetic resources from various provenances into one orchard would help to maintain a high level of genetic diversity in this species.
Documentation
Information management is an important aspect of germplasm acquisition. Background information on the origins of germplasm accessions is commonly referred to as 'passport' data (Hummer 1999). The collection and documentation of such data are of great importance to ex situ conservation efforts. In 2000, a database known as the Forest Genetic Resources Information System (FORGRiS) was established under the Malaysian-German Forest Planting Material Procurement Programme (MGFPMPP). The database is modelled on the Forest Genetic Resources Information Database (FGRID) developed by the ASEAN Forest Tree Seed Centre project. It allows both centralized and decentralized management of information concerning seed and plant procurement.
The FORGRiS database holds important genecology data on approved forest reproductive materials. These include data on resource types, selected plus trees and monthly phenological monitoring, as well as seed collection, seed storage and nursery management (Thai et al. 1999). Initially, data for most of the commercially valuable tree species have been collected, but efforts are now being made to include also rare and endangered species. Most importantly, this system also follows the OECD scheme for certifying forest reproductive materials. To date, 47 mother trees of N. heimii have been selected and documented under the system in eninsular Malaysia, most of them in Terengganu and Perak. Many more have yet to be recorded, particularly in Pasoh Forest Reserve, Negeri Sembilan, and Balok Forest Reserve, Pahang.
Phenology
Most dipterocarp species tend to flower and fruit erratically. Such behaviour limits the supply of reproductive materials, especially seeds. It has been estimated that, in the aseasonal zones of Southeast Asia, the majority of dipterocarp species flower at intervals of 2-5 years (Wood 1956; Ashton 1969; Burgess 1972). Some species flower annually, but only a few mother trees within the population bear fruit.
Bawa (1998) reported that dipterocarps appear to be strongly cross-pollinated, a feature which could account for poor seed production if flowering trees of the same species occur infrequently and are widely dispersed. This appears to be the case in N. heimii, as natural regeneration beneath parent trees is rarely abundant despite annual fruiting (Elouard et al. 2001). Given that seeds are the only feasible method of propagating and regenerating N. heimii, future supplies of timber are likely to be seriously affected. Production through cuttings has not been successful for this species (A. Fauzi pers. comm.). It is important, therefore, to monitor flowering seasons and to optimize the timing of seed collection.
Several studies have been carried out to determine the ecology and biology of flowering in N. heimii, and to attempt to predict flowering events. Ashton et al. (1988) found that a fall in minimum temperatures sustained for at least 5-8 days caused flowering events eight to nine weeks later. Marzalina et al. (2001) analysed data from 20 years of phenological observations of N. heimii. They found that it tends to flower gregariously almost every year, thus confirming the observations of Medway (1972), Appanah (1979) and Yap (1985). Flowering was observed to occur either annually or biannually, and to peak between March and May, and between September and November (Figure 3). In view of the atypical flowering behaviour of N. heimii compared with other dipterocarps, Appanah (1979) suggested that flowering in this species is driven endogenously.
Seed and seedling collection and storage
The timing of seed collection for N. heimii is critical because seeds germinate soon after falling. The seeds are wingless and heavy (5-7g), so the majority can be found under the canopy of the mother tree. The highest number of seedlings is found within 5m of the stem (Siti Rubiah 1990), and seed dispersal does not extend beyond 16m from the mother tree (Elouard et al. 2001). High rates of mortality near the stem could be caused by competition, nsufficient light or species-specific pathogens. Levels of fruit predation are high, mainly because of insect infestations that destroy 23-37% of the seed crop before the fruits fall. Elouard et al. (2001) found that fungal infections, insect predation and water stress caused seedling mortality rates of 31-52%.
There is usually a high risk of losing seed viability during transit. Once collected, the seeds are best placed in the shade in an open container that circulates air readily. This will reduce heating from respiration and limit fungal growth. Marzalina et al. (1999) found that recalcitrant seeds are more likely to survive if they can be transported in a mobile seed-seedling chamber, which can maintain a temperature of 16-20ºC during a ten-day journey.
Germination
Seeds of N. heimii are highly recalcitrant. Table 1 gives moisture levels and germination rates in different freshly collected seed lots.
Table 1. Germination and moisture content of N. heimii seed. Source: Unpublished data, Seed Technology Section, FRIM.
|
Forest Reserve |
Germination Rate(%) |
Peak Germination Period (days) |
Moisture Content(%) |
|
RIM |
100 |
7-9 |
46.70 |
|
Ampang |
100 |
5-7 |
37.78 |
|
Pasoh |
95 |
4-9 |
37.69 |
|
Ulu Gombak |
86 |
4-5 |
43.05 |
|
Bukit Rengit |
68 |
4-5 |
50.54 |
Germination rates range from 68% to 100%, with moisture contents of 37.7-50.5%. Germination peaks after the fourth day of sowing. Both Sasaki and Ng (1981) and Siti Rubiah (1990) found that germination success is related to seed size and weight. Seeds over 7g in dry weight performed better in terms of growth and development than those below 5g. We also found that seed weight is positively correlated with seedling growth. Large food reserves provide an initial boost to freshly germinated seedlings, allowing the development of large root masses essential for adequate nutrient acquisition (Whitmore 1984; Foster 1986). Siti Rubiah (1990) also found that germination was highest under a light intensity of 25%, whereas seedlings grown in full sunlight grew poorly in height and biomass. These findings indicate that N. heimii is shade tolerant and well adapted to a closed-canopy environment.
Seed storage
A low temperature and low moisture content are fundamental conditions for long-term seed storage of most plants. Storage studies of N. heimii seeds have been limited by the low number of seeds available for experimentation. The conventional approach to securing a limited quantity of germplasm is immediate sowing of short-lived recalcitrant seeds in the nursery. This practice is crucial because delays in sowing are known to reduce germination rate, increase the number of abnormal seedlings and lower field emergence (Krishnapillay & Tompsett 1998).
The optimal storage period for N. heimii seeds is no more than 57 days at 14ºC. This yields a germination rate of 80% for seeds that have been treated with a fungicide dressing of 0.2% Benlate before storage (Yap 1981). Seeds do not die if their moisture content is reduced by half (to 28.0% from an initial 46.7%) before storage. Fungicide treatment followed by partial esiccation and storage at low temperature has also yielded good results for mid-term storage of N. heimii seeds.
Seedling storage
Marzalina et al. (1994) tested the use of a seedling chamber to slow down germination and growth of seedlings. Using this procedure, the Seed Technology Section of FRIM carried out an experiment with freshly collected N. heimii seeds. The surface of the seeds was treated with 0.1% Benlate/Thiram fungicide and allowed to germinate under ambient conditions in containers with moist vermiculite. Once the seeds had germinated, these containers were put into a specially built chamber where the temperature was maintained at 16ºC, relative humidity at 80% and the photoperiod at four hours a day. Germinated seeds in this chamber developed slowly, barely reaching a height of 20-25cm. After 12 months of storage, four-fifths of the seedlings were still alive. These were planted into polybags and weaned under progressively brighter conditions (from 75% to 25% shade) for two months. Post-storage survival was 70%. The hardening of seedlings is this manner is necessary before field planting.
Cryopreservation
Another ex situ conservation technique is cryopreservation, which is designed for long-term storage. Using this technique, recalcitrant seeds from more than ten tropical tree species have been stored in a liquid nitrogen phase (Marzalina & Krishnapillay 1999). When whole N. heimii seeds were desiccated to half of their initial moisture content and plunged directly into liquid nitrogen, none of the seeds survived. Most successful cryopreservation techniques have used excised embryos rather than whole seeds. Embryos are used because they are small, more resistant to desiccation and relatively uniform in size and moisture content. Table 2 lists moisture contents for different parts of N. heimii seeds.
Table 2. Moisture content of N. heimii seeds. Source: Jayanthi and Krishnapillay (2000).
|
|
Whole Seed |
Seed Coat |
Cotyledons |
Embryo |
|
Initial moisture content |
52.87% |
31.53% |
55.10% |
70.70% |
These figures indicate that the moisture content of the embryo is higher than other parts of N. heimii seed. Marzalina and Krishnapillay (1999) found that desiccated embryos of recalcitrant species, despite their higher moisture content, were able to recover after cryopreservation. We found, however, that after six hours of desiccation, the moisture content of N. heimii embryos fell to 27% and they were unable to survive cryopreservation. Even when the embryos were subjected to vitrification using a cocktail of 30% dimethyl sulfoxide, 15% ethylene glycol and 15% glycerol before being plunged into liquid nitrogen, their survival rate was less than 5%. More work is needed to determine the optimal combination of vitrification chemicals to prevent ice nucleation. The Seed Technology Section of FRIM has begun to produce artificial seeds based on somatic embryos. Newly emerged shoot tips from germinated seeds are being used as the source of somatic embryos. This work is still at an early stage and has yet to produce any results. However, cryopreservation techniques for the long-term storage of N. heimii may be available in the near future.
Conclusions
Important information for conserving N. heimii has been collected and documented. In recent years, basic information covering the distribution, ecology, biology, reproductive behaviour, flowering, genetic resources, growth and development, propagation and planting of this species has been updated. More research is needed, however, to sustain effective conservation programmes for this species. This research should address:
The mating and dispersal systems of pollen and seed dispersal vectors.
The minimum population size required for in situ conservation.
The maintenance of linkages and corridors between fragmented forest areas to sustain population viability.
Methods to increase seedling production, either through cuttings, artificial seeds or tissue culture.
Protocols for long-term storage.
Establishment of seed orchards.
The importance of in situ conservation notwithstanding, efforts to improve ex situ conservation techniques should not be dismissed. Using the latest developments in biotechnology, the conservation of N. heimii can be enhanced. All of the available techniques should be evaluated, but other options must also be considered. The need for adequate training and resources should also be addressed. Such efforts should not only concern conservationists, but also government agencies, politicians and international bodies.
Acknowledgements
We would like to thank the Director General of FRIM and the Director of FRIM's Plantation Division for supporting the ex situ conservation studies. The budget was maintained by IRPA projects under the Ministry of Science and Technology of Malaysia. We are grateful to all scientists who are working to safeguard the future of N. heimii. We are also grateful to the Forestry Department for providing access to forest reserve areas within participating states. Thanks are due to the IPGRI Regional Office in Malaysia for providing funds to allow the lead author to present this paper at the workshop.
References
Appanah, S. (1979) The ecology of insect pollination of some tropical rainforest trees. Ph.D Thesis, University of Malaya, Kuala Lumpur.
Appanah, S. & Weinland, G. (1994) Planting quality timber trees in Peninsular Malaysia - a review. Malayan Forest Records 38: 1-247.
Ashton, P. S. (1969) Speciation among tropical forest trees: some deductions in the light of recent evidence. Biological Journal of the Linnean Society 1: 155-196.
Ashton, P. S. (1982) Dipterocarpaceae. In Van Steenis, C. G. G. J. (ed), Flora Malesiana Serial 1. Spermatophyta 9: 251-552.
Ashton, P. S., Givnish, T. J. & Appanah, S. (1988) Staggered flowering in the Dipterocarpaceae: New insights into floral induction and the evolution of mast fruiting in the aseasonal tropics. American Naturalist 132 (1): 44-66.
Bawa, K. S. (1998) Conservation of genetic resources in Dipterocarpaceae. In Appanah, S. & Turnbull, J. M. (eds.), A Review of Dipterocarps - Taxonomy, Ecology and Silviculture. Centre for International Forestry Research, Bogor.
Benson, E. E. (1999) An introduction to plant conservation biotechnology. In Benson, E. E. (ed), Plant Conservation Biotechnology. Taylor & Francis Ltd, London.
Burgess, P. F. (1972) Studies on the regeneration of the hill forest of the Malay Peninsula -the phenology of dipterocarps. Malaysian Forester 35 (2): 103-123.
Chan, H. T. & Appanah, S. (1980) Reproductive biology of some Malaysian Dipterocarps. I -flowering biology. Malaysian Forester 43 (2): 132-143.
Chin, T. Y., Nor Akhiruddin, M., Samsuanuar, N., Yong, T. K., Hasnuddin, M. A. & Mohd Nashir, S. I. (1997) Inventori Hutan Nasional Ketiga: Semenanjung Malaysia. Jabatan Perhutanan Semenanjung, Malaysia, Kuala Lumpur.
Chua, L. S. L. (1998) Neobalanocarpus heimii. In Oldfield, S., Lusty, C. & MacKinven, A. (eds.), The World List of Threatened Trees. World Conservation Press, Cambridge.
Elouard, C., Blanc, L. & Appanah, S. (2001) Regeneration strategy and spatial distribution pattern of Neobalanocarpus heimii in the lowland dipterocarp forest of Pasoh, Peninsular Malaysia. In press.
Foster, S. A. (1986) On the adaptive value of large seeds for tropical moist forest trees - a review and synthesis. Botanical Review 52: 260-299.
Hummer, K. E. (1999) Biotechnology in plant germplasm acquisition. In Benson, E. E. (ed), Plant Conservation Biotechnology. Taylor & Francis Ltd, London.
Jayanthi, N. & Krishnapillay, B. (2000) Neobalanocarpus heimii - preliminary results. Danida Forest Seed Centre & IPGRI Newsletter 7: 17.
Konuma, A., Tsumura, Y., Lee, C. T., Lee, S. L. & Okuda, T. (2000) Estimation of gene flow in the tropical-rainforest tree Neobalanocarpus heimii (Dipterocarpaceae), inferred from paternity analysis. Molecular Ecology 9: 1843-1852.
Krishnapillay, B. & Tompsett, P. (1998) Seed handling. In Appanah, S. & Turnbull, J. M. (eds.), A Review of Dipterocarps - Taxonomy, Ecology and Silviculture. Centre for International Forestry Research, Bogor.
Manokaran, N., LaFrankie, J. V., Kochummen, K. M., Quah, E. S., Klahn, J. E., Ashton, P. S. & Hubbell, S. P. (1990) Stand table and distribution of species in the 50-hectare research plot at Pasoh Forest Reserve. FRIM Research Data 1: 1-454.
Marzalina, M., Abd. Khalim, A. S., Siti Hasanah, M. S., Abd. Rahman, A. J. & Kassim, A. (1999) Mobile Seed-Seedling Chamber: mechanism to maintain germplasm viability over long journey. In Marzalina, M., Khoo, K. C., Jayanthi, N., Tsan, F. Y. & Krishnapillay, B. (eds.), Proceedings of IUFRO Seed Symposium 1998 - Recalcitrant seeds. FRIM Publication, Kuala Lumpur.
Marzalina, M., Jayanthi, N. & Ang, K. C. (2001) Flowering prediction based on 20 years of phenological observations of Neobalanocarpus heimii (King) Ashton. In press.
Marzalina, M. & Krishnapillay, B. (1999) Recalcitrant seed biotechnology applications to rainforest conservation. In Benson, E. E. (ed), Plant Conservation Biotechnology. Taylor & Francis Ltd, London.
Marzalina, M., Krishnapillay, B., Haris, M. & Siti Asha, A. B. (1995) A possible new technique for seedling storage of recalcitrant species. In Ratnam, W., Ahmad Zuhaidi, Y., Amir Husni, M. S. & Darus, A. (eds.), Proceedings of the international workshop of Bio-Refor, Kangar, 28 November-1 December 1994. BIO-REFOR/FRIM Publications, Kuala Lumpur.
Medway, L. (1972) Phenology of a tropical rainforest in Malaya. Biological Journal of the Linnean Society 4: 117-146.
Roche, L. (1994) Strategies for genetic conservation - balance between ex situ and in situ. In Drysdale, R. M., John, S. E. T. & Yapa, A. C. (eds.), Proceedings of International Symposium on Genetic Conservation and Production of Tropical Forest Tree Seed, 14-16 June 1993, Chiang Mai, Thailand. ASEAN-Canada Forest Tree Seed Centre, Muak Lek.
Sasaki, S. (1980) Storage and germination of Dipterocarpaceae. Malaysian Forester 43 (3): 290-308.
Saw, L. G. & Raja Barizan, R. S. (1991) Directory of plant genetic resources in Malaysia. Research Pamphlet 109. 1-161.
Siti Rubiah, Z. (1990) Studies on germination and seedling growth of Neobalanocarpus heimii (King) Ashton. M.Sc. Thesis, Universiti Pertanian Malaysia, Selangor.
Symington, C. F. (1943) Foresters manual on dipterocarps. Malaysian Forest Record 16: 1-244.
Thai, S. K., Abdul Rahman, A. J., Marzalina, M. & Schmalen, W. (1999) Forest Genetic Resources Information System (FORGRiS) - Concept. Project Document No. B4, MGFPMPP, Forestry Department, Kuala Lumpur.
Whitmore, T. C. (1984) Tropical rain forests of the Far East. 2nd Edition. Clarendon Press, Oxford.
Wood, G. H. S. (1956) The dipterocarp flowering season in North Borneo, 1955. Malayan Forester 19: 193-201.
Wong, W. C., Miller, R. B. & Dos Santos, G. (1994) Neobalanocarpus P. Ashton. In Soerinegara, I. & Lemmens, R. H. M. J. (eds.), Plant Resources of South-East Asia (PROSEA) 5(1). Timber trees: major commercial timbers. Backhuys Publishers, Leiden.
Wyatt-Smith, J. (1987) Manual of Malayan Silviculture for inland forest. Part III - Chapter 8: Research Pamphlet 101: 1-89.
Yap, S. K. (1985) Gregarious flowering of dipterocarps: observations based on fixed tree populations in Selangor and Negri Sembilan, Malay Peninsula. In Kostermans, A. J. G. H. (ed), Proceedings of 3rd Roundtable Conference on Dipterocarps. UNESCO, Jakarta.
|
[9] Head of Seed Technology
Section, Forest Research Institute Malaysia (FRIM), Kepong 52109, Kuala Lumpur,
Malaysia, Tel: +60-3-6274 2633, Fax: +60-3-6276 5531, E-mail:
[email protected]. |